INTRODUCTION
Over the last few years, there has been a growing interest in using natural compounds produced by plants and understanding their biological significance. Indeed, many of these compounds may have applications in the food, pharmaceutical, or biotechnological industry [1,2]. Among the various organic structures possessed by these biomolecules, polyphenols present in plant foods such as fruits, legumes, and vegetables have been shown to have significant health benefits [3]. Indeed, a vegetable-based diet, rich in polyphenols, has been associated with a decrease in the incidence of many human pathologies, such as cancer, neurodegenerative, and cardiovascular diseases. Polyphenols can be divided into two main groups: flavonoids and non-flavonoids, such as tannins [2]. Flavonoids and condensed tannins (CT) are synthesized in several plant species, including forage plants, which also represent a rich and interesting source of bioactive molecules endowed with therapeutic potential in human health [4]. For instance, the Fabaceae family, including Medicago sativa, Hedysarum coronarium, and Lotus ornithopodioides contains the richest source of polyphenols [4]. These phytochemicals possess anti-cancer properties, acting on enzymes and proteins, regulating cellular and signaling events in growth, and having anti-inflammatory effect and anti-oxidant action [5]. The bioactive components present in these plants possess antioxidant, anti-inflammatory, immunostimulatory, and antiaging properties [6,7]. Among antioxidant effects, M. sativa inhibits the xanthine dehydrogenase/xanthine oxidase conversion and resultant superoxide anion production in ischemia [8]. Hedysarum coronarium is a short-lived legume, found throughout the Mediterranean region both as a wild herb and as a cultivated fodder crop [9]. Hedysarum coronarium is a source of main specialized metabolites within the chemical classes of flavonoids, CT, and saponins, which have various applications in human and animal health and nutrition [10]. Asteraceae is another important plant family cultivated worldwide. Most members of this family have therapeutic applications and a long history in traditional medicine. Many species of Asteraceae exhibit various pharmacological activities, such as anti-inflammatory, antimicrobial, antioxidant, and hepatoprotective properties, attributed to their phytochemical components, including saponins, and polyphenolic compounds [11]. Chicory, or Cichorium intybus L., is a perennial herb of the Asteraceae family and is cultivated worldwide. It is a rich source of bioactive compounds, including inulin, caffeic acid derivatives, flavonoids, and vitamins. Cichorium intybus has anti-inflammatory, antioxidant, immunological, anti-diabetic, anti-cancer, gastro-protective, anti-microbial, and many other properties [12]. Therefore, the properties of the polyphenols present in these Mediterranean forage crops prompted an investigation into their effects towards the activity of some redox enzymes involved in the cellular control of reactive oxygen species (ROS), eventually produced during an uncontrolled electron transport chain [13].
It is known that ROS, although considered important cell-signaling components when present in low amounts [14], can cause serious damage to cellular biomolecules [15] including lipids, DNA, RNA, and proteins, contributing to cell pathophysiology. In particular, hydrogen peroxide, superoxide anion radical, singlet oxygen, and hydroxyl radical are considered the most common ROS acting in biological systems. The uncontrolled endogenous/exogeneous ROS production, called oxidative stress, is a feature commonly observed in several pathologies including cancer [15,16]. Cells contain several redox enzymes, acting as pro- or anti-oxidant systems, and are involved in the fine tuning of a balanced ROS level. Superoxide dismutase, catalase, peroxiredoxin, and glutathione peroxidase are considered the most common antioxidant enzymes [17]. Among these, catalase (CAT) is present in a wide range of aerobic and anaerobic organisms and is highly conserved throughout evolution. CAT plays a crucial role in cell detoxification from ROS, because it catalyzes the dismutation of two hydrogen peroxide molecules into two molecules of water and one of oxygen [18,19]. However, CAT is also capable of scavenging peroxynitrite, another strong ROS responsible for severe damage to biomolecules. Hydrogen peroxide, although produced by superoxide dismutase, is also formed during the activity of xanthine oxidase (XO), a redox enzyme involved in purine catabolism. Together with xanthine dehydrogenase, XO derives from the conversion of a common pro-enzyme called xanthine oxidoreductase; however, XO becomes the predominant form in oxidant environments. XO catalyzes the oxidation of xanthine to uric acid, by producing one molecule of hydrogen peroxide from one molecule of oxygen and one of water. However, in some conditions, XO forms two superoxide anions instead of one hydrogen peroxide, by using two molecules of oxygen and one of water. In this work, CAT and XO have been considered as typical redox enzymes to study the possible effects of the bioactive molecules contained in extracts from leaves of L. ornithopodioides, H. coronarium, M. sativa, and C. intybus.
The working hypothesis of this study was the question of whether extracts from forage crops could be endowed with an inhibitory effect towards typical redox enzymes; in fact, to the best of our knowledge, this type of investigation has not been considered yet. However, previous investigations on plant extracts pointed to the importance of phytomedicine as a coadjuvant in disease treatment [20–22]. Under this concern, the extract from M. sativa displayed significant inhibition of xanthine oxidase, an enzyme frequently used as a target of pharmacological studies aimed at the identification of possible novel drugs. Interestingly, this extract was also endowed with cytotoxicity against human gastric cancer cell lines.
MATERIALS AND METHODS
Materials and reagents
Reagents
Xanthine, hydrogen peroxide (30% v/v stabilized solution), xanthine oxidase from bovine milk (0.5 U/mg protein), catalase from bovine liver (2,000–5,000 U/mg protein), and all other reagents and solvents of high analytical grade were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid, (+)-catechin, and delphinidin were purchased from Sigma-Aldrich (Merk Life Science, Milan, Italy).
Preparation of plant extracts
Leaves of Lotus ornithopodioides and Cichorium intybus were obtained from plants indigenous to Sardinia (Italy), grown as previously reported [23]; those from Medicago sativa were obtained by CRA-FLC (Lodi, Italy) [4]; leaves from Hedisarum coronarium were obtained from cultivated fields of Irsina, Matera (Italy) [4]. Deep-frozen samples were finely powdered, defatted with chloroform, and then subjected to the following extraction procedure on 100–200 mg of defatted material. Concerning samples from L. ornithopodioides and H. coronarium, the procedure included a first step of water/acetone extraction [24], followed by chromatography on Sephadex LH 20 column that was first eluted with aqueous methanol (1:1) to eliminate the lower molecular weight phenols; a second elution step with aqueous acetone (3:7) allowed the obtainment of a source of CT [10]. Regarding M. sativa and C. intybus, samples were extracted with 5 ml of 80% methanol under stirring overnight. After centrifugation (3,000 × g), the supernatant was collected, whereas the residue underwent a second step of extraction and centrifugation, and the supernatant was combined with the previous, thus forming a source of flavonoids [10]. Solvent from all these extracts was eliminated under vacuum and the samples underwent a final freeze-dried step of lyophilization. Stock solutions of these extracts were prepared by dissolving the lyophilized material in dimethylsulfoxide (DMSO). These solutions were used for evaluating the presence of the different groups of phenolic compounds. In particular, the total phenolic content in the extracts was determined according to the adapted Folin–Ciocalteu colorimetric method [25], measuring the amount as gallic acid equivalents; total flavonoids were evaluated with the AlCl3 method [26] as catechin equivalents; the presence of proanthocyanidins were determined by the butanol/HCl assay [27] as delphinidin equivalents.
Biochemical methods
For measuring the activity of catalase from bovine liver (CAT) and xanthine oxidase from bovine milk (XO), the spectrophotometric methods chosen entailed the usage of a Cary 100 UV-Vis Spectrophotometer (Agilent Technologies, Milan, Italy). In the CAT assay, the wavelength was set at 240 nm, because the elimination of hydrogen peroxide by CAT provoked a specific absorbance decrease at this wavelength. On the other hand, in the XO assay, the wavelength was set at 295 nm, because the formation of uric acid by XO provoked a specific absorbance increase at this wavelength. The following reaction mixtures were prepared for the steady-state determination of CAT and XO. In the CAT assay, the 1 ml final volume of a 50 mM potassium phosphate buffer, pH 7.0 (buffer A) contained 32.6 mM hydrogen peroxide and different concentrations of the various plant extracts. In the XO assay, the 500 µl final volume of a 100 mM potassium phosphate buffer, pH 7.8 supplemented with 0.1 mM EDTA (buffer B) contained 75 µM xanthine and different concentrations of the various plant extracts. Moreover, all reaction mixtures contained an identical 1% (v/v) concentration of DMSO as a vehicle. The reaction started at 25°C with the addition of 1 U/ml of CAT or 0.2 U/ml of XO, respectively. The absorbance decrease/increase was kinetically recorded up to 30 seconds, to evaluate the linear part of the kinetics. The effect of each polyphenolic extract was expressed through the ratio of CAT or XO activity measured in the presence of the putative inhibitor over that measured in the absence of the inhibitor. This activity ratio, expressed as a percentage, was directly reported vs the concentration of extract, evaluated as the molarity of gallic acid equivalent [25] in a dose-dependent profile; 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. Moreover, a logarithmic transformation of the activity ratio was realized and semilogarithmic plots were constructed to calculate the inhibitor concentration that caused a 50% reduction of the activity (IC50) through an evaluation of the data from the resulting straight lines [28].
To evaluate the inhibition power and mechanism exerted by the polyphenolic extracts, kinetic measurements of CAT and XO activity were realized. To this aim, the substrate concentration in the reaction mixtures ranged between 3.26 and 32.6 mM hydrogen peroxide or 4 and 30 µM xanthine, in the CAT or XO assay, respectively; the other experimental conditions remained those of the steady-state assays. Furthermore, the kinetics were performed in the absence or in the presence of two fixed concentrations of each polyphenolic extract. The KM for the substrate and Vmax of the reaction were derived either from the direct nonlinear interpolation in the Michaelis–Menten hyperbolic equation of the initial rate of reaction versus the substrate concentration or from a double reciprocal transformation of the kinetic data in Lineweaver–Burk plots. The putative type of inhibition mechanism was tentatively assessed on the basis of the effect of polyphenolic extracts on KM and Vmax values. In particular, a putative competitive mechanism was assigned if the Vmax of the reaction remained essentially unvaried, whereas the KM for the substrate significantly increased in the presence of the inhibitor; vice versa, a noncompetitive mechanism was assigned if the Vmax decreased and the KM remained unvaried; finally, a concomitant decrease of KM and Vmax suggested an uncompetitive inhibition mechanism. The inhibition constant (Ki) was obtained by using the following equations for competitive (a), noncompetitive (b), or uncompetitive (c) mechanism:
Ki = KM × [I]/(K’M – KM)equation (a)
Ki = V’max × [I]/(Vmax – V’max)equation (b)
Ki = V’max × [I]/(Vmax – V’max) and Ki = K’M × [I]/(KM – K’M)equations (c)
where K’M or V’max represents the KM or Vmax measured in the presence of the concentration [I] of the inhibitor.
Cell cultures and treatments
The human gastric adenocarcinoma MKN-28 [29] and AGS cell lines (American Type Culture Collection, Manassas. VA, USA), were cultured in Dulbecco’s modified Eagle medium (DMEM; Microgem Laboratory Research, Milan, Italy) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Microgem Laboratory Research, Milan, Italy), 2 mM L-glutamine, 100 IU/ml penicillin G, and 100 μg/ml streptomycin, in a humidified incubator at 37°C under a 5% CO2 atmosphere. Cancer cells were split and seeded in plates (75 cm2) every 2 days and used for assays during the exponential phase of growth. Cell treatments were always carried out 24 hours after plating.
The cell viability was evaluated as a mitochondrial metabolic activity using the 3-(4,5-dimethylthiazol-2-yl)-2,5-biphenyltetrazolium bromide (MTT) assay, as previously reported [29,30]. Briefly, cells were seeded into 96-well microplates (1 × 104 cells/well), and after 24 hours of incubation, treated with different concentrations of the extracts or with 0.6% (v/v) DMSO as the control vehicle. After 24 hours of treatment, 10 μl of the MTT solution (5 mg/ml) was added to each well in the dark, and the plates were incubated for 3 hours at 37°C under a 5% CO2 atmosphere. At the end of incubation, the culture medium was removed, and 100 μl of 0.1 N hydrochloric acid in isopropanol was added to each well to solubilize the formazan crystals. Finally, the absorbance was measured at a wavelength of 570 nm using a BioTek Synergy H1 microplate reader (Agilent, Santa Clara, CA, USA). The cell viability was expressed as a percentage relative to the untreated cells set as 100%.
Statistical analysis
All the activity measurements were repeated in at least three separate experiments and the resulting data were analyzed with the KaleidaGraph program (Synergy, 5.0 version, Adalta, Italy). The kinetic and inhibition parameters were reported as the mean ± standard error. The statistical significance of nonlinear and linear fittings of the data was evaluated with the correlation coefficient R. The statistical significance of the cell viability data was evaluated with ANOVA with Bonferroni’s Post hoc test, and the significance was accepted when p < 0.05.
RESULTS
Preparation of polyphenolic extracts from leaves of Mediterranean forage crops
Leaves from typical plants of Mediterranean forage crops, such as L. ornithopodioides, H. coronarium, M. sativa, and C. intybus were subjected to extraction procedures to obtain a source of polyphenols. The total polyphenolic content ranged in the 4–50 mg of gallic acid equivalent per mg of defatted material. In particular, the extraction from L. ornithopodioides and H. coronarium aimed at the obtainment of CT and the corresponding samples were called LoCT and HcCT, respectively; vice versa, the extraction from M. sativa and C. intybus aimed at the obtainment of flavonoids (F) and the corresponding samples were called MsF and CiF, respectively. The four extracts displayed a different composition as shown in Table 1. A higher amount of phenolics was found in CiF, representing 66.1% ± 1.1% of the total extract, followed by flavonoids, evaluated as 33.9% ± 1.3% of the total. Flavonoids were quoted as the dominant compounds in MsF, evaluated as 65.4% ± 2.8% of the total extract, while phenolics accounted for 34.5% ± 1.6%. CT were found in HcCT and LoCT where they represent 58.4% ± 2.2% and 27.8% ± 1.0% of the total, respectively, whereas they are completely absent in the other plant extracts. Vice versa, flavonoids (18.2% ± 1.4% and 47.1% ± 1.4%, in HcCT and LoCT, respectively) and phenolics (23.4% ± 1.8% and 25.1% ± 0.4%, in HcCT and LoCT, respectively) were also found in both these plant extracts.
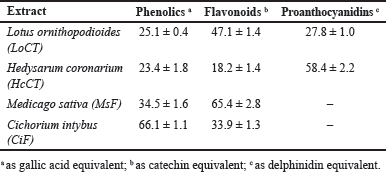 | Table 1. Percentage composition of the main classes of polyphenolic compounds detected in the four plant extracts used in this investigation. [Click here to view] |
Effect of polyphenolic extracts on the steady-state activity of some typical redox enzymes
The effect of the plant extracts on the activity of redox enzymes was investigated. The first redox enzyme considered in this study was CAT and the effect of an increasing concentration of these extracts on the steady-state activity of CAT is illustrated in Figure 1A. All the extracts caused a dose-dependent reduction of the activity, thus suggesting that the polyphenolic compounds present in the extracts were endowed with some inhibitory effect on CAT; however, no great differences seem to emerge in their inhibition strength. After a logarithmic transformation of the activity data (Fig. 1B), an IC50 value was calculated for each extract and reported in Table 2 as the molarity of gallic acid equivalents. These parameters confirm the previous observation on a similar moderate inhibition strength possessed by the various extracts, because all values are comprised in a narrow concentration interval. Indeed, the inhibition strength of HcCT (IC50 28 ± 5 µM) is closely followed by MsF (36 ± 3 µM), CiF (37 ± 5 µM), and LoCT (53 ± 3 µM). These findings suggest that these extracts, containing CT or flavonoids, have a similar moderate inhibition strength towards CAT.
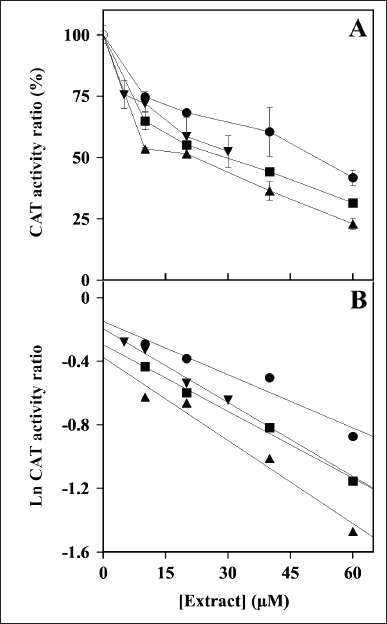 | Figure 1. Effect of LoCT, HcCT, MsF, and CiF extracts on the steady-state activity of bovine liver catalase (CAT). (A) The ratio of CAT activity was assayed in the absence without (empty circle) or in the presence of the indicated concentrations of LoCT (filled circles), HcCT (triangles), MsF (inverted triangles) and CiF (squares) and expressed as a percentage, as indicated in the Methods section. 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. (B) Data analyzed after a logarithmic transformation of the activity ratio. [Click here to view] |
The evaluation of the possible inhibition by the polyphenolic extracts was extended to XO, another crucial enzyme involved in the homeostasis of the cellular ROS levels. Indeed, Figure 2A shows the effect of an increasing concentration of the extracts on the steady-state activity of XO. Among the four extracts, MsF caused a clear dose-dependent reduction of the XO activity, followed at a distance by CiF, the other flavonoid-containing extract. On the other hand, the extracts containing CT, namely LoCT and HcCT, caused only a scarce if any inhibition of the XO activity. After a logarithmic transformation of the data (Fig. 2B), reliable IC50 values were obtained only for MsF and CiF (Table 2). As expected, MsF had a significant inhibition power towards XO with an IC50 value of 15 ± 2 µM, followed at a distance by CiF, having an IC50 of 83 ± 4 µM. For LoCT and HcCT, no reliable IC50 values could be calculated because of their low inhibition strength; a rough evaluation of the IC50 extrapolated for these extracts predicted a value greater than 100 µM (Table 2). Therefore, the extracts containing CT were no longer considered as possible inhibitors of the XO activity, whereas the flavonoid-containing extracts, especially MsF, merited a deeper investigation.
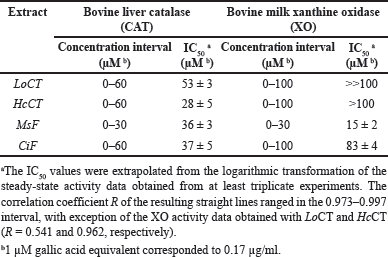 | Table 2. Inhibition by polyphenolic extracts on the steady-state activity of typical redox enzymes. [Click here to view] |
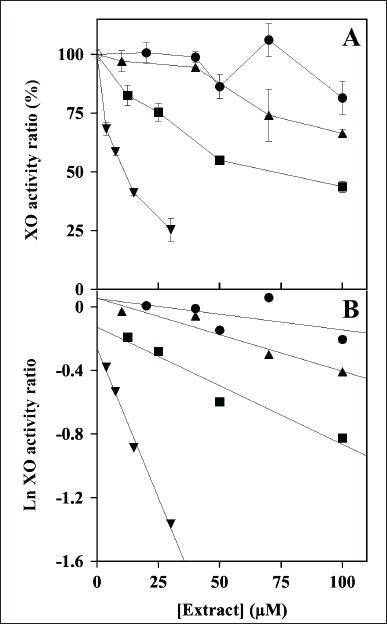 | Figure 2. Effect of LoCT, HcCT, MsF, and CiF extracts on the steady-state activity of bovine milk xanthine oxidase (XO). (A) The ratio of XO activity was assayed in the absence (empty circle) or in the presence of the indicated concentrations of LoCT (filled circles), HcCT (triangles), MsF (inverted triangles) and CiF (squares) and expressed as a percentage, as indicated in the Methods section. 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. (B) Data analyzed after a logarithmic transformation of the activity ratio. [Click here to view] |
Effect of polyphenolic extracts on the kinetic parameters of catalase and xanthine oxidase
The investigation of the inhibition mechanism exerted by the extracts was also realized through kinetic measurements of the CAT and XO activity. Concerning CAT, the time-dependent consumption of hydrogen peroxide was measured at different hydrogen peroxide concentrations in the absence or in the presence of two fixed concentrations of the various extracts. The resulting data of initial velocity were analyzed either in the typical Michaelis–Menten representation (Fig. 3, panels A, C, E, and G) or in Lineweaver–Burk plots (Fig. 3, panels B, D, F, and H). In particular, the analysis of the straight lines drawn in the Lineweaver–Burk plots allowed the tentative assignment of the inhibition mechanism for each extract. In the case of LoCT and MsF, the almost parallel straight lines did not intersect, when crossing neither the abscissa nor the ordinate axis, a behavior corresponding to an uncompetitive mechanism of the CAT activity; vice versa, a noncompetitive mechanism was envisaged for HcCT, because the straight lines apparently intersected when crossing the abscissa axis; finally, in the case of CiF, the intersection occurred when crossing the ordinate axis, thus pointing to a competitive inhibition mechanism. Interestingly, the values of the kinetic parameters KM and Vmax derived from the Michaelis–Menten equation were almost overlapping with those calculated from the Lineweaver–Burk plots. On the basis of the effects observed on these parameters of the CAT activity calculated in the absence or in the presence of two concentrations of extracts, it was possible to obtain the Ki value for each extract (Table 3). Compared to IC50, Ki represents a more accurate measurement of the inhibition power exerted by each extract on the enzyme CAT, because this parameter is independent on the substrate concentration. Among the four samples, the lowest Ki (22.7 ± 1.2 µM) was found with HcCT. The inhibition power of the other samples slightly and progressively decreased with MsF, CiF, and LoCT in the order, because the corresponding Ki values were 36.6 ± 15.1 µM, 45.6 ± 13.0 µM, and 52.2 ± 17.6 µM, respectively. These data confirm that the CT and flavonoids extracted from leaves of common forage crops are endowed with a common evident, although moderate capability to inhibit the CAT activity.
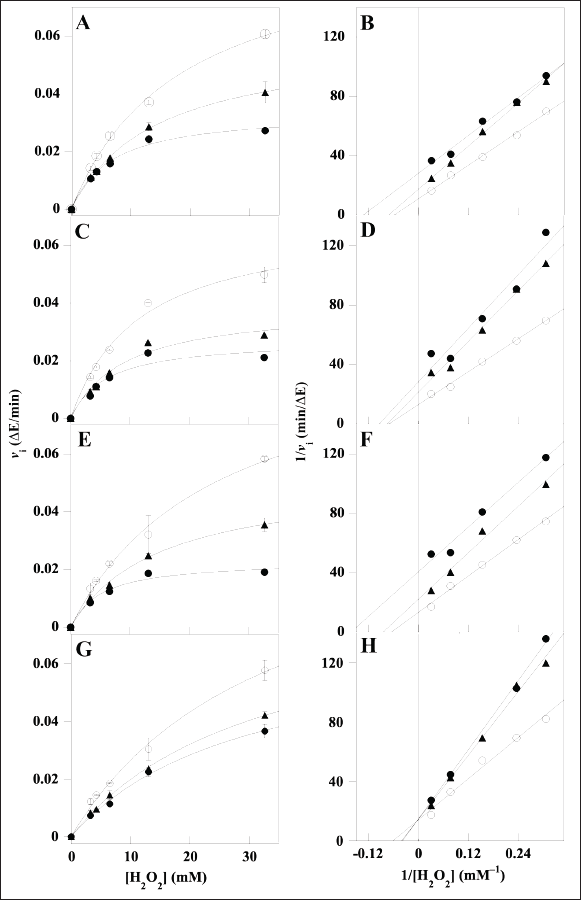 | Figure 3. Kinetic analysis of the CAT inhibition by LoCT, HcCT, MsF, and CiF extracts. The kinetic measurements of CAT activity were realized as reported in the Methods section in the presence of 3.26–32.6 mM H2O2 concentration, without (empty circles) or with the following concentrations of polyphenolic extracts: (A, B) 20 µM (triangles) or 40 µM (filled circles) LoCT; (C, D) 20 µM (triangles) or 40 µM (filled circles) HcCT; (E, F) 20 µM (triangles) or 40 µM (filled circles) MsF; (G, H) 20 µM (triangles) or 40 µM (filled circles) CiF. 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. Data were reported using the hyperbolic Michaelis–Menten equation (A, C, E, G) or the Lineweaver–Burk representation (B, D, F, H). The correlation coefficient R of the hyperbolic or linear equation ranged between 0.994 and 0.999 (A, B), 0.966–0.998 (C, D), 0.988–0.998 (E, F), 0.991–0.999 (G, H). [Click here to view] |
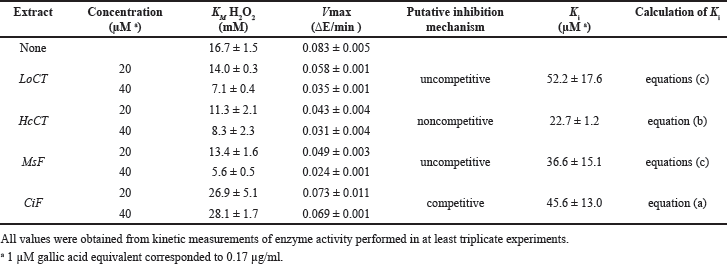 | Table 3. Effect of polyphenolic extracts on the kinetic parameters of bovine liver catalase. [Click here to view] |
Concerning XO, the kinetic analysis of its activity in the presence of the extracts involved only MsF and CiF, because the steady-state measurements of the XO activity indicated that LoCT and HcCT had a scarce if any inhibition strength. Even in this case, the time-dependent formation of uric acid was measured at different xanthine concentrations without or with two fixed MsF or CiF concentrations. The resulting data were analyzed with the Michaelis–Menten (Fig. 4, panels A and C) or Lineweaver–Burk representation (Fig. 4, panels B and D). In particular, the straight lines observed in the Lineweaver–Burk plots seem to intersect when crossing the ordinate axis, a behavior suggesting a tentative competitive inhibition mechanism of the XO activity for both MsF and CiF. Based on the effects of the extracts on the kinetic parameters of the XO activity, namely an increase of the KM with CiF and even more with MsF, while the Vmax remained essentially unvaried, the Ki values were calculated and reported in Table 4. MsF was endowed with a low value of Ki (5.0 ± 1.2 µM), thus pointing to its powerful inhibition strength, whereas CiF had a 9-fold greater value of Ki (44.2 ± 10.9 µM). These data suggest that some flavonoids extracted from leaves of M. sativa may act as powerful inhibitors of the XO activity.
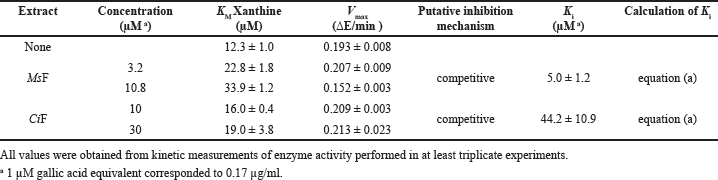 | Table 4. Effect of polyphenolic extracts on the kinetic parameters of bovine milk xanthine oxidase. [Click here to view] |
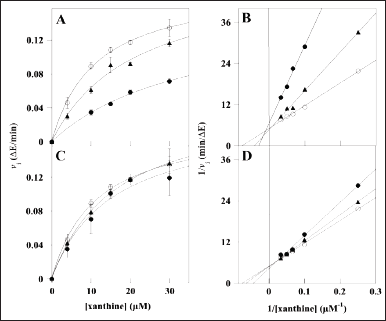 | Figure 4. Kinetic analysis of the XO inhibition by MsF and CiF polyphenolic extracts. The kinetic measurements of XO activity were realized as reported in the Methods section in the presence of 4–30 µM xanthine concentration, without (empty circles) or with the following concentrations of polyphenolic extracts: (A, B) 3.2 µM (triangles) or 10.8 µM (filled circles) MsF; (C, D) 10 µM (triangles) or 30 µM (filled circles) CiF. 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. Data were reported using the hyperbolic Michaelis–Menten equation (A, C,) or the Lineweaver–Burk representation (B, D). The correlation coefficient R of the hyperbolic or linear equation ranged between 0.990 and 0.999 (A, B), 0.990–0.999 (C, D). [Click here to view] |
Effect of polyphenolic extracts on the viability of gastric cancer cell lines
The known anti-cancer activity of plant polyphenols prompted an investigation on the possible cytotoxic effect exerted by the polyphenolic extracts, using an in vitro cell model. In particular, we evaluated whether these extracts could affect the cell viability of the human gastric adenocarcinoma cell lines AGS (from primary tumors), and MKN-28 (from metastatic lymph nodes), using the 3-(4,5-dimethylthiazol-2-yl)-2,5-biphenyltetrazolium bromide MTT assay. Specifically, AGS and MKN-28 cells were incubated for 24 hours with vehicle alone or in the presence of increasing concentrations of the four extracts (Fig. 5). The data show that only MsF displayed an evident and strong concentration-dependent inhibition of cell viability (Fig. 5C). In particular, cell proliferation was progressively reduced starting from low concentrations of this extract and, after treatment with 250 µM MsF, cell viability was reduced to 9% and 18% for AGS and MKN-28, respectively. The extrapolated IC50 value for MsF ranged in the 75–100 µM interval for both cell lines. Conversely, the other extracts were much less effective in reducing proliferation of the cancer cells, because their cell viability remained always greater than 50% even after treatment with the maximum extract concentration. In particular, cell viability of AGS and MKN-28 was 67% and 58%, respectively, after treatment with 250 µM LoCT (Fig. 5A); 74% and 56%, respectively, after treatment with 250 µM HcCT (Fig. 5B); 73% and 65%, respectively, after treatment with 150 µM CiF (Fig. 5D). Furthermore, a general overview of the cell viability data suggested that, compared to AGS, the MKN-28 cell line was apparently more sensitive to the treatments with the various extracts.
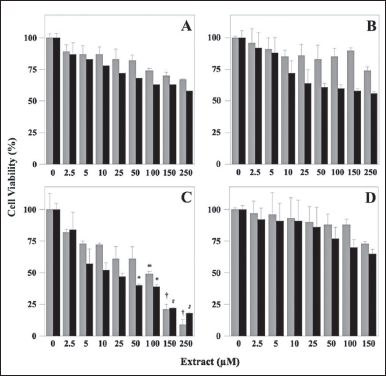 | Figure 5. Cell viability of human adenocarcinoma cell lines after treatment with polyphenolic extracts. AGS (gray columns) and MKN-28 (black columns) cells were treated for 24 hours with the indicated concentrations of LoCT (panel A), HcCT (panel B), MsF (panel C), or CiF (panel D). Control cells were incubated with 0.6 % (v/v) DMSO as a vehicle. Cell viability was determined with the MTT assay, as reported in Materials and Methods. 1 µM gallic acid equivalent corresponded to 0.17 µg/ml. The values, reported as a percentage compared to control cells, represent the mean ± standard error of separate experiments performed in triplicates. The significance was evaluated with p < 0.05 (*), 0.01 (#), and 0.001 (†). [Click here to view] |
DISCUSSION
It is known that the Mediterranean diet may give a great contribution to a healthy status and that the advantages of this eating behavior are probably linked to the presence of several edible plants. Indeed, many plants, including those used as forage crops, are rich in polyphenols, whose consumption produces several benefits [31]. In particular, the antioxidant and anti-inflammatory properties of these compounds are relevant for preventing and/or reducing the incidence of many human pathologies, such as neurodegenerative, cardiovascular, gastric or metabolic diseases, and even cancer. Furthermore, the complementation of synthetic medical drugs with the consumption of natural polyphenols during a therapeutic treatment may be useful to reduce the dosage of compounds that frequently cause adverse side effects, when administered in great amounts and/or for long periods.
Among polyphenolic substances, flavonoids are the most abundant in the human diet. They can be found in flowers, leaves, and seeds of fruits, vegetables, and other food crops [32,33]. It is widely recognized that the regular consumption of flavonoids is beneficial for preventing several diseases and maintaining a good health status [34–37]. Tannins, a heterogeneous group of high molecular weight polyphenolic compounds, are abundant in plants and have been identified in almost all their parts, including fruits, leaves, roots, seeds, wood, and bark [38]. Tannins are classified into hydrolysable and CT; these latter are the most abundant plant-derived polyphenols, and their presence in forage plants has been reported to increase the efficiency of protein digestion in husbandry animals [39].
Typical plants of Mediterranean forage crops are M. sativa, H. coronarium, L. ornithopodioides, and C. intybus. The preparation of extracts from these plants represented a good source of flavonoids and CT, useful for investigating the effects of these polyphenols on the activity of crucial redox enzymes, such as catalase and xanthine oxidase. The studies on steady-state activity and the kinetic parameters of these enzymes presented in this work show that the mixture of polyphenols present in the plant extracts displayed inhibition properties on these target macromolecules. However, significant differences emerged in the inhibition strength between the two enzymes, as well as among the various extracts.
When considering the effects on the activity of bovine liver CAT, the mixture of CT in LoCT and HcCT or flavonoids in MsF and CiF had a moderate inhibition of this enzyme, as emerging from the Ki values calculated for these extracts, all of them ranging in the 22.7–52.2 µM interval. Therefore, independently of a possible different composition, the CT and flavonoids present in the extracts displayed a similar modest inhibition of catalase activity. The putative inhibition mechanism possessed by the four extracts was analyzed and some differences emerged. In particular, LoCT and MsF seemed to act as uncompetitive inhibitors, HcCT as noncompetitive, and CiF as competitive. However, this apparent different behavior could be also explained with a similar modest inhibition power, which impaired an undoubtful ranking. Taken together, all these data suggest that catalase cannot be considered as a promising target enzyme for its inhibition by polyphenolic substances.
Moving to the effects caused by the various plant extracts on the activity of bovine milk XO, a different behavior emerged with respect to CAT. Indeed, the mixture of CT in LoCT and HcCT was essentially ineffective in inhibiting the activity of XO, whereas the flavonoids contained in MsF and CiF were capable to cause an evident inhibition. Furthermore, among these two extracts, a strong difference emerged in the inhibition power, because the Ki of MsF (5.0 ± 1.2 µM) was strikingly lower than that calculated for CiF (44.2 ± 10.9 µM). It seems relevant that both the efficacy of MsF in inhibiting XO (Ki in the micromolar range) and the inhibition mechanism (competitive) was in line with that observed for allopurinol, a xanthine structural homolog already used as a drug, and for polyphenolic extracts obtained from other plants [40,41]. Therefore, MsF could be ranked as a powerful inhibitor of XO, whereas CiF had a 9-fold lower inhibition power. Despite their different inhibition strength, both these extracts displayed a competitive inhibition mechanism towards the XO activity. It is likely that the bioactive compounds contained in our extracts could mimic the purine-based structure typically present in XO inhibitors [28,42]. This finding, together with the powerful inhibition strength displayed by the MsF extract could suggest considering in the future xanthine oxidase as a promising target enzyme for its inhibition by a specific class of polyphenolic substances, such as the flavonoids contained in the extract of M. sativa, a finding already reported for other plant extracts [43,44].
Polyphenols are known to be associated with a reduction in the incidence of many human diseases, including cancer. The human gastric adenocarcinoma cell lines AGS and MKN-28, were previously used as a representative cellular model of the gastrointestinal system, to study the anti-inflammatory and chemo-preventive effects of lemon peel polyphenols [45]. Therefore, these two cell lines were used to evaluate the effects on cell viability exerted by the polyphenolic extracts obtained from leaves of Mediterranean forage crops. The evaluation of the cellular effects exerted by the extracts on MKN-28 and AGS cell lines demonstrated that only the extract of M. sativa was able to cause a significant reduction of cell proliferation. This finding was an additional thrust to consider the polyphenolic substances contained in MsF as bioactive molecules endowed with properties useful for the design of drugs beneficial for human health [46].
CONCLUSION
The data reported in this work were obtained with commercially available bovine enzymes, which share several properties with the corresponding human counterparts. However, this choice should not represent a strong limitation, as these enzymes have already been used as experimental models for inhibition studies by several compounds. Therefore, our findings could reinforce the working hypothesis that polyphenols could represent bioactive molecules useful for the design of drugs beneficial for human health. Under this concern, the extract from leaves from Medicago sativa can be considered as a suitable source of compounds endowed with putative pharmacological properties. Future perspectives of our study include an investigation on the molecular mechanisms underlying the observed effects as well as an extension of the study on the effects on different cellular system models of diseases; under this aspect, cell morphology analysis and investigation of bio-signaling pathways linked to proliferation would clarify the observed effects. Moreover, also in vivo experiments would be necessary, in order to explore the possible use of the extract(s) as integration in functional foods. Furthermore, a deeper investigation on the extract(s) composition would be helpful to shed light in the inhibition mechanism observed, even in terms of synergistic action. Finally, studies on the effect of natural extracts on the activity of crucial redox enzymes could be useful even for people interested in the obtainment of health benefits through an appropriate consumption of vegetables.
ACKNOWLEDGMENTS
Rosarita Nasso was supported by “Fondazione Veronesi”, Italy.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research work was supported by grants from MUR, Fund for the promotion and policy development of the National Research Program (PNR) -DM 737 of 25 June 2021 CUP I55F21003620001 (RA), DM 1275 of 10 December 2021 CUP I69J22001050001 (MM), and Next Generation EU in the framework of PRIN 2022, CUP I53D23004270006 (MM).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hussain G, Huang J, Rasul A, Anwar H, Imran A, Maqbool J, et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: an updated review. Molecules. 2019;24(12):2213. CrossRef
2. Melo LFM, Aquino-Martins VGQ, Silva APD, Oliveira Rocha HA, Scortecci KC. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023;414:135645. CrossRef
3. Maiuolo J, Costanzo P, Masullo M, D’Errico A, Nasso R, Bonacci S, et al. Hydroxytyrosol-donepezil hybrids play a protective role in an in vitro induced Alzheimer’s disease model and in neuronal differentiated human SH-SY5Y Neuroblastoma Cells. Int J Mol Sci. 2023;24(17):13461. CrossRef
4. Tava A, Biazzi E, Ronga D, Pecetti L, Avato P. Biologically active compounds from forage plants. Phytochem. Rev. 2022;21,471–501. CrossRef
5. Usman M, Khan WR, Yousaf N, Akram S, Murtaza G, Kudus KA, et al. Exploring the phytochemicals and anti-cancer potential of the members of Fabaceae family: a comprehensive review. Molecules. 2022;27(12):3863. CrossRef
6. Bora KS, Sharma A. Phytochemical and pharmacological potential of Medicago sativa: a review. Pharm Biol. 2011;49(2):211–20. CrossRef
7. Raeeszadeh M, Mortazavi P, Atashin-Sadafi R. The antioxidant, anti-inflammatory, pathological, and behavioural effects of Medicago sativa L. (Alfalfa) extract on brain injury caused by nicotine in male rats. Evid Based Complement Alternat Med. 2021;2021:6694629. CrossRef
8. Bora KS, Sharma A. Evaluation of antioxidant and cerebroprotective effect of Medicago sativa Linn. against ischemia and reperfusion insult. Evid Based Complement Alternat Med. 2011;2011:792167. CrossRef
9. Burlando B, Pastorino G, Salis A, Damonte G, Clericuzio M, Cornara L. The bioactivity of Hedysarum coronarium extracts on skin enzymes and cells correlates with phenolic content. Pharm Biol. 2017;55(1):1984–91. CrossRef
10. Tava A, Biazzi E, Ronga D, Mella M, Doria F, D’Addabbo T, et al. Chemical identification of specialized metabolites from sulla (Hedysarum coronarium L.) collected in Southern Italy. Molecules. 2021;26(15):4606. CrossRef
11. Rolnik A, Olas B. The plants of the Asteraceae family as agents in the protection of human health. Int J Mol Sci. 2021;22(6):3009. CrossRef
12. Perovi? J, Tumbas Šaponjac V, Koji? J, Krulj J, Moreno DA, García-Viguera C, et al. Chicory (Cichorium intybus L.) as a food ingredient–nutritional composition, bioactivity, safety, and health claims: a review. Food Chem. 2021;336:127676. CrossRef
13. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. CrossRef
14. Averill-Bates D. Reactive oxygen species and cell signaling. Review. Biochim Biophys Acta Mol Cell Res. 2024;1871(2):119573. CrossRef
15. Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97:2499–574. CrossRef
16. Glorieux C, Liu S, Trachootham D, Huang P. Targeting ROS in cancer: rationale and strategies. Nat Rev Drug Discov. 2024 Aug;23(8):583–606. CrossRef
17. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine, 5th ed. New York, NY: Oxford University Press; 2015. CrossRef
18. Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32(1):44–50. CrossRef
19. Galasso M, Gambino S, Romanelli MG, Donadelli M, Scupoli MT. Browsing the oldest antioxidant enzyme: catalase and its multiple regulation in cancer. Free Radic Biol Med. 2021;172:264–72. CrossRef
20. Mileo AM, Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. Oxid Med Cell Longev. 2016;2016:6475624. CrossRef
21. Bolat E, Sar?ta? S, Duman H, Eker F, Akda?çi E, Karav S, et al. Polyphenols: secondary metabolites with a biological impression. Nutrients. 2024;16(15):2550. CrossRef
22. Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, et al. A review on Annona muricata and its anticancer activity. Cancers 2022;14(18):4539. CrossRef
23. Piluzza G, Bullitta S. The dynamics of phenolic concentration in some pasture species and implications for animal husbandry. J Sci Food Agric. 2010;90(9):1452–9. CrossRef
24. Tibe O, Meagher LP, Fraser K, Harding DR. Condensed tannins and flavonoids from the forage legume sulla (Hedysarum coronarium). J Agric Food Chem. 2011;59(17):9402–9. CrossRef
25. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144. CrossRef
26. Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;51:6509–15. CrossRef
27. Porter LJ, Hrstich LN, Chan BC. The conversion of procyanidins and prodelphinidins to cyanidins and delphynidins. Phytochemistry. 1986;25:223–30. CrossRef
28. Rullo R, Cerchia C, Nasso R, Romanelli V, De Vendittis E, Masullo M, et al. Novel reversible inhibitors of xanthine oxidase targeting the active site of the enzyme. Antioxidants (Basel). 2023;12(4):825. CrossRef
29. Arcone R, Palma M, Pagliara V, Graziani G, Masullo M, Nardone G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced Matrix Metalloproteinase-9/2. Biochimie Open. 2016;3:56–63. CrossRef
30. Carrese B, Cavallini C, Sanità G, Armanetti P, Silvestri B, Calì G, et al. Controlled release of doxorubicin for targeted chemo-photothermal therapy in breast cancer HS578T cells using albumin modified hybrid nanocarriers. Int J Mol Sci. 2021;22(20):11228. CrossRef
31. Piluzza G, Sulas S, Bullitta S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: a review. Grass Forage Sci. 2013;69:32–48. CrossRef
32. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69:273–8. CrossRef
33. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. CrossRef
34. Hamer M, Steptoe A. Influence of specific nutrients on progression of atherosclerosis, vascular function, haemostasis and inflammation in coronary heart disease patients: a systematic review. Br J Nutr. 2006;95(5):849–59. CrossRef
35. Serafini M, Villano D, Spera G, Pellegrini N. Redox molecules and cancer prevention: the importance of understanding the role of the antioxidant network. Nutr Cancer. 2006;56(2):232–40. CrossRef
36. Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59(3):113–22. CrossRef
37. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. SciWorld J. 2013;2013:162750. CrossRef
38. Smeriglio A, Barreca D, Bellocco E, Trombetta D. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. 2017;174(11):1244–62. CrossRef
39. Barry TN, McNabb WC. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br J Nutr. 1999;81(4):263–72.
40. Nguyen MT, Awale S, Tezuka Y, Ueda JY, Tran Ql, Kadota S. Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. Planta Med. 2006;72(1):46–51. CrossRef
41. Vitale RM, Antenucci L, Gavagnin M, Raimo G, Amodeo P. Structure-activity relationships of fraxamoside as an unusual xanthine oxidase inhibitor. J Enzyme Inhib Med Chem. 2017;32(1):345–54. CrossRef
42. Šmelcerovi? A, Tomovi? K, Šmelcerovi? Ž, Petronijevi? Ž, Koci? G, Tomaši? T, et al. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur J Med Chem. 2017;135:491–516. CrossRef
43. Orhan IE, Deniz FSS. Natural products and extracts as xantine oxidase inhibitors—a hope for gout disease? Curr Pharm Des. 2021;27(2):143–58. CrossRef
44. El-Tantawy WH. Natural products for the management of hyperuricaemia and gout: a review. Arch Physiol Biochem. 2021;127(1):61–72. CrossRef
45. Pagliara V, Nasso R, Di Donato P, Finore I, Poli A, Masullo M, et al. Lemon peel polyphenol extract reduces interleukin-6-induced cell migration, invasiveness, and matrix metalloproteinase-9/2 expression in human gastric adenocarcinoma MKN-28 and AGS cell lines. Biomolecules. 2019;9(12):833. CrossRef
46. Arcone R, D’Errico A, Nasso R, Rullo R, Poli A, Di Donato P, et al. Inhibition of enzymes involved in neurodegenerative disorders and Aβ1-40 aggregation by Citrus limon peel polyphenol extract. Molecules. 2023;28(17):6332. CrossRef