INTRODUCTION
In the tropical regions, utilization of tannin-containing plant materials for ruminant feeding and rumen manipulation received a greater interest, due to both its potential in the improvement of animal performances and the shortage and the rising costs of purposed feedstuffs. Plant secondary metabolites including condensed tannins (CT) are widely distributed in forages, shrubs, legumes, cereals, and grains. Those plant materials are edible for animals, especially for ruminant animals in grazing systems (Paengkoum and Paengkoum, 2010; Purba et al., 2020a). In comparison, several articles are available, which outline the effects of CT on rumen fermentation and its influence on the animal response which are varied because of their sources and concentrations used (Naumann et al., 2017; Paengkoum et al., 2015; Purba et al., 2020b). They wield enormous influences by CT on animal responses might be determined by their purity property value (Purba and Paengkoum, 2019). However, in the same years, duo studies seemed to suggest that chemical structure and molecular weight (MW) of CT metabolite have a greater influence on their major biological property, protein-binding ability, and their efficacy to manipulate rumen fermentation (Aboagye and Beauchemin, 2019; Petlum et al., 2019). It is, thus, suggested that tannin substances could be degraded in the rumen through hydrolysis of their glycosides and cleavages of heterocyclic compounds (McSweeney et al., 2001), and these degradations ended in hydroxyphenolics, phloroglucinol, and volatile fatty acid shifts that are stored finally as energy to animal-derived products, such as meat and milk (Purba et al., 2020d).
Despite CT being a significant benefit for modulating animal performance, imbalance CT intake affected metabolic disorder, such as decreasing animal palatability, limiting voluntary intake, and inhibiting nutrient digestibility (Liang and Paengkoum, 2019; Osborne and McNeill, 2001; Purba et al., 2020c; Purba et al., 2021), which resulted in a higher risk of detrimental health effects (Waghorn, 2008). Some authors have highlighted scenarios where CT could inhibit protein degradation (at a wide range of pH: 3.5–7.0) released in the abomasum that would be absorbed by the host ruminal animals (Huang et al., 2010, 2011; Naczk et al., 2001). The incorporation of CT to bind protein may result in clinically significant forms in the absorption, distribution, metabolism, and pharmacokinetics of protein (Bumbaca et al., 2019; Schmidt et al., 2010). Thus, CT and animal responses to dietary CT have been extensively discussed for metabolic and therapeutic purposes in animals (Fraga-Corral et al., 2020; Smeriglio et al., 2017). In this context, CT may act the same as a drug or substantial food to improve the maintenance of animals. Since animal feedstuff is, in general, to use leafy material and by-product agriculture, we postulated that those originated sources determine the variability of MW and its protein-binding affinity (PBA). As a consequence, the bioavailability of CT might be a wide variety and has to be monitored, considered, and analyzed. Therefore, the present study aimed to determine the concentration, MW distribution, and PBA of CT from selected tropical plants including leaf peel substances as compared to a commercial CT extract.
MATERIALS AND METHODS
Plant preparation
Tropical plants including leaf and peel materials were selected based on their potential benefits as a dietary source of tannins for ruminant feedstuff and their CT content. Three leaves were cassava (Manihot esculenta Crantz.) leaves (CV), Leucaena (Leucaena leucocephala Lam. de Wit) (LN), and Siamese neem (SN) (Azadirachta indica A. Juss. var. siamensis Valeton) (SN) that were planted in a minigarden near the Suranaree University of Technology Goat and Sheep farm, Thailand (14°52′48″N, 102°00′18″E at an elevation of 243 m above sea level), harvested per month by cutting the tips of young leaves, and samples per month were stored until the last collection. A mangosteen (MS) peel (Garcinia mangostana Linn.) was bought from the local market, Nakhon Ratchasima, Thailand. Four plant materials were subsequently freeze-dried using a Lyophilizer (GAMMA 2-16 LSC, CHRiST, Germany) for 24 hours. All dried samples were ground, strained through a 1.0 mm sieve, and kept in an airtight dark container at 4°C for further analysis. In addition, a commercial quebracho tannin (UNITAN ATO, Buenos Aires, Argentina; purity: 75%–77% tannins) (QB) was used to compare simultaneously the concentration of CT, MW, and PBA throughout experimental leaf peel.
Extraction
The concentration of tannin of experimental leaf peel and QB was extracted with aqueous acetone following a prior methodology (Phuyal et al., 2020), with a minor modification. Briefly, 200 mg of dried-ground samples were dissolved in 10 ml of 70% (v/v) aqueous acetone and kept in a shakable-water bath (MEMMERT, WNB22) for 20 minutes at room temperature. Samples were then subjected to centrifugation for 10 minutes at 3,000 × g at 4°C (Sorvall Legend XT/XF Centrifuge Series, Thermo FisherScientific, Waltham, MA). The supernatant was immediately collected and kept at 4°C for further analysis.
Determination of total phenol, total tannin, and CT
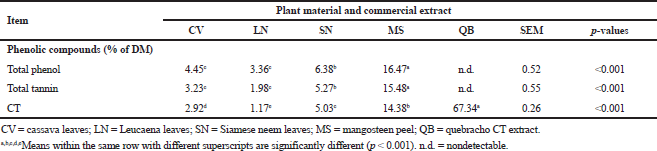
Total phenol and total tannin of experimental leaf peel and QB were determined using the Folin-Ciocalteu method as given by Phuyal et al. (2020). Briefly, 0.1 ml of aliquots of the tannin-containing extract of each extracted sample was added into test tubes, adding distilled water spiking the volume to 0.5 ml. A volume of 0.25 ml of the Folin-Ciocalteu reagent and a volume of 1.25 ml of the sodium carbonate solution was added, and the tubes were vortexed for 1 minute. Total phenol was determined after those tubes were read by a spectrophotometer at 725 nm with 40 minutes prior to incubation (Spectronic 200, Thermo Scientific, Thailand). The amount of total phenol was calculated (n = 3) as tannic acid equivalent and expressed on a dry matter (DM) basis (x%, Table 1).
Total tannin was assayed in polyvinylpolypyrrolidone (PVPP) as a phenolic binder. A mass of 100 mg of PVPP was analytically weighed and transferred to a 100 × 12 mm test tube. Then, 1.0 ml of distilled water and 1.0 ml of each extracted sample were added, mixed, and vortexed for 2 minutes. To preserve tannin precipitated with the PVPP, each tube was stored at 4°C for 15 minutes and subsequently centrifuged at 3,000 × g for 10 minutes. The supernatant which has only simple phenolic other than tannin was collected and measured for the phenolic content following the procedure as mentioned above by taking triple volume as total phenol estimation. The content of nontannin phenol was expressed on a DM basis (y%), and the percentage of tannins was calculated (n = 3) as equal to x−y and expressed as tannic acid on a DM basis (Table 1).
CT content was determined using the Vanillin-HCl method (Hagerman, 2011). By this method, 1 ml of an aliquot of each extract was decanted into a test tube and incubated in a water bath at 30°C for 5 minutes. A volume of 5.0 ml of vanillin reagent was added. A volume of 5.0 ml of 4.0% HCl solution was added at 1 minute after the incubation of the vanillin reagent. After prolonged incubation of the mixture for 20 minutes (n = 3), the final mixture was read at 500 nm to determine CT. The CT content was compared and expressed as a standard curve of catechin equivalent (Table 1).
 | Table 1. Phenolic compounds of each experimental sample. [Click here to view] |
 | Table 2. MW of CT from each experimental sample. [Click here to view] |
Fragmentation and purification of CT
CT from experimental leaf peel and QB was fragmented and purified based on prior protocols (Huang et al., 2010, 2011; Terrill et al., 1992), with a minor modification. Immediately, 200 mg of freeze-drying of experimental samples was assayed in 200 ml of 70% (v/v) aqueous acetone, with a medium shake for 20 minutes. All samples were centrifuged at 3,000 × g for 10 minutes, and the supernatant was collected and then filtered to remove any plant residues under a vacuum. Chlorophylls, pigments, and low MW phenolic acids were removed by washing with similar volume use of diethyl ether in a separation funnel. The mixture was further evaporated under vacuum in a rotary evaporator (Rotavapor R-3, Büchi Labortechnic, Switzerland) at roughly 40°C in order to remove traces of diethyl ether and acetone. Fragmented crude CT of each experimental sample was lyophilized (GAMMA 2-16 LSC, CHRiST, Germany) and kept at 4°C in the dark for further analysis.
Fragmented crude CT of each experimental sample was prepared in an equal volume of 40% (v/v) methanol prior to undergoing the purification step by using Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) as given by Huang et al. (2010). Fragmented crude CT of each experimental sample was subsequently eluted with 40% (v/v) methanol in order to remove low MW phenolics, and then the CT mixture was eluted with 80% (v/v) aqueous acetone. The purified CT was evaporated to remove traces of aqueous acetone by using a rotary evaporator (Rotavapor R-3, Büchi Labortechnic, Switzerland). The purified CT was directly freeze-dried and stored in the dark at 4°C.
Molecular weight determination
MW of the purified CT from each experimental sample was determined by gel permeation chromatography (RID-10A, Shimadzu, Columbia, MD) as described in Huang et al. (2010) equipped in an HSP gel RT 5.0 THF 3 ¼m column (Waters, Milford, MA) and tetrahydrofuran as the mobile phase (at 0.5 ml/minutes, 25°C). Purified CT was assayed in tetrahydrofuran (0.5 mg/ml) and a volume of 20 ¼l of this mixture was injected simultaneously (n = 3). The mean of relative MW was measured based on comparison simultaneously with the prepared calibration curve. The calibration curve was made by preparing serial concentrations of polystyrene (MW standard used) at a range of 162–22,000 Da. The polydispersity index (PDI) was calculated as the MW divided by the mean of MW. The degree of polymerization (DP) was estimated based on the constituent proanthocyanin per acetate MW of approximately 500 (Table 2).
Protein-binding affinity measurement
PBA of the purified CT from each experimental sample was measured by using a protein precipitation assay (Huang et al., 2010). The ability of CT to bind protein was determined by using bovine serum albumin (BSA) as an indicator. The margin number of several concentrations of BSA (0–1.6 mg/ml) was tabulated to make an appropriate standard curve. Standard curve was made from numbers of concentrations that were 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.6 mg/ml dissolved in 500 ml/l aqueous methanol which were assayed each in 0.5 ml BSA buffer (1 mg/ml of BSA in 0.2M acetate buffer, with 0.17M NaCl, at pH 5.0). The assays were mixed gently and underwent centrifugation at 5,000 × g for 20 minutes. The supernatant was carefully discarded and the unbound protein was removed by washing with 0.2M acetate buffer. Hydrolysis was started by prior drying of the tubes in an oven at 100°C overnight and 13.5 M NaOH was subsequently added to tubes, but this step was adjusted to 120°C for 20 minutes. A volume of 1.0 ml of acetic acid was carefully decanted to terminate the drying step. After the temperature at roughly 30°C, the mixture was 0.1 ml of aliquot and combined with 1.0 ml of ninhydrin solution. This aliquot was further incubated in a water bath at 100°C for 20 minutes. Then, its temperature was decreased and subsequently added with 5 ml of deionized water. The mixture was vortexed for 1 minutes and the reading was found to be 570 nm wavelength using a spectrophotometer (n = 3) (Spectronic 200, Thermo Scientific). Nonlinear regression was determined, and it was used to predict an equation for protein-binding data analysis. The curve for purified CT was fitted to a sigmoid curve: Y = a/(1 + b × exp(−c×x)), where Y = mg of BSA precipitated and x = mg of extracted CT incubated. The PBA of CT was expressed as the amount of precipitated protein (Fig. 1).
 | Figure 1. Protein-binding ability of CT in difference of MW of CT from each experimental sample. [Click here to view] |
Statistical analysis
All data including replication per determined parameter were accounted for by one-way analysis of variance procedure of SAS 9.4. Different means among experimental samples were calculated by using Duncan’s procedure, with a difference at p < 0.05.
RESULTS AND DISCUSSION
The occurrence of positive communication between MW of CT and its PBA from various originated sources of CT is evidence that the PBA is origin-dependent. Therefore, the discussion will be counted on the CT profile of each source, MW, and its PBA.
Total phenol, total tannin, and CT
The concentration of phenolic compounds of each experimental sample is presented in Table 1. The concentration of total phenol, total tannin, and CT ranged from 3.36 to 6.38, from 1.98 to 5.27, and from 1.17% to 5.03% of DM, respectively, and those values were the lowest concentration at LN and the highest concentration at SN leaves (p < 0.001). Although MS peels contained significantly a higher concentration of phenolic compounds (p < 0.001) including total phenol, total tannin, and CT (16.47%, 15.48%, and 14.38% of DM basis, resp.) compared to those leafy materials of selected plant species, commercial quebracho CT extract (QB) contained the highest concentration of CT (almost 70% of DM).
In the present study, we highlighted that there are increasing interests to use unconventional local feed materials to replace the traditional feed ingredients in livestock production in the developing countries due to shortage and high cost of the latter. Because of their high nitrogen content, leaves of Leucaena, cassava, and SN are among the local feed resources used for livestock, including ruminant production in the tropical countries (Paengkoum, 2010). Due to their nutrient properties, the presence of secondary phenolic compounds in the above-mentioned feed forages has been shown to exhibit the beneficial capability to increase the flow of rumen undegraded protein (Paengkoum, 2011; Purba et al., 2020b) and mitigate methane production (Beauchemin et al., 2007; Khotsakdee and Paengkoum, 2014).
According to the concentration of some phenolic compounds of selected plant materials, the present results suggest that, among the leaves of tropical plant species, SN had the greatest concentrations of total phenols, total tannins, and CTs followed by CV with LN containing the lowest concentrations of the above compounds. The concentration of CT in LN (approximately 1.17% of DM) which was recorded in the present study was lower compared to the number of 2.76% for Leucaena accession, UHK636 reported by Osborne and McNeill (2001). However, the concentrations of CT of CV (2.92% of DM) and SN (5.03% of DM) derived from the present study were lower than those reported by Wanapat (2003) (4.3% of DM for CV); Paengkoum et al. (2013) (3.9% of DM for LN), and Ngamsaeng et al. (2006) (11.4% of DM for SN). It could be noted that MS is an alternative source for CT supply used in ruminant feeding manipulation because it has an abundance of CT and saponin substances. MS is a tropical fruit-bearing evergreen in countries of Southeast Asia, particularly in the southern region of Thailand. The concentration of tannins of MS peel obtained from the present study (15.48% and 14.38% of DM for total tannins and CT, resp.) is within the range that has been reported at 14.1% total tannin (Moosophin et al., 2010), 7.0%–15.0% crude tannin (Ngamsaeng et al., 2006; Suchitra and Wanapat, 2008), 15.4% of CT (Paengkoum et al., 2015), and 17.7% CT (Norrapoke et al., 2014). Generally, the concentration of CT is affected by various environmental and management factors, including temperature, light intensity, nutrient and water uptake, soil quality, and features of land surfaces. Therefore, the differences in the concentrations of CT among studies are expected.
Condensed tannin molecular weight
MW distribution of CT of each experimental sample is presented in Table 2. The mean of relative MW in peel leaf (Da) was ranked in the descending order as follows: SN > QB > CV > LN > MS with DP ranged from 5.93 to 7.22. Similar to those of the concentration of phenolic compounds, the MW and DP values of CT, compared to those of selected tropical plant leaves, were the lowest at LN and the highest at SN. In addition, among all the CT sources in the present study, MW and DP values of CT from MS were the lowest, while MW and DP values of CT from QB were similar to SN.
The MW of CT has been reported to be a major factor affecting the efficacy of CT to influence rumen fermentation rate, nitrogen balance, and enteric methane production (Huang et al., 2010; Naumann et al., 2013). More recently, Paengkoum et al. (2017) determined the MW of the above three commonly used feed leaves (CV, LN, and SN). The mean of relative MW of CT at CV (3,409 Da), LN (3,222 Da), and SN (3,612 Da) was in agreement with the results in the present study; nevertheless, Paengkoum et al. (2017) and recent study observed the concerned leaves for short-term collection. It indicated that a repeated investigation regarding calculating MW of CV, LN, and SN is not necessary. However, a further study aiming to comprehensively understand the MW distribution investigated for long-term collection and varied approach to extract is highly recommended. Of note, the MW of temperate plants was reported to range between 3,036 and 7,143 Da and was in lower numbers at MW of warm-season perennial legumes (McAllister et al., 2005). The range of MW values of CT extracted from selected tropical leaf peel in the present study from 3,222 to 3,612 Da was higher than those reported for warm-season perennial legumes, which ranged from 552 to 1,483 Da (Naumann et al., 2013). The molecular mass of CT of LN estimated in this study was higher than those reported by Huang et al. (2010) for local L. leucocephala (2,737 Da) and L. leucocephala hybrid (Bahru) (2,872 Da), Huang et al. (2010), and Saminathan et al. (2014) for L. leucocephala hybrid Rendang (1,266 Da) that are grown and harvested in Malaysia. We do not know of any published data on the MW of CV, SN, and QB, thus assuming that the present data are the first reported of MW of CT from CV, SN, and QB.
Protein-binding affinity of CT
The PBA of CT of each experimental sample is depicted in Figure 1. The present study indicated that the amount of protein (BSA) precipitated with CT relatively increased with a higher MW of CT. CT extract from SN had the highest weight-average MW compared to all selected tropical materials studied; thereby, it seemed to have the highest amount of protein precipitated, whereas CT extract from MS had the lowest MW among all materials studied; thereby, it is suggested to have the lowest protein-binding capacity. As a consequence, these present results indicated that a higher MW of CT is suggested to have a greater PBA, and vice versa.
The PBA is the major biological property of CT. It has been well-documented that CT is able to bind and shift the insoluble protein complexes, which influences the defend system towards the inevitably microbial and enzymatic degradation and subsequent ruminal fermentation (Lorenz et al., 2014). It is fairly tough to generate that PBA has a direct influence on the pharmacodynamic of protein; however, the inclusion of CT to perform PBA might relate to the remarkable changes in the absorption, distribution, metabolism, and pharmacokinetics of protein (Bumbaca et al., 2019; Schmidt et al., 2010). Understanding the PBA scenario, herein, could provide an easy-to-understand definition for the inhabitation of protein utilization. Hence, dietary CT by animals could simply reflect the metabolic and therapeutic goals (Smeriglio et al., 2017).
Notably, the molecular size of CT is the major influence on their PBA (McNabb et al., 1998); however, even MW including pH of CT is not a sole factor to be more paid attention to PBA influence; here, other factors such as the monomeric composition of CT could differ the PBA of CT (Huang et al., 2011). According to the literature, it is suggested that PBA of CT differed among plant species and varieties, indicating that the difference of MW resulted in the difference of PBA. For instance, Osborne and McNeill (2001) investigated the ability of CT within different sizes (by size exclusion chromatography procedure) from the genus Leucaena which was expected to bind protein. This present result was in agreement with previous studies by Huang et al. (2010, 2011) who reported that a higher MW fraction of CT extracted from L. leucocephala had been assessed to have a stronger protein-binding capacity compared to those of lower MW. The result showed that the larger-sized CT could precipitate more protein rather than the smaller-sized CT. Therefore, the present result is able to give extensive evidence that each plant material has a varied MW of CT due to differences in fractioned organic constructions such as monomeric composition. CT with a higher monomeric property seems to have a higher power to bind the protein precipitation. However, a more comprehensive understanding to investigate either specific monomer or other agents as protein blocking remains unclear.
CONCLUSION
Based on the current results, the content of phenolic compounds including the proportion of CT was varied in the tropical leaf peel. The experimental group of plant materials in the present result is prepared as commonly feedstuff used. This difference could indicate the divergent profile of MW from the tropical leaf peel. The present study records the difference in MW of CT corresponding to the ability to bind protein. This result showed that there has been further speculation that the aforementioned positive correlation might be due to the link of chemical bonding between an MW and a protein binding by CT as influenced by monomeric property therein. However, its specific monomer itself needs to be assessed in further observation. Leaves of SN seem to have a higher MW of CT corresponding to a higher capacity to bind protein that could be alternative supplementation in animal feeding. However, the size of the dietary intake of SN should be monitored in order to give a negative impact on animal performance. LN may be more suitable to be used for feedstuff purposes due to its content, either the lower MW of CT or the higher protein concentration. LN could help farmers including small-scale farming systems in the tropic or subtropic region, where this feed material is widely available. Collectively, present results presented that a wide variety of PBA from tropical leaf peel could provide a brief reference at least on prohibited protein metabolism by CT tending to the limitation of metabolic and pharmacokinetic of protein.
LIST OF ABBREVIATIONS
CT: condensed tannin; MW: molecular weight; PBA: protein-binding affinity; QB: quebracho tannin; CV: cassava leaves: LN: Leucaena leaves; SN: Siamese neem leaves: MS: MS peel; DM: dry matter; PVPP: polyvinylpolypyrrolidone; PDI: polydispersity index; DP: degree of polymerization; BSA: bovine serum albumin.
ACKNOWLEDGMENTS
The authors would like to thanks Nakhon Ratchasima Rajabhat University and Suranaree University of Technology for their facilities and for financially supporting this research. The authors are truly grateful to all staff of the Centre of Scientific and Technological Equipment, Suranaree University of Technology, and Nakhon Ratchasima Rajabhat University for use of the research facilities. Dr. Rayudika Aprilia Patindra Purba gratefully acknowledges the Institute of Research and Development grant (Contract no. Full-time 61/02/2021) as a full-time Doctoral Researcher to conduct research at Suranaree University of Technology.
AUTHOR’S CONTRIBUTIONS
SP, AP, RAPP, and PP formulated the hypothesis and designed the experiment; AP conducted the experiment and laboratory analysis; AP and RAPP performed the data statistical analysis and interpretation; AP was responsible for writing the first draft of the manuscript. SP and PP were responsible for the correction of the manuscript; SP, RAPP, and PP edited the last version of the manuscript. The manuscript was read and approved by all authors.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest concerning this manuscript.
FUNDING
Funding was provided by Suranaree University of Technology (SUT), Nakhon Ratchasima Rajabhat University (NRRU), and Thailand Science Research and Innovation (TSRI).
REFERENCES
Aboagye IA, Beauchemin KA. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: a review. Animals, 2019; 9:856. CrossRef
Beauchemin KA, McGinn SM, Martinez TF, McAllister TA. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle1. J Anim Sci, 2007; 85:1990–6. CrossRef
Bumbaca B, Li Z, Shah DK. Pharmacokinetics of protein and peptide conjugates. Drug Metab Pharmacokinet, 2019; 34:42–54. CrossRef
Fraga-Corral M, García-Oliveira P, Pereira AG, Lourenço-Lopes C, Jimenez-Lopez C, Prieto MA, Simal-Gandara J. Technological application of tannin-based extracts. Molecules, 2020; 25(3):614. CrossRef
Hagerman, AE. The tannin handbook. New Delhi, India: CBS Publisher and Distributor; Oxford: Department of Chemistry and Biochemistry, Miami University, 2011. CrossRef
Huang XD, Liang JB, Tan HY, Yahya R, Khamseekhiew B, Ho YW. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim Feed Sci Technol, 2010; 159:81–7. CrossRef
Huang XD, Liang JB, Tan HY, Yahya R, Long R, Ho YW. Protein-binding affinity of leucaena condensed tannins of differing molecular weights. J Agric Food Chem, 2011; 59:10677–82.
Khotsakdee J, Paengkoum P. Dietary non-ionic surfactant on rumen fermentation and bacterial population in ruminants: a review. Res J Appl Sci, 2014; 9:17–22.
Liang JB, Paengkoum P. Current status, challenges and the way forward for dairy goat production in Asia – conference summary of dairy goats in Asia. Asian-Australas J Anim Sci, 2019; 32:1233–43. CrossRef
Lorenz MM, Alkhafadji L, Stringano E, Nilsson S, Mueller-Harvey I, Udén P. Relationship between condensed tannin structures and their ability to precipitate feed proteins in the rumen. J Sci Food Agric, 2014; 94:963–8. CrossRef
McAllister TA, Martinez T, Bae HD, Muir AD, Yanke LJ, Jones GA. Characterization of condensed tannins purified from legume forages: chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J Chem Ecol, 2005; 31:2049–68. CrossRef
McNabb WC, Peters JS, Foo LY, Waghorn GC, Jackson FS. Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1). J Sci Food Agric, 1998; 77:201–12.
McSweeney CS, Palmer B, McNeill DM, Krause DO. Microbial interactions with tannins: nutritional consequences for ruminants. Anim Feed Sci Technol, 2001; 91:83–93. CrossRef
Moosophin K, Wetthaisong T, Seeratchakot L, Kokluecha W. Tannin extraction from mangosteen peel for protein precipitation in wine. Asia Pac J Sci Technol, 2010; 15:377–85.
Naczk M, Amarowicz R, Zadernowski R, Shahidi F. Protein precipitating capacity of condensed tannins of beach pea, canola hulls, evening primrose and faba bean. Food Chem, 2001; 73:467–71. CrossRef
Naumann HD, Tedeschi LO, Muir JP, Lambert BD, Kothmann MM. Effect of molecular weight of condensed tannins from warm-season perennial legumes on ruminal methane production in vitro. Biochem Syst Ecol, 2013; 50:154–62. CrossRef
Naumann HD, Tedeschi LO, Zeller WE, Huntley NF. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. R Bras Zootec, 2017; 46:929–49. CrossRef
Ngamsaeng A, Wanapat M, Khampa S. Evaluation of local tropical plants by in vitro rumen fermentation and their effects on fermentation end-products. Pak J Nutr, 2006; 5(5):414–8. CrossRef
Norrapoke T, Wanapat M, Wanapat S, Foiklang S. Effect of Centella Asiatica powder (CAP) and mangosteen peel powder (MPP) on rumen fermentation and microbial population in swamp buffaloes. J Anim Plant Sci, 2014; 24:435–44.
Osborne NJT, McNeill DM. Characterisation of Leucaena condensed tannins by size and protein precipitation capacity. J Sci Food Agric, 2001; 81:1113–9. CrossRef
Paengkoum P. Effects of neem (Azadirachta indica) and Leucaena (Leucaena leucocephala) fodders on digestibility, rumen fermentation and nitrogen balance of goats fed corn silage. J Anim Vet Adv, 2010; 9:883–6. CrossRef
Paengkoum P. Effects of Streblus asper lour foliage on digestibility, rumen fermentation, and nitrogen balance of growing goats. Trop Anim Health Prod, 2011; 43:491–4. CrossRef
Paengkoum P, Paengkoum S. Effects of supplementing rice straw with Leucaena (Leucaena leucocephala) and Madras thorn (Pithecellobium dulce) foliages on digestibility, microbial N supply and nitrogen balance of growing goats. J Anim Physiol Anim Nutr, 2010; 94:e59–65. CrossRef
Paengkoum P, Phonmun T, Liang JB, Huang XD, Tan HY, Jahromi MF. Molecular weight, protein binding affinity and methane mitigation of condensed tannins from mangosteen-peel (Garcinia mangostana L). Asian-Australas J Anim Sci, 2015; 28:1442–8. CrossRef
Paengkoum P, Thongpea S, Paengkoum S. Utilization of concentrate supplements containing varying levels of cassava leaf pellet by growing goats fed a basal diet of pangola hay. Indian J Anim Res, 2017; 51:1091–6. CrossRef
Paengkoum P, Traiyakun S, Paengkoum S. Intestinal digestibility of enriched-protein fodders measured by mobile bag incubated with or without pepsin-HCl and three-step techniques. S Afr J Anim Sci, 2013; 43:511–8. CrossRef
Petlum A, Paengkoum P, Liang JB, Vasupen K, Paengkoum S. Molecular weight of condensed tannins of some tropical feed-leaves and their effect on in vitro gas and methane production. Anim Prod Sci, 2019; 59:2154–60. CrossRef
Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci World J, 2020; 2020:8780704. CrossRef
Purba RAP, Paengkoum P. Bioanalytical HPLC method of Piper betle L. for quantifying phenolic compound, water-soluble vitamin, and essential oil in five different solvent extracts. J Appl Pharm Sci, 2019; 9:033–9. CrossRef
Purba RAP, Paengkoum P, Paengkoum S. The links between supplementary tannin levels and conjugated linoleic acid (CLA) formation in ruminants: a systematic review and meta-analysis. PLoS One, 2020a; 15:e0216187. CrossRef
Purba RAP, Paengkoum S, Yuangklang C, Paengkoum P. Flavonoids and their aromatic derivatives in Piper betle powder promote in vitro methane mitigation in a variety of diets. Cienc Agrotec, 2020b; 44:e012420. CrossRef
Purba RAP, Yuangklang C, Paengkoum P. Enhanced conjugated linoleic acid and biogas production after ruminal fermentation with Piper betle L. supplementation. Cienc Rural, 2020c; 50:e20191001. CrossRef
Purba RAP, Yuangklang C, Paengkoum S, Paengkoum P. Milk fatty acid composition, rumen microbial population and animal performance in response to diets rich in linoleic acid supplemented with Piper betle leaves in Saanen goats. Anim Prod Sci, 2020d. doi:10.1071/AN20182. CrossRef
Purba RAP, Yuangklang C, Paengkoum S, Paengkoum P. Piper oil decreases in vitro methane production with shifting ruminal fermentation in a variety of diets. Int J Agric Biol, 2021; 25:231–40.
Saminathan M, Tan HY, Sieo CC, Abdullah N, Wong CMVL, Abdulmalek E, Ho YW. Polymerization degrees, molecular weights and protein-binding affinities of condensed tannin fractions from a Leucaena leucocephala hybrid. Molecules, 2014; 19:7990–8010. CrossRef
Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci, 2010; 99:1107–122. CrossRef
Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol, 2017; 174:1244–62. CrossRef
Suchitra K, Wanapat M. Effects of mangosteen (Garcinia mangostana) peel and sunflower and coconut oil supplementation on rumen fermentation, milk yield and milk composition in lactating dairy cows. Livest. Res. Rural Dev, 2008; 20 :: supplement.
Terrill, TH, Rowan AM, Douglas GB, Barry TN. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J Sci Food Agric, 1992; 58:321–9. CrossRef
Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—progress and challenges. Anim Feed Sci Technol, 2008; 147:116–39. CrossRef
Wanapat, M. Manipulation of cassava cultivation and utilization to improve protein to energy biomass for livestock feeding in the tropics. Asian-Australas J Anim Sci, 2003; 16:463–72. CrossRef