INTRODUCTION
Alzheimer’s disease (AD) is one of the most progressive neurodegenerative disorders in individuals beyond 65 years of age resulting in loss of neurons, and eventually dementia. It is an untreatable disorder with progressive and longer duration (Goedert and Spillantini, 2006) and represents over 80% cases of dementia in the world in elderly individuals. It results in progressive loss of behavioral, mental, functional decline, and other intellectual abilities (Anand et al., 2019). Age is the most considerable risk factor; as in 2010, 38% of individuals beyond 85 years of age were troubled with AD which is anticipated to escalate to 51% by 2050 (Herbert et al., 2013). Various cardiovascular risk factors, for example, atherogenic dyslipidemia, hypertension, obesity, and diabetes are also associated with AD (Oesterling et al., 2014). Because of its constantly expanding rate and societal aging factor, AD has garnered enormous research interest.
Pathophysiology
AD can be distinguished by significant atrophy of the cerebral cortex as well as loss of cortical and subcortical neurons. The neurotic signs of this disorder are senile plaques that are spheric aggregations of the protein beta-amyloid along with declining activities of neurons (also referred to as cerebral amyloid angiopathy) and neurofibrillary tangles (NFTs), consisting of paired helical filaments and other proteins (Braak and Braak, 1994). Neuronal loss as well as pathology might be observed especially in the amygdala, hippocampus, entorhinal cortex and the cortical associated regions of the frontal, temporal, and parietal cortices, and also subcortical nuclei. The tangles deposition occurs in a specific pattern, beginning from the trans-entorhinal cortex; followed by the entorhinal cortex, the CA1 part of the hippocampus and then the cortical associated regions, where frontal, parietal, and temporal lobes are especially influenced. The degree and position of tangle arrangement associates well with the seriousness of dementia, significantly more than quantities of amyloid plaques (Kumar et al., 2015; Thakur et al., 2018).
Advanced AD is characterized by large number of senile plaques and NFTs mostly in the hippocampus and related areas of the cortex, while regions like the visual and motor cortices are moderately spared. This relates to the clinical characteristics of noticeable disability of memory and theoretical thinking, with conservation of vision and movement. Parameters responsible for the particular susceptibility of specific cortical neurons to the pathological outcomes of AD are still unknown (Hardman et al., 2006).
The accumulation of tau proteins correlates intimately with intellectual decay and atrophy of brain along with hippocampus. The neuronal pathology of AD involves neuronal loss and temporofrontal cortex atrophy, leading to inflammation, deposition of amyloid plaques, and unusual accumulation of fragments of protein and tangled bundles of filaments. This results in a rise in macrophages and monocytes in the cerebral cortex, and activation of the microglial cells in the parenchyma (Thakur et al., 2018). The pathophysiology of AD is exceptionally intricate and relies upon various pathological processes, which have been summed up in Figure 1 and are described in the preceding text.
MANAGEMENT OF AD
The overall management of AD includes pharmacological as well as non-pharmacological treatments. Non-pharmacological approach basically addresses different causes of cognitive impairment and behavioral disturbances. Pharmacological approach, which is described as symptomatic or neuroprotective is based on modulation of disease-related neurotransmitter alterations. At present, approved symptomatic treatment includes cholinesterase inhibitors (ChEIs) and N-methyl-D-aspartate (NMDA) receptor antagonists, (Kumar et al., 2015) that depends on their ability to retard the clinical development of symptoms across intellectual, behavioral, and functional areas. Currently approved some of the disease modifying therapeutic drug targets are highlighted in Table 1.
Initially, pharmacological approach for AD was aimed to increase cholinergic transmission in the brain (cholinergic hypothesis). Among the various approaches utilized to raise synaptic levels of acetylcholine (ACh), hindering the disintegration of ACh by acetylcholinesterase (AChE) inhibition was proved to be successful. Inhibition of butyrylcholinesterase (BuChE) enzyme, which is present in lower amounts in normal brain, is present in greater amount in AD brain may also enhance cholinergic transmission (Thakur et al., 2018). Novel approaches of targeting the Aβ and tau-based therapeutics can be significant keys to cater the disease (Anand et al., 2014).
 | Figure 1. Hypothesis for pathophysiology of Alzheimer’s disease. [Click here to view] |
Non-pharmacological treatments can enhance the quality of life of individuals with AD (Olazaran et al., 2010). A moderate quantity of well-organized randomized controlled trials has evidenced the advantages of different non-pharmacological strategies, such as intellectual training, intellectual rehabilitation, and intellectual stimulation treatment in AD patients (Ballard et al., 2011). Various non-pharmacological approaches were reported in Table 1.
The biomarkers manifest significant importance in drug development for AD for choosing the ideal drug candidate for large and high-cost phase-III clinical trials. Biomarkers are also significant in confirming the drug influence on the basic pathophysiology of the disease, which may be required to designate the drug as a disease-modifier (Thakur et al., 2018). Generally used biomarkers for AD are compiled in Figure 2.
 | Table 1. Pharmacological and non-pharmacological therapy for AD. [Click here to view] |
POTENTIAL RISK FACTORS ASSOCIATED WITH AD
A few studies have exhibited that hypertension, sedentary lifestyles, and lifestyle-related disorders like type 2 diabetes mellitus and obesity can enhance possibility of AD (Jayaraman and Pike, 2014). Several genetic factors are also responsible for its outbreak and their contribution in its pathogenesis. However, there are no incidences recommending a considerable effect of occupational exposures on AD. Various physiological and other risks associated with AD are encapsulated in Table 2.
APPROVED DRUGS FOR THE TREATMENT OF AD—CURRENT STATUS
ChEIs and NMDA receptor antagonists are the drugs which are used most frequently for the treatment of AD. Donepezil, rivastigmine, galantamine, and memantine are the generally used four FDA-approved drugs; however, combination of donepezil and memantine is also used for AD therapeutics. They improve the quality of life by controlling the intellectual loss and thus motor regulation (Harilal et al., 2019). Three of these drugs including donepezil, galantamine, and rivastigmine act on CNS cholinergic pathways. All these have anticholinesterase activity, and galantamine which is a natural-product alkaloid also acts as an allosteric modulator at nicotinic acetylcholine receptors. These drugs are frequently prescribed for individuals in early predementia stage having considerable continuous memory impairment depending on intellectual testing reports (Graham et al., 2017). AChE inhibitors may cause g.i. adverse effects such as vomiting and nausea that may be reason for discontinuation of treatment (Santos et al., 2015).
Rivastigmine co-inhibits acetylcholinesterase and butyrylcholinesterase and is specific for the G1 rather than G4 form of the enzyme. As compared to other approved drugs, it does not metabolize in liver and, subsequently, does not cause adverse drug reactions of other drugs generally given to elderly individuals with AD. Galantamine shows lower AChE selectivity; vomiting and nausea are its most frequent adverse effects, which are usually self-limiting (Atri, 2019; National Institute for Health and Clinical Excellence, 2011).
In contrast to all other drugs listed above, donepezil has significant effect in AD therapeutics and is given as 5–10 mg tablets once in a day before bed, because of its longer half-life. It selectively inhibits AChE, leading to well response from AD patients with enhancement in intellectual, behavior, and general function. These may be the reason for its more frequent use as compared to other drugs mentioned above (Atri, 2019).
 | Figure 2. Biomarkers for Alzheimer’s disease. [Click here to view] |
 | Table 2. Risk factors associated with Alzheimer’s disease. [Click here to view] |
 | Table 3. Drugs approved by FDA for treatment of Alzheimer’s disease. [Click here to view] |
Memantine, a recently approved drug for AD, aims to target the NMDA receptors and glutaminergic pathways. Application of memantine in chronic treatment decreases the concentration of Aβ both in aged animals and in AD models. This results in decreased Aβ production. However, in 2005, USFDA refused to extend the application of memantine for mild AD; because of significant adverse effects, e.g. confusion, dizziness, headache, and constipation (Carvalho et al., 2015). Riluzole, a glutamate release inhibitor and post synaptic glutamate receptor signaling, is under phase II trial in mild AD patients (Thakur et al., 2018).
The combination of memantine and donepezil has also been permitted by USFDA for treating moderate-to-severe AD in individuals who were on 10 mg of donepezil hydrochloride treatment. When memantine is given along with stable cholinesterase inhibitor therapy in patients with AD, a good safety profile was found (Ito et al., 2017). Table 3 compiles an overview of pharmacokinetics of drugs used in AD.
DRAWBACKS OF CONVENTIONAL THERAPY
Conventional drug delivery systems including tablet, capsules, powder, or liquid dosage forms have critical restrictions, namely requirement of high-dose, rapid/extensive first pass metabolism, and unfavorable pharmacokinetics leading to low/poor bioavailability (Mudshinge et al., 2011). In treatment of AD, drugs administered orally are expected to cross various gastrointestinal barriers, undergo effective absorption, sustain the drug in the systemic circulation, and successfully transport the drug into the blood–brain barrier (BBB), to reach the target site (Stegemann et al., 2007). Breakdown of the drug moiety in the gastrointestinal tract accelerates drug elimination, reduces half-life, and reduces bioavailability, thus mitigating the predicted beneficial effects. Additionally, sustained interaction or unpremeditated activation of drug substances at non-specific target sites resulting in several side effects, namely nausea, vomiting, gastric problems, etc. (Sainsbury et al., 2014). Few examples are memantine causes dizziness, confusion, constipation, and vomiting (Kornhuber et al., 2007). Acetylcholinesterase inhibitors are associated with vomiting and nausea which frequently leads to discontinuance of therapy (Raina et al., 2008). Owing to a short half-life of 0.5–3 hours, tacrine needs four administrations in a day (Watkins et al., 1994). Furthermore, the physicochemical properties of the drug molecule including the solubility, molecular weight, polarity, partition coefficient, and dissociation constant play an important part in therapeutic effect or drug failure (Alyautdin et al., 2014; Sainsbury et al., 2014). Furthermore, several bioactive substances like proteins, peptides, and other biological macromolecules have poor solubility and are poorly absorbed in the g.i.t, which is responsible for their lower therapeutic effectiveness and hence clinical trials (Munin and Edwards-Levy, 2011).
NANOCARRIERS IN AD THERAPEUTICS
The primary reasons for frequent therapy failure can be correlated to the adverse pharmacodynamics and pharmacokinetics of drugs, gastrointestinal instability of drugs, and toxicity (hepato-, neuro-, or renal toxicity) to the tissues (Arias, 2015; Suri et al., 2015). Currently approved drugs for AD therapeutics are based on improving neurotransmission or enzyme modulation. The drugs conventionally used to target CNS suffer from the primary constraints of the inability to cross the “BBB” or the “B-CSF barrier” efficiently; and higher drug efflux because of P-glycoprotein activity. Nanotechnology, in conjugation with therapeutics, can be used to control various obstacles faced by drug molecules in the treatment of neurodegenerative diseases. Nanotechnology based dosage forms radically regulate these characteristics of the drug molecules and enhance the therapeutic potential applying various functional aid by utilizing nanotechnology-based dosage forms (Brambilla et al., 2011; Nazem and Mansoori, 2008). Nanoformulations not only result in the improvement of pharmacodynamics and pharmacokinetics of drugs, they also have potential to minimize toxicity (Orive et al., 2003; Parveen and Sahoo, 2006). Nanoformulations overcome the obstacles by safely carrying the drug through the biological environment with enhanced permeability, thus providing maximum efficiency at a comparatively lower dose. An essential feature of nanoformulations is the controlled drug release at the target site (Safari and Zarnegar, 2014).
Substantial research reports have evidenced the capability of nanoformulations to circumvent oral and intestinal absorption barriers and carry drugs to the site of action (Ensign et al., 2012; Lundquist and Artursson, 2016). This is achievable owing to their small size (1–1,000 nm), surface charge, high surface to volume ratio (Singh and Lillard, 2009). In CNS delivery, the BBB barrier presents a great obstacle for the nanoformulations targeting neuronal systems (Misra et al., 2003). Altering the surface characteristics of nanformulations enables crossing the BBB by avoiding phagocytic opsonization, thus enhancing the drug levels in the brain (Gidwani and Singh, 2014). The nanosized dosage forms can be classified on the basis of nature of the carrier material as (i) inorganic (gold, carbon, and silica) and (ii) organic (solid-lipid NPs, dendrimers, emulsions, liposomes, and polymers).
Various nanotechnology-based strategies such as polymeric nanocarriers, carbon nanotubes, lipidic nanocarriers, liposomes, nanoemulsions, dendrimers, and metal-based nanocarriers have been evolved over the past decade, focusing on both arresting neurogeneration and neuroprotective for the treatment of AD. Herein, we discuss emerging nanodrug delivery systems for AD therapeutics.
ACROSS THE BLOOD–BRAIN BARRIER
Newly discovered drugs that might be efficacious for the AD treatment may face various hindrances that other drugs used for non-CNS systems may not confront, one of which is the –BBB. The BBB is a highly complex and specialized structure that permits only those substances which are essential for brain activity (Banks, 2012). It is made up of tight junctions in the endothelial membrane that permits just certain molecules to pass through. The tight intersections of BBB are at high transendothelial electrical resistance in comparison to other tissues. There are specialized mechanisms such as specific enzyme systems, protein receptors, and glucose transporters for transporting substances across the BBB. The BBB obstructs undesirable substances from crossing by utilizing tight intersections between cells, extra degradative enzymes, and pumps to eliminate undesirable substances that do pass. Receptors and transporters permit nutrients required for normal functioning to move through to the brain parenchyma (Oesterling et al., 2014). The BBB does not have transporters for conventional drugs and so they are generally not able to pass or are actively pumped back out after crossing. This turns into a significant issue for the therapy of neurodegenerative disorders (Edwards, 2001).
Although conventional methods are suitable for treatment of neurodegenerative disorders, they do not have optimum efficacy. This necessitates for the development of therapeutic alternatives for AD. Various approaches can be prodrug formation and the utilization of carrier-mediated transport systems, for example, carbohydrates, peptides, antibodies, gene delivery vectors, nanoparticles, micelles, and liposomes. The mechanisms by which nanocarriers can improve BBB penetration are efflux transport inhibition, nanocarrier cationization, paracellular transport enhancement pharmacologically, or using the hyperosmotic strategy and olfactory delivery of nanocarriers (Shah et al., 2013). To cross the BBB, various nanotherapeutic approaches have been adapted (Fig. 3). The approaches include
(i) The affinity and binding of lipophilic nanocarriers for endothelial cells improve the transportation of drug molecule via lipophilic transcellular pathways or endocytosis;
(ii) The adsorptive property of nanocarriers towards blood vessels provided a sustained drug release in the systemic circulation with increased possibility for transport of drug across BBB;
(iii) Additionally, functionalized nanocarriers trigger receptor mediated transcytosis and carrier protein mediated transport of drug candidates across BBB (Saraiva et al., 2016).
NANOPARTICLES IN DRUG DELIVERY SYSTEM AND ITS IMPORTANCE
Nanoparticulate drug delivery system is an advanced technology that could be applied to deliver drug substances directly into the brain and has been shown to be extremely efficacious against some CNS disorders. Nanosized systems have garnered considerable interest owing to their favorable features, namely capability to prevent chemical and enzymatic drug degradation, improve drug solubility, and facilitate its transport across the biological membranes. Targeted systems carry the drug straight to the site of action, reduce adverse reactions of the drug, and improve the therapeutic index (Mehmood et al., 2015). Various nanoparticulate systems like polymeric nanoparticles, nanoemulsions, liposomes, antibody tethered nanocarriers, dendrimers, etc., are used for drug delivery and some of them are depicted in Figure 4.
While nanoformulations have been investigated for efficacious drug delivery through various routes such as oral, parenteral, topical, vaginal, rectal, etc., they can also play an important part in diagnosis, and imaging. Nanocarriers are also amenable for nasal mucosal vaccination and nasal drug delivery. The nanocarrier systems allow the effective antigen recognition; especially nanocarriers based on the lipid and polymer are used for nasal delivery of antigen (Le et al., 2019). Nanomedicines played a vital role in the nose to brain delivery. For treating the brain related pathologies, the main focus is on the drug’s ability to cross the BBB. The nanoparticulate systems are considered efficient in to achieve brain targeting as they exhibit better penetration of the drugs in the brain. While the need for devices that can allow deposition of the dosage form to the upper part of nose is essential for nose to brain delivery, surface modification of the nanocarrier can act as a strategic approach for perfect nose to brain targeting. Several reports account for targeting of drugs to brain via different nanoparticulates. Of the various nanocarrier systems investigated for brain therapeutics and theranostics in recent years, are either polymeric or lipidic in nature. Polymeric nanoformulations include polymeric nanoparticles, polymeric micelles, and carbon based nanovehicles. Lipidic nanoformulations are liposomes, niosomes, transferosomes, solid lipid nanoparticles, and nanostructured lipid carriers. Nanogels and nanoemulsions have also garnered considerable attention of researchers for transendothelial delivery of neuropharmaceuticals (Oesterling et al., 2014).
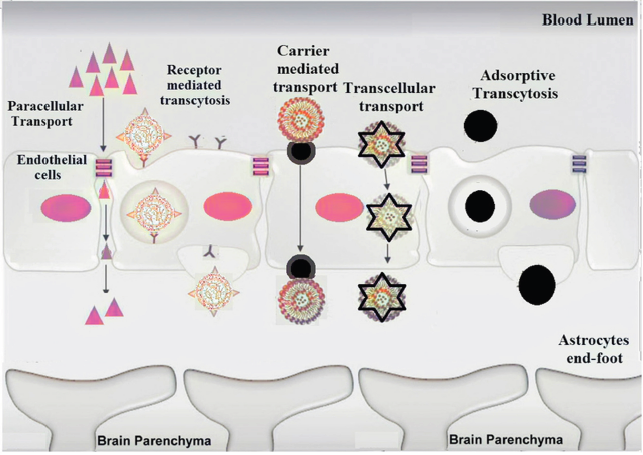 | Figure 3. Schematic representation of potential pathways involved in nanocarrier-mediated drug trafficking across BBB associated with AD-nanotherapeutics. [Click here to view] |
 | Figure 4. Versatile nanocarriers adopted for mitigation of Alzheimer’s disease. [Click here to view] |
The BBB is enriched with various transport systems which can transport substances in and out of the brain. The prominent ones are receptor-mediated transport system, transporter-mediated system, adsorptive-mediated transport system, active efflux-mediated transport system, and peptide-mediated transport approach. Mostly, molecules use either of these transport systems to enter and leave the brain with the exception of certain small lipid soluble molecules that cross the BBB transcellularly. These transport systems could serve as efficient routes for the transport of drugs to the brain by improving its permeability (Sweeney et al., 2018).
Biological therapeutic agents such as nucleic acids, peptides, proteins, and monoclonal antibodies are being aggressively researched since they have potential to restore the damaged cells and decelerate the progression of AD (Sharma et al., 2012). Researchers have developed vaccines for AD and some of them are under clinical trial. Most of the vaccines act on the tau protein in the AD which prevents the functional damage in the brain. Of lately, nano-vaccines (antigen loaded in the nanoparticles as vehicles) have emerged that confer protection to the antigen and result in enhanced efficacy (Wisniewski et al., 2016). The drug delivery systems mediated by nanotechnology are primarily based on the brain’s transportation of therapeutic agents. Currently the focus is on both specific and non-specific processes for mechanisms to target research of brain locations.
Polymeric nanoparticles
Nanoparticles of 10–1,000 nm can easily cross through various biological and physiological barriers to facilitate rapid transport of drug molecules. The NPs can entrap hydrophilic as well as hydrophobic drugs, and also due to excellent release profile they serve as optimistic drug delivery systems (Kamaly et al., 2016). In context to brain delivery, owing to smaller size, NPs can be delivered to cerebellum via olfactory bulb to olfactory cortex and cerebral hemisphere has a caudal pole which carries to the cerebrum. The low efficiency of BBB transportation can be overcome by surface modification of these NPs with antibodies, surfactant, or transferrin (Ghalamfarsa et al., 2016).
Muntimadugu et al. (2016) investigated encapsulation of the drug tarenflurbil, and recommended for the treatment of AD but failed in the phase III clinical trial due to very low drug permeability in the brain. Tarenflurbil was enclosed in two different nanocarriers, namely solid lipid nanoparticles and poly (lactide-co-glycolic) nanoparticles. A particle dimension of less than 200 nm assured transcellular transport along the olfactory axons (diameter ~ 200 nm) and then paving a direct transport to brain. This was confirmed by pharmacokinetic studies in male Sprague Dawley rats that proved prolonged circulation of the nanoparticles, and the absolute bioavailabilities of intranasally administered poly (lactide-co-glycolytic) nanoparticles was highest followed by solid lipid nanoparticles and drug solution. Similar pattern was recorded for drug targeting efficiency (DTE) and drug transport percentage (DTP). The poly (lactide-co-glycolytic) nanoparticles showed higher DTE (287.24) and DTP (65.18) than solid lipid nanoparticles (% DTE: 183.15 and DTP: 45.41) among all other tested groups. These results confirmed direct transport of tarenflurbil in therapeutic concentration, to brain through olfactory pathway after intranasal administration of lipidic and polymeric NPs.
Elnaggar et al. (2015) elaborated intranasal chitosan NPs for targeting piperine into the brain. The oral delivery of neuroprotective piperine suffers due its pre-systemic metabolism and hydrophobicity. The optimized drug loaded chitosan nanoparticles significantly improved the intellectual functions as efficiently as donepezil injection (standard) with additional benefits of dual mechanism (AChE inhibition and antioxidant effect). The nanoparticles alleviated piperine associated nasal irritation, and was devoid of brain toxicity. The authors claimed 20-fold decreases in oral dose of the drug for AD therapeutics.
Likewise rivastigmine, an AChE as well as BuChE enzyme inhibitor, can be given for AD treatment. Nonetheless, the drug has limited entry into the brain because of its hydrophilicity, thus repeated dosing becomes necessary. So, rivastigmine loaded chitosan nanoparticles were prepared by ionic gelation method to improve the uptake of RHT to the brain through intranasal delivery and enhance the bioavailability (Fazil et al., 2012). The intranasally administrated drug loaded chitosan NPs generated stronger fluorescent signals in brain as compared to intravenously injected NPs. The brain/blood ratio of rivastigmine for intranasally administered chitosan NPs at 10–480 minutes was 1.35–2.16, higher than that of intranasally administered rivastigmine solution or intravenously injected NPs. The DTE of 355% and DTP of 71.8% suggested better brain targeting efficiency of the developed system.
Amyloid peptides (A beta) (amyloid plaques) are recognized as targets for developing the biomarkers for AD diagnosis. For the sake of developing efficacious in vivo probes, polymeric n-butyl-2-cyanoacrylate (PBCA) NPs were formulated and enclosed with the radiolabeled I125-clioquinol to enhance its delivery to the brain and retention in the amyloid plaque. The nanocarrier was preferentially taken up by AD brain sections compared to cortical control sections. Furthermore, the I125-clioquinol PBCA nanoparticles crossed the BBB in wild type mouse, verifying an increased brain uptake measured in terms of percent injected dose/g compared to I125-clioquinol. I125-clioquinol PBCA nanoparticles demonstrated enhanced brain retention in the AD transgenic mice against wild type controls. The study exhibited specificity of I125-clioquinol PBCA nanoparticles for A beta plaques both in vitro and in vivo, thus offering a promising delivery vehicle for amyloid imaging (Kulkarni et al., 2010).
Lipidic nanoparticles
The nanocarriers, SLNs can also encapsulate both hydrophilic and hydrophobic pharmaceutical drugs (Chen et al., 2001). Being in the dimensions ranging from 120 to 200 nm, SLNs are not quickly captured by RES cells and, therefore, are cleared by liver and spleen filtration. Moreover, by adding a specific ligand on their surface, their drug-targeting ability can be improved. In fact, many pharmaceutical molecules, other than those intended for AD therapeutics, have been delivered to the brain via receptor-mediated transcytosis mechanism (Grumezescu AM et al., 2016; Mozafari M, 2020).
These lipidic systems are efficient nanocarriers that can competently upload versatile therapeutics and bioactive agents. The system quite resembles with lipid emulsion, liposomes, and polymeric nanoparticles. Literature envisaged that SLNs behave as low-density lipoproteins, and, therefore, get interacted with LDL receptors present on blood–brain barrier (Ghasemiyeh and Mohammadi-Samani, 2018). The created network of SLNs and receptors let entry of SLNs inside BBB (Fig. 5).
These formulations are feasible to fabricate without use of organic solvents, are cost effective, have reproducibility, and offer several advantages over other nanocarriers, i.e., controlled release (for weeks), site specific drug delivery (neurons, cancer cells), stability (~ 3 years), etc. Nanoscaled size range (50–200 nm) enables these particles to circumvent liver/spleen filtration, assists to escape from RES (reticuloendothelial system), and facilitates to cross tight junctions of BBB (Mukherjee et al., 2009). Table 4 summarizes few research reports on SLNs for AD targeting.
Magnetic nanoparticles
In the recent years, magnetic NPs have been extensively explored and are applied for both diagnosis and therapeutic reasons. Their inert nature associated with low toxicity suggests implications in brain pathologies including AD. Kong et al. (2012) in an experiment in a mouse model suggested that magnetic NPs can permeate the normal BBB when an external magnetic field is applied. On systemic administration, an applied strategic external magnetic field navigates the magnetic nanoparticles to cross the BBB and aggregate in a perivascular zone of the brain parenchyma. Internalization by endothelial cells was suggestive of transcellular trafficking as the BBB crossing mechanism. Furthermore, remote radio frequency magnetic field can be utilized to release drug from silica coated magnetic nanocapsules. In conjunction, the results suggest a meticulous approach for manipulating the biodistribution of MNPs in the brain via an external magnetic field.
 | Figure 5. Schematic illustration of composition of SLN and its interaction with LDL receptors. [Click here to view] |
 | Table 4. SLNs for the treatment of AD. [Click here to view] |
In a strategic approach, an electromagnetic actuator was designed for guiding magnetite containing drugs (Do et al., 2016). These magnetic nanoparticles were capable to cross BBB after applying external electromagnetic fields [28 mT (0.43 T/m)]. Additionally, the brain uptake and transport rates of magnetic nanoparticles were considerably improved by applying a pulsed magnetic field. The localization of NPs monitored using fluorescent magnetic nanoparticles demonstrated the feasibility of the magnetic nanocontainers as valuable targeting system for AD diagnosis and therapy.
Nanoparticle conjugates
Functionally integrating proteins, peptides, antibody, DNA, and other nucleic acids with NPs have developed a large variety of composite nanomaterials which in many cases, display augmented properties because of synergistic effect of both components. These capabilities are drawing increased attentiveness from researchers searching of new nanosize options for diverse applications that include therapeutics, cellular delivery, and diagnostics (Jeong et al., 2018). Table 5 compiles few promising approaches containing nanoconjugates to combat neurological disorders.
Xiong et al. (2017) developed peptide-gold NPs comprising two inhibitory peptide sequences (VVIA and LPFFD) for amyloid-β aggregation. The system was targeted to inhibit accumulation of Aβ proteins. The two peptide sequences were conjugated onto the gold NP surfaces and ordered/oriented in optimal conformation to effectively inhibit amyloid-β protein accumulation. Using the two different peptides on a single NP was greatly synergistic, prohibiting amyloid-β proteins accumulation more strongly with less cytotoxicity, compared to the free peptides.
AD is distinguished by the cerebral aggregation of extracellular amyloid plaques and can target on a dual-functional nanoparticle (TQNP) to deliver biotechnological drugs, namely the H102 peptide, a β-sheet breaker, to AD lesions precisely. A spherical, dual-function, drug delivery system loaded with H102 (TQNP/H102) was designed by Zheng et al. (2017). Two targeting peptides, TGN and QSH, were linked to the surfaces of NPs to allow BBB transport and Ab42 targeting, respectively. The study revealed that TQNP could be taken up via various routes, including caveolae-mediated endocytosis, indicating that some of TQNP could cross the BBB intact.
A hybrid system for amyloid plaques targeting siRNA delivery was developed using PEGylated Poly (2-(N, N-dimethylamino) ethyl methacrylate) (PEG-PDMAEMA) joined with two d-peptides, a CGN for brain penetration, and a QSH for β-amyloid binding. The hybrid complex composed of 25% QSH-PEG-PDMAEMA, 50% CGN-PEG-PDMAEMA, and 25% MPEG-PDMAEMA encapsulating siRNA showed negligible cytotoxicity and conferred protection to siRNA from enzymatic degradation. It was determined that the complex was taken up by neuron cells suggesting that the complex was capable of escaping from lysosomes, releasing siRNA in the cytoplasm, and, therefore, establishing effective gene silence (down-regulated protein level to 18.5%). Post i.v. injection, the intact hybrid complex penetrated into the brain and was localized around the amyloid plaques in transgenic AD mice. The precise delivery leads to increased therapeutic activities, which was affirmed by the poor yield of enzyme-digested products sAPPβ (−42.6%), the strong mRNA (36.4%) knockdown of BACE1 (a therapeutic target of AD), as well as the better neuronal protection than the single component complexes. The results are suggestive of an efficient and precise nanocarrier for delivery of siRNA to the AD lesion that may be future candidate for gene therapy for AD (Zheng et al., 2017).
Cubosomes
These are liquid crystalline nanostructured particles consisting of biocompatible carriers. A cubosome is comprised of a three dimensionally organized bicontinuous curved lipid bilayer separated by two aqueous channels within which the bioactive ingredients and proteins come into contact (Fig. 6) Their distinguishing characteristics are that they can encapsulate substances that are hydrophobic, hydrophilic, and amphiphilic, maintain controlled release of the drug and bioadhesion, are thermodynamically stable, and are promising vehicles for various drug administration routes (Karami and Hamidi, 2016).
 | Table 5. Research highlights of few nano-conjugates developed for treatment of Alzheimer’s disease. [Click here to view] |
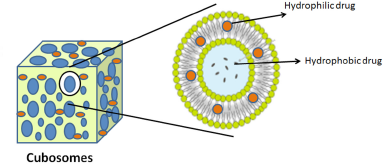 | Figure 6. Composition of cubosome drug delivery system. [Click here to view] |
Wu et al. (2012) developed lectin modified non-immunogenic odorranalectin (small peptide) cubosomes. The system was conjugated with streptavidin through incorporating maleimide PEG oleate and aimed for effective nose to brain drug delivery. The relative uptake of odorranalectin cubosomes was 3.46 times higher compared to the bare cubosomes in brain tissues. Pharmacodynamic study on rats after intranasal administration revealed enhanced efficacy of streptavidin conjugated odorranalectin cubosomes for the mitigation of Alzheimer’s disorder that suggested a potential non-invasive peptide and protein delivery approach in brain tissues.
Piperine, a memory enhancing natural alkaloid, was used for developing novel oral cubosomal delivery system by Elnaggar et al. (2015) for the treatment of neurological AD. Various bioactive surfactants (Tween, cremophore, and poloxamer) modified crystalline cubosomes were evaluated for pharmacokinetic and pharmacodynamics assessments. Among them, Tween 80 modified piperine cubosomes exhibited effective anti-apoptosis and prominent anti-inflammatory activity performed through Caspase-3 assay. Nanoscaled dimension (~167 nm), low polydispersity index (0.18), and optimum zeta potential of developed cubosomes exhibited high entrapment efficiency (~88.7%) and superior stability. Developed novel oral cubosomes depicted potential for inhibition of AD progression.
Inorganic nanoparticles
Gold and silica nanoparticles are designated for the impressive management of AD owing to their exclusive transcytosis movement through endothelial cells of brain without surface modification. These positive charged nanoparticles are self-sufficient for the carriage of bioactive agents across targeted brain tissues. A lot of inorganic NPs are reported that successfully crossed BBB, designed for nose to brain delivery without surface modifications. Intranasal silicon coated NPs (mean particle size 200 nm) were developed for effective brain targeting (Masserini, 2013).
PEG coated iron oxide NPs were demonstrated by Wadghiri et al. (2013) for improved BBB permeation. Although inorganic nanoparticles are highly concerned with immunotoxicity, inorganic NPs (silicon oxide) have been extensively explored for brain targeting owing to their biocompatibility and minimal cytotoxicity. In this context, silicon quantum dots (QDs) are widely explored as theranostic system that serves both as therapeutic and diagnostic agent for the management of neurological diseases. QDs proficiently cater drug delivery at the target sites, monitor cellular uptake with less cytotoxicity. Moreover, the fluorescent silicon QDs have been utilized for cellular imaging and diagnosis of the neuronal diseases (Sivasankarapillai et al., 2019).
Silica nanoparticles (SiNPs) were designed as novel drug delivery systems for BBB targeting due to their profound cellular uptake efficiency and localization in the cytoplasm. These NPs were significantly deposited in the intracellular amyloid cells (Aβ1-42), thus facilitating alleviation of AD (Yang et al., 2014). Developed SiNPs have potential to reduce cellular apoptosis and reactive oxygen in the intracellular region in dose dependent manner. Amyloid peptide deposition and increased tau phosphorylation are the two distinct pathological hallmarks of AD. Both were notified for the activity of silica nanoparticles to check their efficiency for diminishing Aβ (1–42) plaque formation and hyperphosphorylation. However, cellular uptake study performed in mouse neuroblastoma cells and human brain model (SK-N-SH) revealed neurotoxicity after administration of 10 μg/ml SiNPs for 24 hours. The developed system got deposited in the cytoplasm, reduced cell viability, and increased pathogenesis of AD.
Gold nanoparticles (Au NPs) have been extensively used to mitigate neurological disorders owing to their efficiency to cross BBB that project important role in retrieval of depreciated behavioral aspects of brain cells in AD. Developed AuNPs significantly suppressed amyloidosis, checked aggregation of oligomers, and avoided fibril formation within 72 hours (Chiang et al., 2020). Sanati et al. (2019) worked for the development of Au NPs and investigated their impact on memory cells. The outcomes revealed improvement in acquisition and retention in AD. The study was conducted in rat animal model (Morris water maze) to investigate restoration of memory cells after intraperitoneal and intrahippocampal injections of developed AuNPs. The results showed reduction in retention time of memory and spatial learning cells by AuNPs in rats, i.e., ~25.7 seconds compared to non-treated memory cells (39.6 seconds). Moreover, other brain derived factors such as Brain-derived neurotrophic factor (BDNF), cAMP response element binding protein (CREB), and neural survival rates were also modified interpreting improved neural behavior. The developed system displayed promising efficiency for AD outbreak.
Antibody-tethered nanoparticles
Of lately, researchers from University of Cambridge have disclosed a methodology that demonstrates designing of an antibody that can recognize and quantify toxic particles (amyloid beta oligomers) associated with destruction of human healthy brain tissues/cells. Antibodies are proteinaceous molecules that identify and neutralize pathogens/microbes by the virtue of their affinity towards oligomers (Fig. 7). This innovation in healthcare sector would pave of management strategy against the AD (Francesco et al., 2020).
Antibody conjugated novel delivery approach is considered as promising clinical strategy for mitigation of AD. These antibody tethered systems reduce amyloid protein/plaque deposition at the presynaptic phase of the neurological disorder and hence facilitate retrieval of diminished memory cells of brain. In this context, monoclonal antibodies (MAb) have displayed satisfactory capability to target amyloid β peptides in AD affected transgenic mice and human brain tissues. Numerous MAbs, i.e., solanezumab, bapineuzumab, and crenezumab have been explored for the evolution of brain drug delivery systems (Watt et al., 2014).
 | Figure 7. Monoclonal antibody-oligomer interaction to neutralize amyloid plaque. [Click here to view] |
MAbs sweep away undesirable amyloid protein either by passive immunization or through complement activation and check neurodegeneration process. Although, solanezumab and bapinezumab were reported to non-specifically target the amyloid protein and delayed the AD treatment time comparative to crenezumab monoclonal antibody (Prins et al., 2015).
Rosse (2017) presented a series of conjugates composed of antibody-drug to combat wide range of inflammatory diseases, namely, AD, atherosclerosis, Parkinson’s disease, rheumatoid arthritis, etc. These conjugates were fabricated through phosphate-based linkers associating immunoglobulin proteins (CD74/CD163) and therapeutics as steroids (Rosse, 2017).
Another, antibody tethered PBD-C06 formulation (anti-pGlu3-Aβantibody) has been developed through grafting murine antigens on light and heavy chains of antibody. This immunotherapeutic approach was designed to target pGlu3-Aβepitope to mitigate the pathology of AD. Developed PBD-C06 has affinity for oligomers, monomers, fibrils, and clustered Aβpeptide that efficiently targeted neurotoxic peptides at site. Significant reduction of inflammation in the intracellular cells was reported that suggested potential clinical application in vasogenic edema (Hettman et al., 2020).
Nanocomposites
In AD, most of the therapy and therapeutics are focused on amyloid Aβ peptide as it hinders functions of memory cells and causes dementia. In this perspective, tau pathway is extensively correlated with AD symptoms, pathology, and clinical development.
Methylene blue, an inhibitor of tau aggregation, was loaded on CeNC-IONC-MSN-T807 to make nanocomposite. Developed system exhibited profound affinity towards tau and checks AD pathogenesis. Moreover, the designed system depicted symptomatic relief through hindering process of hyperphosphorylation and alleviating mitochondrial oxidative stress. A significant reduction in neuronal cells was observed that rescued destruction of memory cells in AD affected rats (Chen et al., 2018).
Karaboga et al. (2020) developed nanocomposite biosensor comprising of reduced graphene oxide and gold nanoparticles. The surface of nanocomposite was functionalized with 11-mercaptoundeconoic acid to target tau-441 protein accountable for degeneration of memory cells. Developed functionalized nanocomposite has potential to capture tau-441 proteins at a concentration 0.091 pg/ml that offered targeting proteins present in serum and cerebrospinal fluid.
Quercetin, a flavonoid is reported to inhibit Aβ plaque deposition owing to its antioxidant property. However, poor water solubility and extensive first pass metabolism restrict the usage of quercetin in the management of neurological disorders. Selenium nanocomposite containing quercetin and sodium selenite was synthesized and modified with polysorbate 80. The developed template possessed high aqueous solubility and capacity to cross BBB efficiently in comparison to plain quercetin. The outcomes suggested improved inhibition of Aβ fibrillation and antioxidant activity (Qi et al., 2020).
Zhao et al. (2019) demonstrated novel synthesis of nanocomposites to eliminate Aβ induced neurotoxicity in AD affected mice. Nanodimensional clusters containing cross linked KLVFF proteinaceous body were developed through in situ polymerization. The prepared bioactive system altered the morphology of amyloid peptide aggregates and reduced the population of pathological oligomers at the infected site. The nanocarriers system lessened neuronal injuries caused by amyloid aggregation and plaque deposition. Thus, nanoscaled composites presented feasible approach for protection of AD affected hippocampal neurons and enabled fast recovery of endocranial microglia efficiency (Zhao Y).
Nanoemulsions
Thermodynamically stable nanosized emulsions have been explored for effective import of bioactive agents across BBB owing to their advanced pharmaceutical designing. Innovative surfactants and co-surfactants enable nanoemulsion as potential drug delivery approach for the management of neuronic disorders. Their small particle size (50–500 nm) let uniform dispersion and higher payload of therapeutics for the site specific delivery.
Memantine embedded nanoemulsion has been reported for impressive intranasal drug delivery. This approach bypassed BBB and was found to be effective for AD therapeutics. Formulated nanoemulsion depicted mean globular size of approximately 11 nm, with 80% drug release in simulated nasal fluid. On intranasal administration, memantine loaded nanoemulsion exhibited amplified antioxidant efficiency and superior cellular uptake in brain cells of experimental rats (Kaur et al., 2020a). The oil in water donepezil hydrochloride loaded nanoemulsion was prepared utilizing 10% each of labrasol and glycerol. Nanoscaled, uniform, and stable particles of nanoemulsion impacted the AD infected brain cells. Cytotoxicity behavior investigated on Sprague Dawley rats through nasal route revealed dose dependent efficacy without disturbing the morphology of cells. The antioxidant and radical scavenging efficacy revealed promising potential of donepezil hydrochloride nanoemulsion for treating neurological diseases. Outcomes of scintigram defined maximum cellular uptake by brain cells (Kaur et al., 2020b).
Recently, selective AChE inhibitor, Huperzina A, was explored for nose to brain delivery to mitigate AD. Extensive researches on conventional dosage of Huperzina A demonstrated its noticeable adverse effects in the peripheral cholinergic region and gastrointestinal tract. Huperzina A nanoemulsion modified with lactoferrin (Lf-HupA-NE) was investigated against in vitro brain model (Hcmec/D3 cells). Lactoferrin, an iron binding glycoprotein, is widely expressed in brain endothelial cells and highly utilized in the treatment of age related neurodisorders. Thus developed nanoemulsion on intranasal administration was targeted to brain cells and tissues. The results notified impressive drug release in the brain parenchyma and suggested brain targeted drug delivery systems (Jiang et al., 2019).
Md et al. (2018) revealed neuroprotective property of naringenin bioflavonoid. Its restricted permeation coefficient across biological membrane limits its pharmaceutical applications. Naringenin loaded nanoemulsion was investigated against amyloid peptide induced toxicity in the brain cells (SH-SY5Y). The outcomes defined alleviation of amyloid induced reactive oxygen species and exhibited neuroprotective action in SH-SY5Y neuroblastoma cells in the brain. Designed nanoemulsion depicted promising approach for reducing phosphorylated tau level and amyloidogenesis related to AD.
Liposomes
Liposomes are self-aggregating lipid nanosystems that amalgamate and supply the CNS with lipophilic drugs, hydrophilic drugs, protein-based drugs, and nucleic acid components (Pashirova et al., 2018). The lipophilic characteristics of liposomes assist the transportation of drugs across the endothelial membrane of brain cells and provide them with outstanding brain uptake characteristics. Drug diffusion and liposome endocytosis are the two prominent mechanisms engaged in drug release from liposomes (Wei et al., 2014). Although, vesicle size and lipid composition do affect their transmission in the systemic circulation and in cellular uptake in brain cells (Montesinos, 2017). Liposomes offer high pay loads both for hydrophilic and hydrophobic therapeutics (Fig. 8).
Research on liposomes demonstrates its versatile pharmacological actions, i.e., neuroprotective, anti-ischemic, anti-epileptics, and antimicrobials. These formulations have been explored as cargo for import of therapeutics across BBB through active targeting. Austen et al. (2008) carried out a study based on inhibition of Aβ aggregation and toxins in the fibrils on brain cells. They developed stable retro-inverso peptides capable to crossing BBB. The system was modified with fluorescein labeled inversion to amplified anti-amyloid aggregation property. In vivo study on transgenic mice (TG2576) after peripheral injection revealed protection of memory cells of brain.
Behl et al. (1994) formulated curcumin loaded liposomes for the impressive management of AD. Antioxidative curcumin liposomes offered anti-amyloid effects. The outcomes demonstrated neuroprotective efficacy against amyloid toxins released in brain endothelial region. Adorned phenolic group in curcumin therapeutic got bound with amyloid proteins, diminished plaque deposition, and facilitated aggregation free environment around brain cells.
Arumugam et al. (2008) demonstrated treatment of AD through rivastigmine loaded liposomes administered intranasally. The delivery system sustained the drug release effect that could reduce frequency of drug administration and ultimately patient compliance. Augmented half-life and higher concentration of rivastigmine in brain cells were reported after oral and intranasal administration.
Zheng et al. (2015) encapsulated H102, a novel peptide (βsheet breaker) in liposomes to avoid degradation and enhance brain penetration through intranasal drug delivery for AD therapeutics. The formulated liposomes had ability to penetrate Calu-3 cell monolayers consistently. After drug administration through nasal route, H102 was effectively delivered to the brain and produced the three times greater effect in the brain. The delivery produced excellent spatial memory. The liposomal intranasal brain delivery approach for H102 was stable, effective, and safe.
Donepezil encounters hurdles in crossing BBB, hence is not preferred in conventional dosage formulations. To avoid this problem, donepezil was loaded in liposomes and intranasally administered which reduced the risk of first-pass metabolism, unwanted side effects, and persisted rapid drug delivery to the CNS. Pharmacokinetic study of both donepezil loaded liposomes and free donepezil administered through oral and intranasal routes revealed improved bioavailability of the former owing to its better absorption via intranasal administration. A twofold increase in the AUC of donepezil liposomes administered intranasally was observed compared to oral route. Microphotographs of brain and olfactory bulb revealed no morphological changes in the visceral tissues after intranasal administration of liposomes after intranasal administration in rats. Donepezil loaded liposomes given through intranasal route showed improved bioavailability in brain, enhanced cellular uptake, and reduced cellular toxicity (Al-Asmari et al., 2016).
Dendrimers
The three dimensional, spherical, branched architectural entities (dendrimers) are emerging pharmaceutical nanocarriers, widely worked out for designing numerous targeted drug delivery system owing to their versatile properties, i.e., nanosize, low polydispersity index, high payload efficiency, improved solubility, less viscosity, biocompatibility, non-immunogenicity, and stability. The morphology of dendrimers is comprised of functional groups containing linkers attached with therapeutic agents on their periphery (Fig. 9). Their specific structure facilitates site specific drug delivery. For the effective treatment of neurogenerative disorders, extensive research and formulations based on dendrimers are reported. Presence of multiple functional groups or polyvalent recognition sites enable high pay load of therapeutics (Abbasi et al., 2014).
 | Figure 8. Nanocarrier: liposomes and its salient features. [Click here to view] |
 | Figure 9. Drug loaded dendrimers for treatment of AD. [Click here to view] |
In AD, several molecular mechanisms are involved such as apoptosis, inflammation, and oxidative stress. These processes should be targeted through designing nanoengineered dendrimers. Numerous bioactive agents, i.e. peptides/protein, nucleic acids, genes, and biosensors are frequently loaded in dendrimers for brain delivery (Aliev et al., 2019).
Katare et al. (2015) stated that drug delivery to the brain is challenging because of the low aqueous solubility and bioavailability. The haloperidol dendrimer was delivered to the brain through the nasal route of administration. The developed preparation showed hundred times high aqueous solubility. Cataleptic and locomotor studies were evaluated after intraperitoneal injection administration of haloperidol dendrimers. Haloperidol response given through nasal and intraperitoneal route was compared. It was mentioned that 6.7 times less dose was required for nasal delivery to obtain same behavioral response compared to intraperitoneal route. The study suggested improvement in aqueous solubility of poorly soluble drug through dendrimers for the amplified locomotors action in AD cases. The study confirmed that the dendrimers given via nasal route is suitable delivery for targeting brain for poorly aqueous soluble drugs.
Nazem and Mansoori (2011) described various biomedical applications of dendrimers due to their shape and size. The anti-myeloid strategy was recommended for the dendrimers. The strategy was designed in such a way that the peptide monomer was attached to the end of fibrils which resulted in the prevention of cytotoxic effects. The most frequently researched dendrimers for the therapy of brain diseases are polyamidoamine (PAMAM) dendrimers. The effect of PAMAM dendrimers on prion peptide PrP185-208 and Alzheimer’s peptide Aβ1-28 was assessed using thioflavin T dye. Outcomes of the fluorescence and electron microscopy revealed high degree of amyloid aggregation and signs of fibril disruption. The interaction of globular and branched dendrimers with amyloid proteins showed inhibition of fibril formation in AD (Klajnert et al., 2006).
Another anti-inflammatory and antioxidant therapeutic N-acetyl-l-cysteine was explored for the synthesis of poly (amidoamine) (PAMAM) dendrimers. The synthesized system was capable of intracellular drug delivery at the site of inflammation. Presence of detachable disulfide bonds in the integrity of dendrimers enabled linking with intracellular glutathione and microglial cells. Higher payload facilitated desirable amount of drug release at local cells and exhibited improved antioxidant activity (Navath et al., 2006).
Klementieva (2019) discussed exclusive brain cell protective activity of poly propyl imine (PPI) dendrimers functionalized with maltose in transgenic mice. PPI dendrimers modified with maltose got bound with amyloid proteins, hindered their aggregation and checked fibril deposition at brain cells. The study revealed that unmodified PPI dendrimers cause cellular toxicity, whereas maltose modified dendrimers did not show intrinsic toxicity. The research highlighted that polysaccharide coated dendrimers were non-toxic to the neuroblastoma cells. Dendrimers containing cationic PAMAM and phosphorous have been fabricated through polymerization technique to investigate efficacy against deposited amyloid peptides over neuroblastoma cells. The intrinsic toxicity due to positive charge of PAMAM restricted the performance of dendrimers. Biocompatible sugar coated glycodendrimers have also been reported. The modified dendrimers were capable of interacting with amyloid proteins with minimum cell toxicity. The developed sugar coated glycodendrimers were taken account for symptomatic relief in AD owing to their high surface group density over protofibrils G4 of brain cells (Benseny-Cases et al., 2012).
Nanosuspensions
Pharmaceutical nanoscaled biphasic suspensions are acceptable drug delivery systems for hydrophobic drug therapeutics owing to their stable and well dispersed appearance. Availability of a series of natural/synthetic surfactants and the ease of preparation methodologies enable nanosuspensions for targeted drug delivery through oral, parenteral, pulmonary, ocular, and topical routes (Yadollahi et al., 2015).
Donepezil nanosuspensions prepared through ionic crosslinking method with chitosan were investigated in Sprague Dawley rats for olfactory pathway following nose to brain administration. Fine nanoscaled particles (mean size 200 nm) and low polydispersity index (0.341) provided better absorbance and bioavailability in brain. The animal study revealed effective donepezil responses to the brain cells (Cmax 7.2 ng/ml) and plasma (82.2 ng/ml) by developed nanosuspension. The formulation was safe as no animal loss or distinct change in cell morphology or intrinsic toxicity was observed in experimental animals. The formulation served as a novel approach for drug administration to combat symptomatic relief from AD (Bhavna et al., 2014).
Li et al. (2018) developed meloxicam nanosuspensions for reduction in brain that efficiently reduced inflammation in brain cells. Meloxicam embedded bovine serum albumin suspension was formulated through acid/base neutralization method. Uniformly dispersed biphasic suspension consisted fine particles of mean size 78.67 nm that were physically stable with 6 months shelf-life. Spherical, smooth, and regular surface coating of bovine-serum albumin over drug particles (mean particle size = 78.67 ± 0.22 nm) was revealed by transmission electron microscopy. The pharmacokinetic parameters, half-life, mean residence time, and AUC0-∞ got improved by 169.8%, 150.1%, and 148.8% after intravenous administration. Though the authors did not prove its utility in AD therapeutics, they claimed its usefulness for symptomatic relief in neurodegenerative disorders. The concentration of meloxicam was found to be higher in inflamed brain tissues after injecting a nanosuspension in Sprague Dawley rats left and right paws. The graph plotted between concentration of drug in endothelial cells and time exhibited higher meloxicam concentration (~450 ng/ml) from nanosuspension compared to meloxicam solution (225 ng/ml) after 2 hours. The results exhibited novel application of bovine-serum albumin coated meloxicam for alleviation of inflammation in brain neurons.
RECENT UPDATES ON PATENTS AND CLINICAL TRIALS ON ALZHEIMER’S TREATMENT
Nanotechnology based dosage forms are being the most explored area for efficient delivery of the drugs for AD. The USFDA has recently approved Tauvid (flortaucipir F18) for i.v. injection, the first drug used to help image a distinctive characteristic of AD in the brain called tau pathology. Tauvid is a radioactive diagnostic agent for adult patients with intellectual impairment and is being evaluated for AD. Tauvid is suggested for positron emission tomography imaging of the brain to determine the distribution and density of aggregated tau NFTs, a primary marker of AD (USFDA, 2020). Luthman et al. (2019) patented methods for bringing down clinical reduction in an individual having early AD, methods of converting an amyloid positive individual during initial stages to amyloid negative, methods for lowering brain amyloid level in an individual, and methods of preventing AD, the methods comprising administering a composition comprising a therapeutically effective amount of at least one anti-Aβ protofibril antibody. Gelmont et al. (2019) have been assigned a patent which provides, among other aspects, methods for the treating AD in individuals in need thereof, the method including administration of a therapeutically effective amount of a pooled human immunoglobulin G (IgG) and/or an anti-beta amyloid monoclonal antibody composition to an individual having moderately severe AD.
Various patents have been granted/published in this field depending on the nanocarrier systems, including multiple dosage forms, liposomes, nanoparticles, solid lipid nanoparticles, nano-emulsion, etc., (Patel et al., 2017) which are compiled in Table 6. Castor et al. (2019) got patented an AD drug candidate, APH-1104, a potent analog of Bryostatin-1, and is neuroprotective by α-secretase activation via novel protein kinase C (PKC) isoforms, down-regulation of pro-inflammatory and angiogenic processes, and the substitution of β-amyloid for its soluble and harmless relative, sAPP-α at concentrations which are orders of magnitude lower than conventional APP modulators. These nanoparticles protect Bryostatin-1 in its transit to the stomach, are resistant to stomach acids, and increase residence time and efficacy once transported to the circulation system in the duodenum. Castor (2020) has been assigned a patent for the combination therapeutic consisting of nanospheres co-encapsulating Bryostatin-1 and a Retinoid to improve synergistically α-secretase production and reduce β-amyloid plaque generation. Bryostatin-1 stimulates the production of some isoforms of PKC which enhances the production of α-secretase resulting in soluble amyloid precursor protein, and inhibits the formation of β amyloid plaques. Retinoids such as all-trans retinoic acid (ATRA, retinoic acid) enhance α-secretase activity via increased levels of expressed ADAM10 protein.
Improvement in therapeutic mediation for treating individuals with AD is of prime importance for both clinicians and pharmaceutical industries. Hence collection and investigation of clinical information is required for designing new protocols and/or the advancement of upgraded therapeutics for treating the disorder. Table 7 presents a compilation of the drugs that are in phase III clinical trials, while the drugs utilized in phase II clinical trials are summarized in Table 8 (Romano, 2018).
FUTURE PERSPECTIVE
Nanocarriers seem to offer a novel way of facilitating research attempts by explicitly regulating various pathways in focused areas. An interesting target for the development of better AD therapeutics can be mitochondrial dysfunction. Magnetic NPs are a productive area for research and have numerous existing as well as potential technological applications. Dendrimers and nanoemulsions are also two classifications of nanoparticulate systems which ought to be investigated additionally for transport of CNS drug. A few issues are required to be resolved before nanomedicines for AD comes to clinical setting. They are (i) the general and the biggest hurdle is low targeting efficiency, which may restrict the therapeutic effect and cause harm to other organs and (ii) another concern is the distribution of nanomaterials into the brain. To attain specific targeting in the brain, sequentially targeted nanomaterials require consideration. Objective should be to target not only BBB, but to also target the diseased site, to prevent distribution in whole brain.
Various in vitro investigations have reported the potential adequacy of nanocarriers; however, future in vivo studies of these nanocarriers can possibly disclose a long-term systemic efficacy or potential toxicity in biological systems which can be correlated with in vitro systems. Despite the fact that the registration of patents related to nanotechnology-based systems is presently rising, clinical studies are required to assess their clinical efficacy and potential toxicological effects in humans. A detailed focus on the stability and safety in terms of biological retention, exposure time, nanocarrier size, dose, and metabolites of various polymers of the nanocarriers is also required. Subsequently, an assessment of the safety and adequacy of appropriate nanocarriers by conducting clinical studies in humans can result in encouraging cost-effective AD therapeutics.
 | Table 6. Patents on drug delivery systems based on nanotechnology for treatment of AD. [Click here to view] |
 | Table 7. Drugs used in phase III clinical studies for the treatment of AD patients. [Click here to view] |
 | Table 8. Drugs used in phase II clinical studies for the treatment of AD patients. [Click here to view] |
CONCLUSION
Significant advancement has been achieved in AD therapeutics, but the entire currently available approaches target on mitigating symptoms instead of curing, implying that AD can still be considered as an unresolvable neurodegenerative disorder and needs interim advancement. Nanotechnological applications offer a lucrative efficient strategy for the AD treatment. A plethora of nanocarriers have been developed that have ability to cater to the limitations of conventional therapy, can assist in preliminary diagnosis, enhance therapeutic efficacy and bioavailability, offer negligible cytotoxicity in animal models, and present newer possibilities for development of superior formulations of potent drugs intended for AD therapeutics.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett, 2014; 9:Article number 247. CrossRef
Al-Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Devel Ther, 2016; 10:205–15. CrossRef
Aliev G, Ashraf GM, Tarasov VV, Chubarev VN, Leszek J, Gasiorowski K, Makhmutovа A, Baeesa SS, Avila-Rodriguez M, Ustyugov AA, Bachurin SO. Alzheimer's disease—future therapy based on dendrimers. Curr Neuropharmacol, 2019; 17:288–94. CrossRef
Allon N, Gavish M, Veenman JA. Liposomes for in vivo delivery. WO Patent No 2014/ 0776709 A1, 2014.
Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int J Nanomed, 2014; 9:795–811. CrossRef
Anand A, Arya M, Kaithwas G, Singh G, Saraf SA. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model. J Drug Deliv Sci Technol, 2019; 49:219–26. CrossRef
Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology, 2014; 76:27–50. CrossRef
Arias JL, Key aspects in nanotechnology and drug delivery. In: Nanotechnology and drug delivery. Vol. 1: Nanoplatforms in drug delivery. Boca Raton, FL: CRC Press, pp 1–27, 2015. CrossRef
Arumugam K, Subramanian GS, Mallayasamy SR, Averineni RK, Reddy MS, Udupa N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm, 2008; 58:287–97. CrossRef
Atri A. Current and future treatments in Alzheimer’s disease. Semin Neurol, 2019; 39:227–40. CrossRef
Atti AR, Palmer K, Volpato S, Winblad B, Ronchi DD, Fratiglioni L. Late-life body mass index and dementia incidence: nine year follow-up data from the Kungsholmen Project. J Am Geriatr Soc, 2008; 56:111–6. CrossRef
Austen BM, Paleologou KE, Ali SA, Qureshi MM, Allsop D, El-Agnaf OM. Designing peptide inhibitors for oligomerization and toxicity of Alzheimer’s β-amyloid peptide. Biochemistry, 2008; 47:1984–92. CrossRef
Ballard C, Khan Z, Clack H, Corbett A. Nonpharmacological treatment of Alzheimer disease. Can J Psychiatry, 2011; 56:589–95. CrossRef
Banks WA. Drug delivery to the brain in Alzheimer's disease: consideration of the blood-brain barrier. Adv Drug Deliv Rev, 2012; 64(7):629–39. CrossRef
Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell, 1994; 77:817–27.
Benseny-Cases N, Klementieva O, Cladera J. Dendrimers antiamyloidogenic potential in neurodegenerative diseases. New J Chem, 2012; 36:211–6. CrossRef
Beydoun MA Wang Y, Forno GD, Zonderman AB. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am J Epidemiol, 2008; 168:1179–89. CrossRef
Bhavna, Md S, Ali M, Ali R, Bhatnagar A, Baboota S, Ali J. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int J Biol Macromol, 2014; 67:418–25. CrossRef
Braak H, and Braak E. Pathology of Alzheimer's disease. In: Neurodegenerative Diseases. (Calne, D.B., ed.) Saunders, Philadelphia; 1994:585-614.
Brambilla D, Le Droumaguet B, Nicolas J, Hashemi SH, Wu LP, Moghimi SM, Couvreur P, Andrieux K. Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. Nanomedicine, 2011; 7:521–40. CrossRef
Breteler MM. Vascular involvement in cognitive decline and dementia: epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann N Y Acad Sci, 2000; 903:457–65. CrossRef
Carvalho KM, Winter E, Antunes AMDS. Analysis of technological developments in the treatment of Alzheimer’s disease through patent documents. Intell Inf Manag, 2015; 7:268–72. CrossRef
Castor TP, Alexander JS, Purdum G, Rios JD, Schrott LM, Tyler TA, Vizcaino MI. Drug delivery system and method for the treatment of neuro-degenerative disease. US patent No 10,485,766, 2019.
Castor TP. Combination therapeutics and methods for the treatment of neurodegenerative and other diseases. US patent No 10,828,276, 2020.
Chen DB, Tian Y, Lu WL, Zhang Q. In vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull, 2001; 49:1444–7. CrossRef
Chen Q, Du Y, Zhang K, Liang Z, Li J, Yu H, Ren R, Feng J, Jin Z, Li F, Sun J, Zhou M, He Q, Sun X, Zhang H, Tian M, Ling D. Tau-targeted multifunctional nanocomposite for combinational therapy of Alzheimer's disease. ACS Nano, 2018; 12:1321–38. CrossRef
Chiang MC, Nicol CJB, Cheng YC, Yen C, Lin CH, Chen SJ, Huang RN. Nanogold neuroprotection in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction. Neuroscience, 2020; 435:44–57. CrossRef
Chin-Chan M, Navarro–Yepes J, Quintanilla–Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci, 2015; 9:124–30. CrossRef
Cimini A, D’angelo B, Das S, Seal S. Nanoparticles of cerium oxide targeted to an amyloid-beta antigen of Alzheimer’s disease and associated methods. US Patent No 2014/ 8877207 B2, 2014.
Claudio P, Reatul K, Brigitte E, Geraldine P. Drug-delivery nanocarriers to cross the blood-brain barrier. In: Nanobiomaterials in drug delivery, Elsevier, Amsterdam, The Netherlands, 2016; doi:10.1016/B978-0-323-42866-8.00010-1.
Cohen D, Nabirochkin S, HAJJ R, Brureau A. Idalopirdine-based combinatorial therapies of alzheimer's disease. WO Patent No 2018/197383A1, 2018.
Corot C. Use of metal nanoparticles in the diagnosis of Alzheimer’s disease. US Patent No 2013/8349293 B2, 2013.
Dara T, Vatarnara A, Sharifzadesh M, Khani S, Vakilnezhad MA, Vakhshiteh F, Meybodi MN, Malvajerd SS, Hassani S, Mosaddegh MH. Improvement of memory deficits in the rat model of Alzheimer's disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol Learn Mem, 2019; 166:107082. CrossRef
Do TD, Ul Amin F, Noh Y, Kim MO, Yoon J. Guidance of magnetic nanocontainers for treating Alzheimer's disease using an electromagnetic, targeted drug-delivery actuator. J Biomed Nanotechnol, 2016; 12:569–74. CrossRef
Edwards RH. Drug delivery via the blood-brain barrier. Nat Neurosci, 2001; 4:221–2. CrossRef
Elbayoumi T, Kuo F, Markatos P, Faucher K. Nanoemulsion formulations for direct delivery. US Patent No 2011/0045050 A1, 2011.
Elnaggar SRY, Etman SM, Abdelmonsif DA. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer's disease: optimization, biological efficacy, and potential toxicity, J Pharm Sci, 2015; 104:3544–56. CrossRef
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev, 2012; 64:557–70. CrossRef
Fazil M, Md S, Haque S, Kumar M, Baboota S, Kaur J, Ali J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci, 2012; 47:6–15. CrossRef
Francesco A, Sormanni P, Podpolny M, Chhangur S, Needham LM, Ruggeri FS, Perni M, Limbocker R, Heller GT, Sneideris T, Scheidt T, Mannini B, Habchi J, Lee SF, Salinas PC, Knowles TPJ, Dobson CM, Vendruscolo M. Rational design of a conformation-specific antibody for the quantification of Aβ oligomers. Proc Natl Acad Sci USA, 2020; 117:13509–18. CrossRef
Frautschy S, Gregory C. Bioavailable curcuminoid formulations for treating Alzheimer’s disease and other age-related disorders. US Patent No 2015/9192644 B2, 2015.
Frenkel D, Maron R, Burt D, Weiner HL. Compositions and methods for treating neurological disorders. Europe Patent No 2332570 A1, 2011.
Gard T, Taquet M, Dixit R, Brach N, Lazar SW. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front Aging Neurosci, 2014; 6:76. CrossRef
Gelmont DM, Singer J, Fritsch S, Schwarz HP. Treatment of Alzheimer's disease Subpopulations with Pooled Immunoglobulin G. Europe Patent No 2994160 B1, 2019.
Ghalamfarsa G, Hojjat-Farsangi M, Mohammadnia-Afrouzi M, Anvari E, Farhadi S, Yousefi M, Jadidi-Niaragh F. Application of nanomedicine for crossing the blood-brain barrier: Theranostic opportunities in multiple sclerosis. J Immunotoxicol, 2016; 13:603–19. CrossRef
Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci, 2018; 13:288–303. CrossRef
Gidwani M, Singh AV. Nanoparticle enabled drug delivery across the blood brain barrier: in vivo and in vitro models, opportunities and challenges. Curr Pharm Biotechnol, 2014; 14:1201–12. CrossRef
Goedert M, Spillantini MG. A century of Alzheimer's disease. Science, 2006; 314:777–81. CrossRef
Graham WV, Bonito–Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med, 2017; 68:413–30. CrossRef
Hardman JG, Limbird LE, Goodman GH. The Pharmacological basis of therapeutics. 11th edition. McGraw Hill Publications, New York, pp 253–54, 2006.
Harilal S, Jose J, Parambi DGT, Kumar R, Mathew GE, Uddin MS, Kim H, Mathew B. Advancements in nanotherapeutics for Alzheimer's disease: current perspectives. J Pharm Pharmacol, 2019; 71:1370–83. CrossRef
Hettmann T, Gillies SD, Kleinschmidt M, Piechotta A, Makioka K, Lemere CA, Schilling S, Rahfeld J, Lues I. Development of the clinical candidate PBD-C06, a humanized pGlu3-Aβ-specific antibody against Alzheimer’s disease with reduced complement activation. Sci Rep, 2020; 10:3294. CrossRef
Hoffman LB, Lesser GT, Beeri MS, Purohit DP. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology, 2009; 72:1720–6. CrossRef
Ieni J, Pratt R. Methods and compositions using cholinesterase inhibitors. US Patent No 2006/0018839 A1, 2006.
Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx, 2004; 1:226–34. CrossRef
Ito K, Tatebe T, Suzuki K. Memantine reduces the production of amyloid–beta peptides through modulation of amyloid precursor protein trafficking. Eur J Pharmacol. 2017;798:16–25. CrossRef
Jalili-Baleh L, Nadri H, Forootanfar H, Samzadeh-Kermani A, Küçükkılınç TT, Ayazgok B, Rahimifard M, Baeeri M, Doostmohammadi M, Firoozpour L, Bukhari SNA, Abdollahi M, Ganjali MR, Emami S, Khoobi M, Foroumadi A. Novel 3-phenylcoumarin-lipoic acid conjugates as multi-functional agents for potential treatment of Alzheimer's disease. Bioorg Chem, 2018; 79:223–34. CrossRef
Jayaraman A, Pike CJ. Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep, 2014; 14:476–82. CrossRef
Jeong WJ, Bu J, Kubiatowicz LJ, Chen SS, Kim Y, Hong S. Peptide-nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg, 2018; 5:Article number 38.
Jiang Y, Liu C, Zhai W, Zhuang N, Han T, Ding Z. The optimization design of lactoferrin loaded HupA nanoemulsion for targeted drug transport via intranasal route. Int J Nanomed, 2019; 14:9217–34. CrossRef
Kamaly N, Yameen B, Wu J, Farokhzad OC, Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release, Chem Rev, 2016; 116:2602–63. CrossRef
Karaboga S, Nur M, Kemal SM. Analysis of Tau-441 protein in clinical samples using rGO/AuNP nanocomposite-supported disposable impedimetric neuro-biosensing platform: towards Alzheimer's disease detection. Talanta, 2020; 219:121257. CrossRef
Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today, 2016; 21(5):789–801. CrossRef
Katare YK, Daya RP, Gray CS, Luckham RE, Bhandari J, Chauhan AS, Mishra RK. Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Mol Pharm, 2015; 12:3380–8. CrossRef
Kaur A, Kuldeep N, Srivastava S, Tyagi A, Dang S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J Microencapsul, 2020a; 37:1–30. CrossRef
Kaur A, Nigam K, Bhatnagar I, Sukhpal H, Awasthy S, Shankar S, Tyagi A, Dang S. Treatment of Alzheimer's diseases using donepezil nanoemulsion: an intranasal approach. Drug Deliv Transl Res, 2020b. doi: 10.1007/s13346-020-00754-z. CrossRef
Kay DG, Maclellan A. Composition and method for improving cognitive function and brain bioavailability of ginseng and ginsenosides and treating neurodegenerative disease and neurological disorders. WO Patent No 2018/148821A1, 2018.
Klajnert B, Cortijo-Arellano M, Cladera J, Bryszewska M. Influence of dendrimer's structure on its activity against amyloid fibril formation. Biochem Biophys Res Commun, 2006; 345:21–8. CrossRef
Klementieva O. Glycodendrimers as potential multitalented therapeutics in Alzheimer’s disease. InTech, London, UK, 2019; doi:10.5772/intechopen.88974. CrossRef
Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J Control Release, 2012; 164:49–57. CrossRef
Kornhuber J, Kennepohl EM, Bleich S, Wiltfang J, Kraus T, Reulbach U, Meineke I. Memantine pharmacotherapy: a naturalistic study using a population pharmacokinetic approach. Clin Pharmacokinet, 2007; 46:599–612. CrossRef
Kulkarni PV, Roney CA, Antich PP, Frederick J Bonte, Anjanapura V Raghu, Tejraj M Aminabhavi Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer's disease. Nanomed Nanobiotechnol, 2010; 2:35–47. CrossRef
Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep, 2015; 67:195–203. CrossRef
Le MQ, Carpentier R, Lantier I, Ducournau C, Fasquelle F, Dimier-Poisson I, Betder D. Protein delivery by porous cationic maltodextrin-based nanoparticles into nasal mucosal cells: Comparison with cationic or anionic nanoparticle. Int J Pharm: X, 2019; 1:100001. CrossRef
Lehrer S. Method for the prevention and treatment of Alzheimer’s disease. US Patent No 2015/0086616 A1, 2015.
Li Q, Chen F, Liu Y, Yu S, Gai X, Ye M, Yang X, Pan W. A novel albumin wrapped nanosuspension of meloxicam to improve inflammation-targeting effects. Int J Nanomed, 2018; 13:4711–25. CrossRef
Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, Sevin E, Fenart L, Gosselet F, Coelho MA, Pereira MC. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer's disease. Molecules, 2017; 13:277. CrossRef
Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev, 2016; 106:256–76. CrossRef
Luthman J, Swanson CJ, Zhang Y, Dhadda S, Wang J. Methods of treatment and prevention of alzheimer's disease. WO Patent No 2019/023530A3.
Makhaeva GF, Nadezhda VK, Natalia PB, Sofya VL, Elena VR, Tatyana SS, Alexey AT, Igor VS, Alexey NP, Eugene VR, Vladimir AP, Sergey OB, Rudy JR. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: Synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg Chem, 2020; 94:103387. CrossRef
Maron R, Frenkel D, Weiner HL, Burt D. Novel composition in the form of a medicament for neurological disorders. Indian Patent No. 257811, 2007.
Masserini M, Re F, Sancini G, Forloni G, Salmona M. Liposomes active in vivo on neurodegenerative diseases. US Patent No 2015/0017235 A1, 2015.
Masserini M, Re F, Sesana MS. Liposomes capable of effectively binding the beta amyloid peptide. WO Patent No 2009/150686 A1, 2009.
Masserini M. Nanoparticles for Brain Drug Delivery. Int Sch Res Notices, 2013; 2013:238428. CrossRef
Maurel JC. Reverse micelle microemulsion comprising metal ions and use thereof. Europe Patent No 2550020 B1, 2015.
Mazed M, Mazed S. 2009. Nutritional supplement for the prevention of cardiovascular disease, Alzheimer’s disease, diabetes, and regulation and reduction of blood sugar and insulin resistance. US Patent No 2009/0252796 A1.
Md S, Gan SY, Haw YH, Ho CL, Wong S, Choudhury H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int J Biol Macromol, 2018; 118(Pt A):1211–9. CrossRef
Mehmood Y. Brain targeting drug delivery system: a review. Int J Basic Med Sci Pharm, 2015; 5:32–9.
Mezei M, Turi A, Gaal J, Szekacs G, Szebeni G, Marmarosi T, Magyar K, Lengyel J, Szatmari I. Liposome composition containing selegilin. Canada Patent No 02203513, 2001.
Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci, 2003; 6:252–73.
Montesinos RN. Liposomal drug delivery to the central nervous system. Intechopen, London, UK, 2017; doi:10.5772/intechopen.70055,. CrossRef
Mozafari N, Farjadian F, Mohammadi Samani S, Azadi S, Azadi A. Simvastatin-chitosan-citicoline conjugates nanoparticles as the co-delivery system in Alzheimer susceptible patients. Int J Biol Macromol, 2020; 156:1396–407. CrossRef
Mrak RE, Griffin W. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis, 2005; 8:369–75. CrossRef
Mudshinge SR, Deore AB, Patil S, Bhalgat CM. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J, 2011; 19:129–41. CrossRef
Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci, 2009; 71:349–58. CrossRef
Munin A, Edwards-Levy F. Encapsulation of natural polyphenolic compounds: a review. Pharmaceutics, 2011; 3:793–829. CrossRef
Muntimadugu E, Dhommati R, Jain A, Challa VGS, Shaheen, M, Khan W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur J Pharm Sci, 2016; 92:224–34. CrossRef
National Institute for Health and Clinical Excellence (Great Britain). Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer’s Disease: Review of NICE Technology Appraisal Guidance 111. National Institute for Health and Clinical Excellence, 2011.
Navath RS, Kurtoglu YE, Wang B, Kannan S, Romero R, Kannan RM. Dendrimer-drug conjugates for tailored intracellular drug release based on glutathione levels. Bioconjug Chem, 2008; 19:2446–55. CrossRef
Nazem A, Mansoori GA. Nanotechnology solutions for Alzheimer’s disease: advances in research tools, diagnostic methods and therapeutic agents. J Alzheimers Dis, 2008; 13:199–223. CrossRef
Nazem A. Mansoori GA. Nanotechnology for Alzheimer's disease detection and treatment. Insciences, 2011; 1:169–93. CrossRef
Nowick JS, Kreutzer AG, Spencer RK, Salveson PJ. Synthetic beta-amyloid peptides capable of forming stable antigenic oligomers. US Patent No 10662226, 2016.
Oesterling BM, Gulati A, Joshi MD. Nanocarrier-based approaches for treatment and detection of Alzheimer's disease. J Nanosci Nanotechnol, 2014; 14:137–56. CrossRef
Olazaran J, Reisberg B, Clare L. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord, 2010; 30:161–78. CrossRef
Orive G, Hernández RM, Rodríguez Gascón A, DomıÌnguez-Gil A, Pedraz JL. Drug delivery in biotechnology: present and future. Curr Opin Biotechnol, 2003; 14:659–64. CrossRef
Pacheco-Quinto J, Pappolla MA Sambamurti K. Hyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid b peptide levels. Neurobiol Dis, 2006; 22:651–6. CrossRef
Parveen S, Sahoo S. Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs. Clin Pharmacokinet, 2006; 45:965–88. CrossRef
Pashirova TN, Zueva IV, Petrov KA, Lukashenko SS. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: the acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloid Surf B, 2018; 171:358–67. CrossRef
Patel RB, Thakore SD, Patel MR. Patents survey: treatment of alzheimer’s disease through nanotechnology-based drug delivery system. In: Nanotechnology applied to pharmaceutical technology. Springer International Publishing AG, Cham, Switzerland, pp 335–58, 2017. CrossRef
Pinheiro RGR, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer's disease. Eur J Pharm Sci, 2020; 148:105314. CrossRef
Prins ND, Scheltens, P. Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alz Res Ther, 2013; 5:56. CrossRef
Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv, 2020; 27:745–55. CrossRef
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med, 2008; 148:379–97. CrossRef
Romano G. Alzheimer’s disease—clinical trials. Mater Methods, 2018; 8:2675.
Rosse G. Antibody–Drug conjugates for the treatment of inflammation. ACS Med Chem Lett, 2017; 8:992–4. CrossRef
Safari J, Zarnegar Z. Advanced drug delivery systems: Nanotechnology of health design: a review. J Saudi Chem Soc, 2014; 18:85–99. CrossRef
Sainsbury F, Zeng B, Middelberg APJ. Towards designer nanoemulsions for precision delivery of therapeutics. Curr Opin Chem Eng, 2014; 4:11–7. CrossRef
Sanati M, Khodagholi F, Aminyavari S, Ghasemi F, Gholami M, Kebriaeezadeh A, Sabzevari O, Hajipour MJ, Imani M, Mahmoudi M, Sharifzadeh M. Impact of gold nanoparticles on amyloid β-induced Alzheimer's disease in a rat animal model: Involvement of STIM Proteins. ACS Chem Neurosci, 2019; 10:2299–309. CrossRef
Santos BF, Daflon MP, Chorilli GM. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed, 2015; 10: 4981–5003. CrossRef
Saraiva C, Praca C, Ferreira R, Santo T, Ferriera L Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Control Release, 2016; 235:34–47. CrossRef
Satlin A, Fukushima T. Composition comprising an anti-abeta protofibril antibody and a beta-secretase bace1 inhibitor for the treatment of alzheimer's disease. WO Patent No 2018 /081460A1, 2018.
Selley ML. Increased homocysteine and decreased adenosine formation in Alzheimer’s disease. Neurol Res, 2004; 26:554–7. CrossRef
L. Shah, S. Yadav, and M. Amiji. Nanotechnology for CNS delivery of bio-therapeutic agents. Drug Deliv Transl Res, 2013; 3:336–51. CrossRef
Sharma HS, Castellani RJ, Smith MA, Sharma A. The blood-brain barrier in Alzheimer's disease: Novel therapeutic targets and nanodrug delivery. Int Rev Neurobiol, 2012; 102:47–90. CrossRef
Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol, 2009; 86:215–23. CrossRef
Sivasankarapillai VS, Jose J, Shanavas MS, Marathakam A, Uddin MS, Mathew B. Silicon quantum dots: Promising theranostic probes for the future. Curr Drug Targets, 2019; 20:1255-1263. CrossRef
Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med, 2006; 260:211–23. CrossRef
Stegemann S, Leveiller F, Franchi D, Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci, 2007; 31:249–61. CrossRef
Streeter CC Jensen JE, Cabral HJ, Renshaw PF. Yoga Asana sessions increase brain GABA levels: a pilot study. J Altern Complement Med, 2007; 13:419–26. CrossRef
Suri K, Wolfram J, Shen H, Ferrari M. Advances in nanotechnology-based drug delivery platforms and novel drug delivery systems. In: Singh M, Salnikova M (eds.). Novel approaches and strategies for biologics, vaccines and cancer therapies. Academic Press, San Diego, CA, pp 41–58, 2015. CrossRef
Sweeney M, Sagare A, Zlokovic B. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol, 2018; 14:133–50. CrossRef
Thakur AK, Kamboj P, Goswami K, Ahuja K. Pathophysiology and management of alzheimer’s disease: an overview. J Anal Pharm Res, 2018; 7:226‒35. CrossRef
Thakur G, Micic M, Yang Y,Li W, Movia D, Giordani S, Zhang H, Leblane R. Conjugated Quantum dots inhibit the amyloid β (1-42) fibrillation process. Int J Alz Dis, 2011; 2011:502386. CrossRef
USFDA. FDA Approves First Drug to Image Tau Pathology in Patients Being Evaluated for Alzheimer’s Disease, 2020. Available via: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-diseas.
Vakilinezhad MA, Amini A, Javar AH, Bahaaddini B, Zarandi BF, Montaseri H, Dinarvand R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer's disease animal model by reducing Tau hyperphosphorylation. DARU, 2018; 2:165–77.
Varghesea NM, Venkatachalam S. Potential nanocarriers for the delivery of drugs to the brain. Chapter 19, In: Nanoengineered biomaterials for advanced drug delivery. Woodhead Publishing Series in Biomaterials, Sawston, UK, pp 449–72, 2020.
Wadghiri YZ, Li J, Wang J, Hoang DM, Sun Y, Xu H, Tsui W, Li Y, Boutajangout A, Wang A, De- Leon M, Wisniewski T. Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. PLos One, 2013; 8: e57097.
Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA, 1994; 271:992–8.
Watt AD, Crespi GA, Down RA, Ascher DB, Gunn A, Perez KA, McLean CA, Villemagne VL, Parker MW, Barnham KJ, Miles LA. Do current therapeutic anti-Aβ antibodies for Alzheimer's disease engage the target? Acta Neuropathol, 2014; 127:803–10.
Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharmacol Sin, 2014; 4:193–201.
Wisniewski T, Drummond E. Developing therapeutic vaccines against Alzheimer's disease. Expert Rev Vaccines, 2016; 15:401–15.
Wu H, Li J, Zhang Q, Yan X, Guo L, Gao X, Qiu M, Jiang X, Lai R, Chen H. A novel small Odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β25–35-treated rats following intranasal administration. Eur J Pharm Biopharm, 2012; 80s:368–78.
Xiong N, Zhao YJ, Dong XY, Zheng J, Sun Y. Design of a molecular hybrid of dual peptide inhibitors coupled on AuNPs for enhanced inhibition of amyloid beta-protein aggregation and cytotoxicity. Small, 2017; 13:13–20.
Yadollahi R, Vasilev K, Simovic S. Nanosuspension technologies for delivery of poorly soluble drugs. J Nanomater, 2015; 2015:216375.
Yang X, He C, Li J, Chen H, Ma Q, Sui X, Tian S, Ying M, Zhang Q, Luo Y, Zhuang Z, Liu J. Uptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol Lett, 2014; 229:240–9.
Yusuf M, Khan M, Khan R, Ahmad B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J Drug Target, 2012; 3:300–11.
Zhang C, Chen J, Feng C, Shao X, Liu Q, Zhang Q, Pang Z, Jiang X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer's disease. Int J Pharm, 2014a; 461(1–2):192–202.
Zhang C, Zheng X, Wan X, Shao X, Liu Q, Zhang Z, Zhang Q. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer's disease. J Control Release, 2014b; 192:317–24.
Zhao Y, Cai J, Liu Z, Li Y, Zheng C, Zheng Y, Chen Q, Chen H, Ma F, An Y, Xiao L, Jiang C, Shi L, Kang C, Liu Y. Nanocomposites inhibit the formation, mitigate the neurotoxicity, and facilitate the removal of β-amyloid aggregates in Alzheimer's disease mice. Nano Lett, 2019; 19:674–83.
Zheng X, Pang X, Yang P, Wan X, Wei Y, Guo Q, Zhang Q, Jiang X. A hybrid siRNA delivery complex for enhanced brain penetration and precise amyloid plaque targeting in Alzheimer's disease mice. Acta Biomater, 2017; 49:388–401.
Zheng X, Shao X, Zhang C, Tan Y, Liu Q, Wan X, Zhang Q, Xu S, Jiang X. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer's disease. Pharm Res, 2015; 32:3837–49.
