INTRODUCTION
Breast cancer is the most common cancer and the leading cause of death from cancer in females worldwide [1]. Despite the unfortunate outcome that gained global attention, breast cancer is still late diagnosed until the advanced stages due to women’s negligence in performing breast self-inspection and clinical examination [2]. Poor prognosis was associated with locally advanced and metastatic breast cancer, with an average survival rate of less than 5 years. Furthermore, 10% of newly diagnosed breast cancer progressed to a metastatic stage [3]. Hence, it set a limitation on the early stage first choice of treatment by surgical tumor resection [4]. Chemotherapy served as the second option after the surgery, though it came along with adverse side effects. Not only targeting malignant cells, but these drugs are also toxic to healthy growing cells. It faces a major drawback as some tumors have developed chemotherapy resistance [3]. New approaches and anti-tumor compounds are constantly sought, and natural sources such as plants provide an abundance of promising anti-cancer drugs [5].
Apoptosis, defined as programmed cell death, is an important body mechanism for removing excess, damaged, or harmful cells [6]. Apoptosis dysregulation, which is a hallmark of cancer, disrupts the established balance of cell proliferation and cell death [7]. Numerous molecules, particularly the caspase signaling cascade, play roles in regulating cell apoptosis [1]. Caspase-3 is a key pro-apoptotic caspase [6]. Aberrant Caspase-3 expression is linked with several cancer types [1]. Former research displayed that high caspase-3 expression is associated with a more favorable prognosis in hepatocellular carcinoma and nonsmall cell lung cancer [8].
The process of programmed cell death is crucial in the metastatic process. Cancer cells must be able to avoid various forms of cell death to metastasis [9]. Metastasis occurs when uncontrollable growth cells infiltrate adjacent body parts or spread to other distant body organs [10]. Diagnosed with metastatic cancer is associated with the worst prognosis [11]. Multiple cell processes, including epithelial-mesenchymal transition (EMT), extracellular matrix degradation, and tumor angiogenesis have been demonstrated to be significant parts of cancer metastasis [9].
EMT is a biological process that enables mesenchymal phenotype changes in polarized epithelial cells that normally interact with the basement membrane through the basal surface [12–14]. Epithelial cells lose cell-to-cell junctions, apical-basal polarity, and epithelial markers; however, the cells acquire cell motility, a spindle-cell shape, and mesenchymal markers [14,15]. Mesenchymal markers such as Vimentin, Snail, and N-cadherin were discovered, while epithelial markers such as E-cadherin, occludins, and desmoplakin were lost during EMT [15,16]. Overexpression of Vimentin and Snail has been linked to increased tumor growth, invasion, and poor prognosis, as well as being a predictor of an aggressive tumor phenotype [17,18].
Caulerpa racemosa, widely addressed as sea grapes, is marine widely consumed seaweed. The high bioavailability of seaweeds displayed a potent bio-resource not only for functional food but also as medicine due to a broad range of biological activities [19,20]. These compounds, such as alkaloids, caulerpin, racemosins, and other interesting discoveries, gain interest as they possess various bioactivities: anti-inflammatory, cytotoxic, anti-proliferative, and anti-metastatic [19,21–23]. A previous study demonstrated C. racemosa’s anti-cancer and anti-metastatic potential in HeLa cells by inhibiting cancer cell migration and lowering Snail and Vimentin expression [24]. However, there has been limited research into C. racemosa’s potent anti-cancer activity against breast cancer. The activity of C. racemosa extract from Indonesia as an anti-cancer and anti-metastatic agent in apoptosis induction, upregulation of caspase-3 expression, and influences on Snail and Vimentin protein expression levels in breast cancer cells was investigated in this study.
MATERIALS AND METHODS
The in vitro study was performed on MCF-7 cells, at the Biomedical and Biomolecular Laboratory, Faculty of Medicine, Brawijaya University, Malang, Indonesia. The study protocol was approved by the Institutional Health Research Ethic Committee, with approval number: 18/EC/KEPK/01/2022 dated: January 31, 2022.
MCF-7 breast cancer cell culture
MCF-7 human breast cancer cells were obtained from the Biomedical Laboratory, Medical Faculty, Brawijaya University. The MCF-7 cells were cultured in 24-well plates containing Dulbecco’s Modified Eagle Medium (DMEM) media (Invitrogen, Massachusetts) supplemented with 10% fetal bovine serum (FBS), and antibiotic (100 UI/ml Penicillin and 100 μl/ml Streptomycin), then maintained at a pH of 7.2–7.4 (Fig. 1). After the cultured cells reached 80% density, they were incubated at 37°C in an incubator with 5% CO2. Cells were periodically harvested using trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA) solution [25].
Caulerpa racemosa extract preparation
Sea grapes (C. racemosa) were obtained from the Mantehage Sea, North Sulawesi, Indonesia. Species identification and authentication were confirmed at the Pharmacology Department, Faculty of Mathematics and Sciences, Sam Ratulangi University, Manado, Indonesia. Finalization of the extract was conducted in the Biochemistry Laboratory, Faculty of Medicine, Brawijaya University. Raw C. racemosa was rinsed in water, dried at room temperature, and ground with an electric grinder. The coarse powder (1,000 g) was macerated in 96% ethanol for 72 hours and evaporated in a 40°C oven to produce a thick extract (with a 34% yield) and then dissolved in dimethyl sulfoxide. Each extraction process was carried out in triplicate. The crude extract was filtered with the Whatman 41 filter paper. The filtered crude extract was concentrated at 40°C with a rotary evaporator (RV 8, IKA Rotation Evaporator) under low pressure (100 millibars) for 90 minutes. The crude extracts were stored in a refrigerator at 10°C [26]. Based on the previous study, the metabolite profile content of C. racemosa extract was examined using liquid chromatography-mass spectrometry and yielded a mass of 39,813,278 (39,712,257 m/z), indicating the presence of the antioxidant compound caulerpin in the extract [27]. Caulerpin is a secondary metabolite of C. racemosa that is a potent anti-cancer compound [27]. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay to investigate the safety profile of C. racemosa, and the LC50 value for a macerated extract of C. racemosa after 24 hours of incubation was 914.78 g/ml. The safety profile result indicates that C. racemose extract was safe to develop into various products.
Trypan blue exclusion test
The cultured cells were first transferred to a 24-well plate (Matsunami, Japan) and incubated for 24 hours. MCF-7 cells were administered with C. racemosa extract containing caulerpin at the concentration of 0 μg/ml as a control, 100 μg/ml, 200 μg/ml, and 400 μg/ml. Viable cells were examined using the trypan blue exclusion method after being treated with C. racemosa extract containing caulerpin. The cells on the well plate were placed into a tube and grouped according to the extract dose. They were centrifuged, while the supernatant was discarded. A mixture of 10 μl of cells, 10 μl of complete medium, and 20 μl of trypan blue was evaluated using a hemacytometer under a binocular microscope (Fig. 1) [28]. To minimize the risk of bias, the number of cell viability was calculated three times for each treatment group.
Annexin-V/PI staining
Utilizing the Annexin-V-FITC-PI kit (Sigma-Aldrich, St. Louis, MO), flow cytometry was applied to assess the extent of cell apoptosis. After being washed twice with cold cell staining buffer, MCF-7 cells were resuspended in Annexin-V binding buffer at a concentration of 0.5–1.0 × 107 cells/ml. The cell suspension was subsequently transferred to a 5 ml test tube along with 5 l of FITC Annexin-V and 10 l of a propidium iodide solution at a concentration of 20 g/ml. Following that, the cells were slowly vortexed and incubated in the dark for 15 minutes at room temperature (25°C). After that, 400 l of Annexin-V binding buffer was added to each tube, which was subsequently analyzed through a flow cytometer set to the suitable machine settings (Fig. 1). Cell Pro Quest software was used to analyze the data.
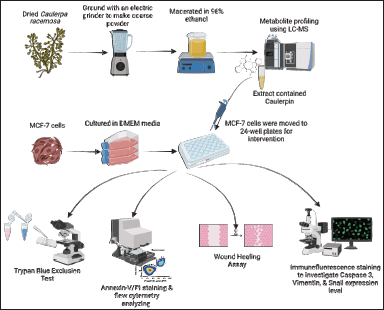 | Figure 1. Methods for testing the anticancer effect of C. racemosa on MCF-7 cells. [Click here to view] |
Wound healing assay
MCF-7 cells were cultured in DMEM media until they formed monolayers in 24-well plates. A micropipette tip was used to scratch a single line to form a space between cells in each well. DMEM medium was used to wash away the dislodged cells. After the scratch generated a space between cells, the cells were grown in starvation media (DMEM with FBS 0.1%) to ensure the cells could not proliferate and could only migrate to close the space. Then, varying doses of extract (0, 100, 200, and 400 μg/ml) were added. Every 3 hours, the wound-closing process was monitored until the scratch in the control was completely closed. ImmunoRatio ImageJ application was utilized to calculate the scratch width between MCF-7 cells.
Observation of caspase-3, Vimentin, and Snail expression by immunofluorescent assay
Immunofluorescence staining was used to look at the expression of caspase and other apoptosis-related proteins in cell samples at the same time. This method was also used to assess the expression level of Snail and Vimentin proteins. Cell cultures were grown in 24-well plates with a circular glass cover with an 18-mm diameter fitted to the bottom of each well (Matsunami, Japan). Cells were washed twice with Phosphate-buffered saline (PBS) before being fixed in 4% formaldehyde in PBS for 15 minutes at room temperature. Following that, the cells were washed twice with PBS and permeabilized for 5 minutes in PBS with 2 ml 0.1%–0.5% Triton X-100 at 4°C. Then, triton X-100 was aspirated, and cells were washed three times with PBS. Afterward, a 200 μl blocking buffer (10% goat serum, 2% bovine serum albumin, 0.2% Triton-X) was added to the slide. Then, the slides were incubated for 2 hours at room temperature. After incubation, the slides were rinsed once again with PBS.
A primary antibody with a ratio 1:100 was applied to each slide. The primary antibodies used were anti-rabbit cleaved caspase-3 (Cell Signaling Technology, Danvers, USA) to stain caspase-3 protein, anti-rabbit Vimentin Cell Signaling Technology, Danvers, MA) to stain Vimentin protein, and anti-mouse FITC Snail-1 (Santa Cruz, CA) to stain Snail protein. Slides without primary antibodies were prepared as negative controls. After that, the slides were incubated in a humid room overnight at 4°C. The next day, the slides were irrigated with PBS three times for 10 minutes and then dried at room temperature. The slides were then treated with 100 μl of secondary antibody diluted 1:1,000 in PBS. The secondary antibodies used were Alexa Fluor-488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) and Alexa Fluor-488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA). The nucleus was stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO) 1 g/ml for 15 minutes and then washed 6 times with PBS. The evaluation was performed using an Olympus IX71 inverted fluorescence microscope with a magnification of 200× [25,29].
Data collection and statistical analysis
ImageJ software was used for the result quantification of cleaved caspase-3, Snail, and Vimentin protein expression. The data outcome was the intensity of these proteins expressed as pixels. Statistical analysis of scratch width, cleaved caspase-3, Snail, and Vimentin proteins expression data was carried out using GraphPad Prism 8 software and IBM SPSS version 26 for MacBook. Shapiro-Wilk test was performed to evaluate data distribution. If the data were normally distributed (p > 0.05), a One-Way ANOVA test was conducted to examine the difference in average between treatment groups. Otherwise, the Kruskal-Wallis test would be performed if the data were not normally distributed (p < 0.05). If the test results are significant (p < 0.05), the data will be analyzed further using Dunn’s Post hoc test to determine the significance between groups. Subsequently, post-tests and correlation tests were carried out. The data were presented in the form of a diagram.
RESULTS
Results of cell viability test using Trypan blue exclusion method
The Trypan blue viability test revealed that the 400 μg/ml dose group had the least amount of viable MCF-7 cancer cells compared to the other dose groups. Furthermore, there was a significant difference in post-treatment viable cells compared to the control group (p < 0.05). Although there was no significant difference in cell viability between the 100, 200, and 400 μg/ml dosage groups, a consistent decrease in cell viability was observed. In general, the mean and SD of viable cell amounts in each group were as follows: 0 μg/ml (990 × 103 ± 26.46), 100 μg/ml (836 × 103 ± 55.08), 200 μg/ml (796 × 103 ± 153.7), and 400 μg/ml (546 × 103 ± 58.59). The viability test results demonstrated that a dose of 400 g/ml was the best dose with the lowest number of cancer cell viability, indicating high apoptotic activity (Fig. 2). There was a significant negative correlation between the dose and the viability of MCF-7 cells (r = −0.877). Thus, the higher concentration of C. racemosa displayed stronger activity to reduce the viability of MCF-7 cells.
Cleaved caspase-3 expression level after administration of C. racemosa extract
Figure 3 shows that increasing the dose of C. racemosa extract was associated with increased expression of cleaved caspase-3 in MCF-7 cells. Caspase-3 expression was significantly higher in cells treated at 400 g/ml compared to the control group (p < 0.05) (Table 1). In line with previous findings, the dose-dependent relationship between C. racemosa concentration and caspase-3 expression revealed a dose-dependent effect, indicating that the higher the dose of C. racemosa, the higher the caspase-3 expression in MCF-7 cancer cells. The lowest mean and SD were in the control group (0 ± 0) and the highest was in the 400 μg/ml group (44.83 ± 19.18). In addition, Figure 3 can also be observed qualitatively, the high intensity of caspase-3 can be seen from the red scattering of immunofluorescent staining at doses of 200 and 400 μg/ml.
Data showing an increase in the expression level of caspase-3 protein through immunofluorescence staining using the anti-rabbit cleaved caspase-3 primary antibody after administration of C. racemosa extract was strengthened by data from flow cytometry of MCF-7 cells (Fig. 4). The results of MCF-7 cell flow cytometry showed an increase in the percentage of total late apoptosis in accordance with increasing of extract concentration (Table 2). This shows a correlation that increasing the level of cleaved caspase-3 protein expression after administration of the extract will induce apoptosis of MCF-7 cells.
Cell migration assessment using wound healing assay
After 24 hours of incubation, the scratch test (wound healing assay) was performed to determine the rate of cell migration activity. ImageJ software was used to measure the width of the scratches. After 24- and 48-hour interventions, the width (in pixel units) between scratch cells was measured (Table 3). There were significant differences in cell gap width between the 0 g/ml group and the 100, 200, and 400 g/ml groups after 24 hours of intervention (p < 0.001). This scratch test revealed that an increase in C. racemosa concentration was associated with a wider gap between two MCF-7 cell populations that had previously been separated by the scratch. There was an inhibition of MCF-7 cancer cell migration rate as a consequence of the treatment (Fig. 5). A similar result was obtained after 48 hours of intervention, in which statistically significant differences were seen between all groups of 0, 100, 200, and 400 μg/ml (p < 0.0001). A strong positive correlation (r = 0.808) between C. racemosa extract dose and a wide range was displayed from the scratch test 24- and 48-hours of MCF-7 cancer cells.
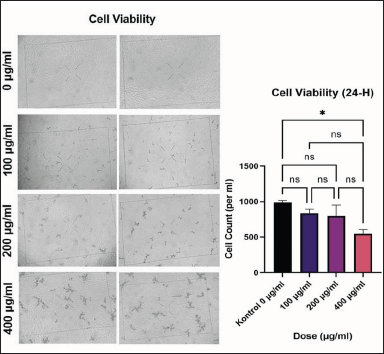 | Figure 2. Cell viability of MCF-7 after 24 hours of treatment and diagram of comparison of total cell viability between treatment groups *p < 0.05. [Click here to view] |
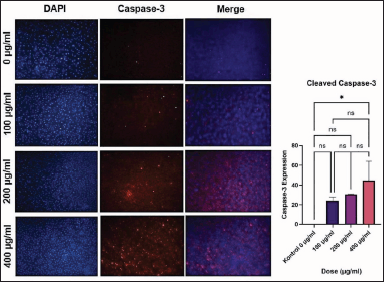 | Figure 3. The intensity of caspase-3 expression of MCF-7 cells by immunofluorescence staining (red) and diagram of comparison of caspase-3 intensity between treatment groups. *p < 0.05. [Click here to view] |
Snail protein expression level after administration of C. racemosa extract
The result of the Kruskal-Wallis test showed that the independent variable (dose of C. racemosa extract) and the dependent variable (Snail protein expression level) were statistically significant with a p-value of 0.003 (p < 0.05) (Table 1). Dunn’s Post hoc test showed a significant p-value of 0.003 (p < 0.05) between cells treated at a dose of 400 μg/ml compared to control. The Spearman correlation test revealed a negative association between C. racemosa extract dose with Snail expression with a very strong degree of negative correlation (r = −0.958). According to this correlation test, the higher the dose of C. racemosa extracts administered, the lower the amount of Snail expression (Fig. 6).
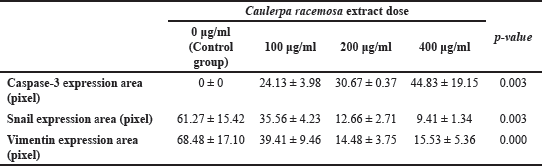 | Table 1. The difference in caspase-3, Snail, and Vimentin expression area between varying doses of C. racemosa extract. [Click here to view] |
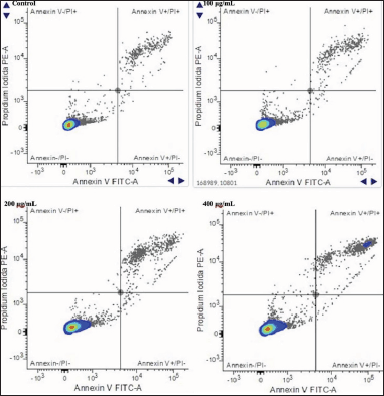 | Figure 4. Increasing the apoptotic activity of MCF-7 cells after 24 hours of incubation with C. racemosa extract. Total apoptosis in MCF-7 cells was measured using flow cytometry with annexin-V/PI staining. Annexin-/PI- : MCF-7 viable cells; Annexin-V-/PI+ : necrotic cells; Annexin-V+/PI- : early apoptosis; Annexin-V+/PI+ : late apoptosis. [Click here to view] |
 | Table 2. Apoptosis activity rate in MCF-7 cells between varying doses of C. racemosa extract. [Click here to view] |
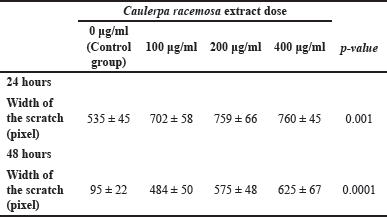 | Table 3. Post 24- and 48-hours scratch test shows cell scratch width (pixels). [Click here to view] |
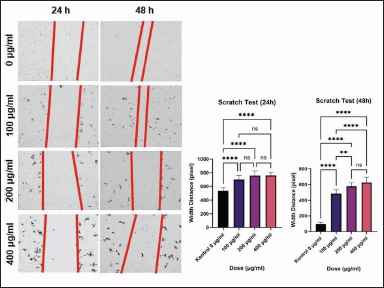 | Figure 5. Diagram of comparison of scratch test between treatment groups and MCF-7 cells migration activity by wound healing assay or scratch test. **** p < 0.001 (24 hours); **** p < 0.0001 (48 hours); **p < 0.05. [Click here to view] |
Vimentin protein expression level after administration of C. racemosa extract
The ANOVA test revealed a significant relationship between Vimentin expression and C. racemosa extract, with a p-value of 0.000 (p < 0.05) (Table 1). A significant difference was found when the overall treatment dose groups were compared using Tukey Multiple Comparison (p < 0.05). Pearson correlation test revealed a negative association between C. racemosa extract dose with Vimentin expression with a very strong degree of negative correlation (r = −0.914). According to this correlation test, the higher the dose of C. racemosa extracts administered, the lower the amount of Vimentin protein expression (Fig. 7).
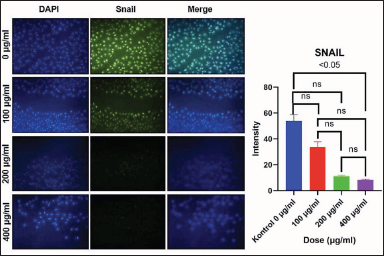 | Figure 6. The intensity of Snail expression of MCF-7 cells by immunofluorescence staining and diagram of comparison of Snail intensity between treatment groups. Decreased Snail protein after C. racemosa extract treatment. MCF-7 were stained for Snail (green). DAPI (blue) indicates MCF-7 cell nuclei. [Click here to view] |
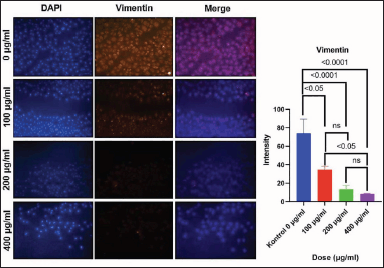 | Figure 7. The intensity of Vimentin expression of MCF-7 cells by immunofluorescence staining and diagram of comparison of Vimentin intensity between treatment groups. Decreased Vimentin protein after C. racemosa extract treatment. MCF-7 was stained for Vimentin (orange). DAPI (blue) indicates MCF-7 cell nuclei. [Click here to view] |
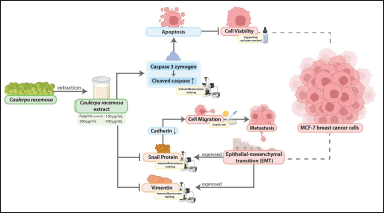 | Figure 8. Graphical abstract. Multiple effects of C. racemosa extract to breast cancer cells in MCF-7 cells model. Enhance the presence of active cleaved caspase-3 thus inducing apoptosis and reducing a Snail and Vimentin protein expression causing inhibition in the metastasis process. [Click here to view] |
DISCUSSION
The most common cancer in women is breast cancer [30]. Varying previous research identified secondary metabolites and crude extracts of the Caulerpa species as promising anti-cancer candidates [19,24,27]. Caulerpin, the major constituent of C. racemosa demonstrated in-vitro anti-tumor activity [7]. Caulerpenyne (Cyn), possesses anti-neoplastic, anti-mitotic, and anti-proliferative activity. It was found to be cytotoxic to several human tumor cells [19]. Breast cancer action is mediated by estrogen hormone and estrogen receptors (ERs) [31]. The presence of ER alpha is critical for a favorable prognosis and response to hormonal therapy. Molecular in silico studies of the ER protein with caulerpin and the standard drug, mitoxantrone, revealed lower binding (−11.18 kcal/mol) compared to standard drugs (−8.45 kcal/mol) [32]. The findings of this study support the possibility of developing this extract as a breast cancer treatment.
A study of C. lentillifera extract in human breast cancer MCF-7 cells revealed the role of caulerpin in the activation of caspase-3 and caspase-9, which subsequently induced apoptosis [7,19]. Polyphenols and flavonoids derived from Caulerpa sp. influence the expression of proteins involved in the cell cycle, resulting in cell cycle arrest [5]. The antioxidant activity of phenols and flavonoids as chemoprevention is capable of neutralizing free radicals (ROS) to prevent cell damage and further progression into malignancy [5]. However, “there has been no study” of C. racemosa extract as an apoptosis inducer by altering the expression level of caspase-3 and anti-metastatic by altering EMT’s protein regulators for breast cancer, particularly in the MCF-7 cell model.
This study revealed the potent anti-cancer effect of crude C. racemosa extracts in MCF-7 human breast cancer cells by inducing apoptosis. Administration of various doses of the crude extract increased the percentage of apoptotic activity in the MCF-7 cells shown in the cell viability test (Fig. 2). Enhanced apoptotic activity along with increasing crude extract dose was evidenced by correlation value r = 0.877 and p < 0.05. This experiment’s results are consistent with previous studies that demonstrated the anti-apoptotic activity of C. racemosa crude extract in neuroblastoma, cervical, and colon cancer cell lines [19,21,33]. Based on the biomolecular view of apoptosis activity, the mechanism of apoptosis involves various proteins to regulate and operate the apoptosis process. The most important protein for apoptosis, thus, as a marker for apoptotic activity is a caspase protein.
Caspase proteins are currently classified into three types: inflammatory caspase, initiator caspase, and effector caspase [18]. Caspase-3 is an effector protein for an apoptotic process that directly leads a cell death [1,8,18]. As an inactive dimer, caspase is activated by proteolytic cleavage which turns caspase-3 zymogen into cleaved caspase-3 [6]. The pro-apoptotic activity of C. racemosa extracts was linked to an increase in the expression of the pro-apoptotic protein caspase-3 (Fig. 8). Increased cleaved caspase-3 indicated apoptosis induction [8]. Cleaved caspase-3 was evaluated with immunofluorescence staining (Fig. 3). There was an increase in caspase-3 expression, respectively, to C. racemosa extracts dose increment in MCF-7 cells (r = 0.783, p < 0.05). Caspase-3 expression was also dose-dependent, with a higher dose of C. racemosa extracts inducing higher caspase-3 expression in MCF-7 cancer cells (Table 1). Based on the experiment work, it can be concluded that administration of a crude extract from C. racemosa is able to enhance the expression of a caspase-3 protein that has a role as an effector protein to leading cell death in MCF-7 cells. It is in accordance with another experiment result that showed an administration of a crude extract could induce an apoptosis activity proven by decreasing MCF-7 cancer cell viability (low cell amount). Based on the findings, we can conclude that crude extract of C. racemosa is capable of inducing apoptosis in MCF-7 cells by increasing caspase-3 expression.
The experiment also demonstrated that crude extract has anti-metastatic properties by inhibiting cell migration. The inhibitions of migration along with the increase of the extract doses given were evidenced by the correlation value r = 0.808 and p < 0.001 (Fig. 5). MCF-7 was treated with 400 μg/ml doses is the most effective in inhibiting cell migration as evidenced by the difference in the width of the scratch between pre- and post-treatment, which means that cell migration is successfully inhibited when compared to cells without treatments (Table 3). Metastasis of breast cell cancer involves a process called EMT [19,21]. EMT will alter the characteristics of epithelial cells and give rise to mesenchymal cells. Besides that, polarity and attachment of epithelial cells to the basal membrane are lost, and then, it makes the cell apart from basal to circulation and metastasis occurs [24]. Based on several pieces of literature, there are three proteins that are involved during the EMT process such as Vimentin and Snail [25, 34–36].
Snail protein is a zinc finger protein family that has a main role in regulating cancer cell metastasis [25,37]. Snail protein is able to bind with a gene promoter which encodes a cadherin protein and acts as an inhibitor protein, thus the transcription rate of cadherin can be decreased through disruption of RNA polymerase binding [25,38,39]. cCadherin protein is a class of transmembrane glycoproteins that serve as anchors for cells in tissues or cells with basement membranes [40]. According to the statement, the cadherin protein is required for cell attachment in tissue. In a cancer cell, the level expression of Snail protein is higher than in the normal cell, thus it influences the decreasing expression level of cadherin and makes the cells detach each other easily [25,37,39]. Snail protein expression level was evaluated with immunofluorescence staining (Fig. 6). Treating MCF-7 cells using crude extract of C. racemosa is able to decrease the expression level of Snail protein (Table 1). This result is strengthened with a significant p-value (p = 0.003) and r-value (r = −0.958) in statistical analysis. Decreasing of Snail expression level leads to an increase in the cadherin expression level [25]. Eventually, the adhesion between cells is strengthened, thus, migration activity becomes lower (Fig. 8). It is in accordance with the wound healing assay or scratch test result that shows the decrease of cell migration along increasing in extract dose (Fig. 5).
According to recent research, carcinoma cells that have completed their EMT process will develop a mesenchymal phenotype and express mesenchymal markers such as Vimentin [25,34,35]. Vimentin levels will eventually rise as a result of increased EMT activity in carcinoma cells [25,35]. Treatment of C. racemosa extract is able to decrease Vimentin expression in MCF-7 cells (Table 1). This expression was observed using immunofluorescence and Vimentin expression was seen to be significantly decreased when treated with C. racemosa extract, especially in the dose of 400 μg/ml (Fig. 7). This result is strengthened with a significant p-value (p = 0.000) and r-value (r = −0.914) in statistical analysis. According to these findings, C. racemosa extract can inhibit EMT in breast cancer cells indicating with decrease in the presence of Vimentin protein in the cells (Fig. 8). This is a preliminary study that reveals caulerpin of C. racemosa extract has potent pro-apoptotic activity against MCF-7 human breast cancer cells. However, further steps of the experiment include continuing to in-vivo study to strengthen the findings of this experiment before entering the clinical trial. Furthermore, the toxicity and adverse effects of C. Racemosa could be investigated further.
CONCLUSION
Caulerpa racemosa extract exhibited a potential anti-cancer agent through pro-apoptotic activity in MCF-7 breast cancer cells. The extract reduced MCF-7 cell viability by increasing the expression of pro-apoptotic protein, cleaved caspase 3. Furthermore, an anti-metastatic activity of C. racemosa extract was demonstrated in the experiment through wound healing assay by inhibition of Snail protein expression level and also decrease of Vimentin expression level indicating that the extract is capable of inhibiting the progression of MCF-7 cells during the EMT process for their metastasis.
ACKNOWLEDGMENTS
The authors would like to thank all contributors who cannot be named individually for their outstanding assistance in formatting this paper and research.
AUTHOR CONTRIBUTIONS
HKP, NA, FRQ, and NIT conducted an experiment, analyzed data, conceptualized, and designed the study, and wrote the manuscripts. DIH, ADS, VMY, SAN, WR, and WB contributed to processing the pictures, graphical abstracts, data interpretation, writing manuscripts, and editing. All authors have read and approved the final manuscript.
FINANCIAL SUPPORT
The entire experiment and works received a fund from Badan Penelitian dan Pengabdian Masyarakat Fakultas Kedokteran Universitas Brawijaya with Dean Letter No. 135 Year 2022.
CONFLICTS OF INTEREST
All authors and contributors (HKP, NA, FRQ, DIH, ADS, NIT, VMY, SAN, WR, and WB) to the study declare no conflicts of interest.
ETHICAL APPROVALS
The study protocol was approved by the Health Research Ethic Committee, Faculty of Medicine, Brawijaya University with approval number 18/EC/KEPK/01/2022 on January 31, 2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ke H, Wang X, Zhou Z, Ai W, Wu Z, Zhang Y. Effect of weimaining on apoptosis and caspase-3 expression in a breast cancer mouse model. J Ethnopharmacol. 2021 Jan 10;264:113363. CrossRef
2. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50:1–23. CrossRef
3. Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020 Jul 1;84:106535. CrossRef
4. Wadasadawala T, Vadgaonkar R, Bajpai J. Management of isolated locoregional recurrences in breast cancer: a review of local and systemic modalities. Clin Breast Cancer. 2017 Nov 1;17(7):493–502. CrossRef
5. Murugan K, Iyer VV. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine algae. In Vitro Cell Dev Biol Anim. 2013 May;49(5):324–34. CrossRef
6. Lei Q, Huang X, Zheng L, Zheng F, Dong J, Chen F, et al. Biosensors for caspase-3: from chemical methodologies to biomedical applications. Talanta. 2022 Apr 1;240:123198. CrossRef
7. Mehra R, Bhushan S, Bast F, Singh S. Marine macroalga Caulerpa: role of its metabolites in modulating cancer signaling. Mol Biol Rep. 2019;46:3545–55. CrossRef
8. Pu X, Storr SJ, Zhang Y, Rakha EA, Green AR, Ellis IO, et al. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis. 2017 Mar 1;22(3):357–68. CrossRef
9. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:1–4. CrossRef
10. Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7(5):1016.
11. Bhutada JKS, Hwang AE, Liu L, Tsai KY, Deapen D, Freyer DR. Risk of presenting with poor-prognosis metastatic cancer in adolescents and young adults: a population-based study. Cancers (Basel). 2022 Oct 1;14(19):4932. CrossRef
12. Lim RBT, Loy EY, Lim GH, Zheng H, Chow KY, Lim ST. Gender and ethnic differences in incidence and survival of lymphoid neoplasm subtypes in an Asian population: secular trends of a population-based cancer registry from 1998 to 2012. Int J Cancer. 2015 Dec 1;137(11):2674–84. CrossRef
13. Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008 Jun 5;2(6):511–2. CrossRef
14. Savagner P. The epithelial–mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010 Oct 1;21(SUPPL. 7):vii89–92. CrossRef
15. Lai X, Li Q, Wu F, Lin J, Chen J, Zheng H, et al. Epithelial-mesenchymal transition and metabolic switching in cancer: lessons from somatic cell reprogramming. Front Cell Dev Biol. 2020;8:760. CrossRef
16. Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15(1):1–4. CrossRef
17. Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. CrossRef
18. Lundgren K, Nordenskjöld B, Landberg G. Hypoxia, snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009 Nov;101(10):1769–81. CrossRef
19. Tanna B, Yadav S, Mishra A. Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol Biol Rep. 2020 Oct 1;47(10):7403–11. CrossRef
20. Permatasari HK, Bulain S, Amar N, Azizah MR, Muslim FZ, Daud VPA, et al. Anticancer properties of Caulerpa racemosa: a review study. Nutr Clin Diet Hosp. 2022;42:110–21.
21. Yang P, Liu DQ, Liang TJ, Li J, Zhang HY, Liu AH, et al. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg Med Chem. 2015 Jan 1;23(1):38–45. CrossRef
22. Shah MD, Maran BAV, Shaleh SRM, Zuldin WH, Gnanaraj C, Yong YS. Therapeutic potential and nutraceutical profiling of North Bornean seaweeds: a review. Mar Drugs. 2022;20(2):101. CrossRef
23. Nurkolis F, Taslim NA, Qhabibi FR, Kang S, Moon M, Choi J, et al. Ulvophyte green algae Caulerpa lentillifera: metabolites profile and antioxidant, anticancer, anti-obesity, and in vitro cytotoxicity properties. Molecules [Internet]. 2023 Jan 31;28(3):1365. Available from: https://www.mdpi.com/1420-3049/28/3/1365 CrossRef
24. Permatasari HK, Barbara Ulfa EN, Adyana Daud VP, Sulistomo HW, Nurkolis F. Caulerpa racemosa extract inhibits HeLa cancer cells migration by altering expression of epithelial-mesenchymal transition proteins. Front Chem. 2022 Nov 21;10:1052238. CrossRef
25. Permatasari HK, Effendi AB, Qhabibi FR, Fawwaz F, Dominique A. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J Appl Pharm Sci. 2021 May 1;11(5):49–53.
26. Kuswari M, Nurkolis F, Mayulu N, Ibrahim FM, Taslim NA, Wewengkang DS, et al. Sea grapes extract improves blood glucose, total cholesterol, and PGC-1α in rats fed on cholesterol- and fat-enriched diet. F1000Res. 2021 Aug 2;10:718. CrossRef
27. Permatasari HK, Wewengkang DS, Tertiana NI, Muslim FZ, Yusuf M, Baliulina SO, et al. Anti-cancer properties of Caulerpa racemosa by altering expression of Bcl-2, BAX, cleaved caspase 3 and apoptosis in HeLa cancer cell culture. Front Oncol. 2022 Sep 20;12:964816. CrossRef
28. Paramee S, Sookkhee S, Sakonwasun C, Na Takuathung M, Mungkornasawakul P, Nimlamool W, et al. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement Altern Med. 2018 Jun 11;18(1):1–3. CrossRef
29. Sorrells S, Toruno C, Stewart RA, Jette C. Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated caspase 3. J Vis Exp. 2013 Dec 20;(82):e51060. CrossRef
30. Pattar SV, Adhoni SA, Kamanavalli CM, Kumbar SS. In silico molecular docking studies and MM/GBSA analysis of coumarin-carbonodithioate hybrid derivatives divulge the anticancer potential against breast cancer. Beni Suef Univ J Basic Appl Sci. 2020 Dec 1;9(1):1. CrossRef
31. Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;2012:1–8. CrossRef
32. Dissanayake IH, Bandaranayake U, Keerthirathna LR, Manawadu C, Silva RM, Mohamed B, et al. Integration of in vitro and in-silico analysis of Caulerpa racemosa against antioxidant, antidiabetic, and anticancer activities. Sci Rep. 2022 Dec 1;12(1):20848. CrossRef
33. Yap WF, Tay V, Tan SH, Yow YY, Chew J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics. 2019 Sep 1;8(3):152. CrossRef
34. Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs. 2007;185(1–3):191–203. CrossRef
35. Usman S, Waseem NH, Nguyen TKN, Mohsin S, Jamal A, Teh MT, et al. Vimentin is at the heart of epithelial mesenchymal transition (Emt) mediated metastasis. Cancers. 2021;13(19):4985. CrossRef
36. Yu H, Shen Y, Hong J, Xia Q, Zhou F, Liu X. The contribution of TGF-β in epithelial–mesenchymal transition (EMT): down-regulation of E-cadherin via snail. Neoplasma. 2015;62:1–15. CrossRef
37. Wu Y, Zhou BP. Snail: more than EMT. Cell Adh Migr. 2010;4:199–203. CrossRef
38. von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, et al. E-Cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009 Jul 1;137(1):361–71.e5. CrossRef
39. Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–63. CrossRef
40. Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A Transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006 Sep 1;131(3):830–40. CrossRef