INTRODUCTION
The treatment of ailments such as hypertension, nephrotic syndrome, cirrhosis, and heart conditions includes diuretics in order to flush out excessive fluids within the body (Bart et al., 2012). They exert the required effect by regulating the elimination of salts in correspondence with water levels via urine. In hypertensive conditions, for instance, increased salt concentration leads to an elevation of fluid build-up, resulting in increased blood pressure. On the contrary, treatments with diuretic agents reverse this effect by the reduction of fluid build-up in the body, primarily by targeting the nephrons and regulation of the composition of the filtrate during urine formation (Aliti et al., 2013).
Diuretics commonly administered are thiazide, loop, potassium-sparing osmotic diuretics, and carbonic anhydrase inhibitors. The role of these diuretics is to primarily flush out excess fluids from the body in order to ensure homeostasis. However, considering their chemical origin, they are often associated with certain adverse effects, such as electrolyte imbalance owing to excess urination. Metabolic acidosis, hyperkalemia, hypernatremia, and hypokalemia constitute among a few serious illnesses caused by these drugs. In addition, kidney stones are also a serious issue caused by the administration of potassium-sparing, one of the diuretic agents which causes an elevated calcium excretion in urine. Such drugs are, therefore, administered along with thiazide diuretics in order to lower calcium excretion (Somasekharan et al., 2012). Drugs add up and lead to many other adversities due to their cross reactions. In addition, they pose a greater burden to the economy of the country and, therefore, are extremely important to identify a potential diuretic agent with fewer adverse effects. In order to address the side effects associated with synthetic drugs, plant-based medicines are gaining popularity in the recent times. As they are a rich source of phenolic acids, flavonoids, and polyphenols, they have an added advantage of antioxidant properties to fight against the reactive oxygen species (ROS) generated. Natural antioxidants present abundantly in various plant sources inhibit oxidative stress and are capable of protecting against various diseases, including degenerative kidney diseases (Halliwell, 2012).
Clitoria ternatea, also known as butterfly pea or blue pea, is a well-known herb used in traditional medicine from the family Fabaceae. Various parts such as the shoots, roots, leaves, and seeds are known for various traditional uses, such as an anxiolytic, memory enhancer, anticonvulsant, anti-stressor, nootropic, antidepressant, and tranquilizing and sedative agent (Gupta et al., 2010). Clitoria ternatea possesses potential health beneficiary properties, such as antioxidant, anticancer, antipyretic, anti-inflammatory, hypolipidemic, respiratory, cardiovascular, immunological, analgesic, insecticidal, and others (Mukherjee et al., 2008). This literature review showed that the alcoholic extracts of the roots of C. ternatea revealed diuretic activity in dogs (Piala et al., 1962). No study has been carried out using the leaf extract of the plant; therefore, the present study focused to evaluate the diuretic activity of the leaf extract from C. ternatea.
MATERIALS AND METHODS
Plant material
Leaves of C. ternatea (CT) were collected from Mysore, Karnataka, India, in Feb 2018 and were authenticated at the Pharmacognosy Department, JSS College of Pharmacy, Mysore. A voucher specimen has been deposited and preserved in the herbarium of JSS College of Pharmacy, Mysore.
Preparation of the plant extract
Shade-dried leaves of C. ternatea were subjected to successive extraction using various solvents [chloroform (CCTL), methanol (MCTL), and water] in increasing order of polarity. MCTL (100%) and CCTL (100%) extracts were extracted with the Soxhlet apparatus (De Castro et al., 1998), whereas the aqueous (ACTL) extract (1:10 w/v) was prepared using the maceration method (by immersing the dried powder of the leaf in water inside an air-tight container) for 7 days at room temperature (Zimman et al., 2002). The extracts were concentrated using the rotary evaporator and dried under vacuum. The percentage yield of MCTL, CCTL, and ACTL extracts was 11.69%, 9.48%, and 10.32%, respectively.
The experiments were in accordance with the guidance from the Institutional Animal Ethics Committee of JSS College of Pharmacy, JSS AHER, Mysuru (JSSMC/Pharma/P5-260/2017). Male Wistar rats (8–10 weeks old) weighing 200–250 g were considered for the study and handled as per the guidelines issued by the Institutional Animal Ethics Committee, JSS College of Pharmacy, Mysuru, Karnataka. After procuring them from a local breeder, the animals were acclimatized to the experimental conditions and maintained at 23°C ± 3°C temperature and 50% ± 5% humidity inside polypropylene cages with paddy husk as bedding material. Each cage contained six animals and all were provided with standard food pellets and water ad libitum.
Chemicals and reagents used
Folin–Ciocalteu (FC) reagent, sodium acetate trihydrate buffer, 2,4,6-tripyridyl-S-triazine, hydrochloric acid (HCl), 20 mM ferric chloride solution, 2, 2-diphenylpicrylhydrazyl (DPPH), Normal saline, sodium CMC 2%, and furosemide 20 mg were used.
Phytochemical analysis
Phytochemical analysis was carried out using standard tests (Harborne, 1973). The MCTL, CCTL, and ACTL extracts of the leaf extract of C. ternatea were subjected to preliminary phytochemical analyses.
Estimation of total phenolic content (TPC) by FC reagent (FC method)
The total phenol content was estimated using the FC method, which is estimated by a blue-colored phosphotungsticphosphomolybdenum complex. This complex was measured at an absorbance of 765 nm (Shan et al., 2005).
Measurement of the antioxidant activity using the ferric-reducing antioxidant power (FRAP) method
The antioxidant potential of the extracts was evaluated using the FRAP assay as described by Benzie and Strain (1999). The FRAP assay is primarily carried out to assess the reduction of ferric 2, 4, 6-tri-pyridyl-s-triazine complex to its ferrous form under conditions, such as low pH. This reduction is measured as a function of change in absorbance at 593 nm.
Antioxidant capacity using the DPPH scavenging assay
The antioxidant potential of the extracts was evaluated using the DPPH radical scavenging capacity as described previously with slight modifications (Patel and Patel, 2011). In brief, five different concentrations of the extract (0.5 ml) taken along with 3.5 ml DPPH solution was incubated for a period of 30 minutes in dark at room temperature (RT). The absorbance of the sample, 95% ethanol or water, was recorded at 536 nm. The activity was calculated using the following formula:
where A0 is the absorbance of the blank and A1 is the absorbance of the standard or test.
Diuretic activity
The diuretic activity of the extracts was evaluated using the Lipschitz model (Lipschitz et al., 1943). The experiment was carried out in five groups with six animals in each group. All the rats were fasted and deprived of water for 18 hours before the commencement of the experiment. Group 1 (control) was treated with vehicle (sodium CMC 2% in normal saline), whereas group 2 was treated with a standard dose of furosemide 20 mg/kg (in normal saline). Group 3 a, b, c; Group 4 a, b, c; and Group 5 a, b, c were orally treated with three different extracts of MCTL, ACTL, and CCTL with dosage of 150 mg, 300 mg, and 450 mg for each extract, respectively. The experimental animals were then housed in diuretic cages (designed to separate urine and feces), with two rats in each cage, containing a netted floor and maintained at room temperature (25°C ± 0.5°C). However, the administration of water and food was restricted during this period. Urine was collected in a conical flask placed below the polythene funnel of the diuretic cage. Extreme care was taken to avoid contamination of urine with fecal matter. 5 hours post-dosing, urine was collected from the cages and assessed for its volume, ion concentrations of Na+, K+ (cations) using flame photometer, and Chloride anion (Cl–)(anions) using Argentometric titration. The determined volume of urine was in terms of ml/5 hours, whereas the concentration of electrolytes was expressed as mmol/l. The diuretic index and diuretic activity (Vogel et al., 2008) were calculated as follows:Diuretic index = Urinary excretion of treated group/Urinary excretion of control group
Diuretic activity = Diuretic action of test drug/Diuretic action of standard
Nucleation assay
Spectrophotometric assay was carried out in order to evaluate the nucleation inhibition of CaOx crystals by the extracts and standard cystone. The addition of CaCl2 and Na2C2O4 to synthetic urine led to crystallization. For this, solutions of 4 mmol/l CaCl2 and 50 mmol/l Na2C2O4 were prepared using 0.05 mol/l Tris buffer in 0.15 mol/l NaCl under physiological temperature and pH. Different concentrations of the extracts (400, 600, 800, and 1,000 µg/ml) were taken and 1 ml each was mixed with 3 ml CaCl2 solution, followed by the addition of 3 ml Na2C2O4 solution. Final mixtures were incubated for 1, 2, and 3 hours at 37°C. The absorbance of the mixtures was then measured at 620 nm wavelength for 3 different hours. Percent inhibition of nucleation by each fraction was calculated by using the following formula:
Aggregation assay
The effect of various solvent fractions on CaOx crystal aggregation was determined by means of an aggregation assay. CaCl2 and Na2C2O4 solutions (50 mmol/l each) were mixed together, heated to 60°C in a water bath for 1 hour, and then incubated overnight at 37°C to prepare seed CaOx crystals. After drying, CaOx crystal solution (0.8 mg/ml) was prepared in a 0.05 mol/l Tris-HCl and 0.15 mol/l NaCl buffer (pH 6.5). Different concentrations of the extracts (400, 600, 800, and 1,000 µg/ml) from each solvent fraction were added to 3 ml CaOx solution, vortexed and then incubated at 37°C for 3 different hours (1, 2, and 3 h). OD of the final mixtures was then read at 620 nm wavelength and percent inhibition of aggregation was then calculated as described for nucleation assay as follows: % Inhibition = [1− (OD test/OD control)] × 100
Statistical analysis
The experiments were carried out in triplicates and the results were expressed as mean ± SD. Statistical comparisons between the control and treatment groups were conducted by oneway analysis of variance (ANOVA), followed by Tukey’s post-hoc and Dunnett’s tests by employing Statistical Package for the Social Sciences software (version 21.0, Chicago, IL). Graph Pad PRISM software (version 4.03) was used for calculating Half-maximal inhibitory concentration (IC50) values.
RESULTS AND DISCUSSION
Preliminary phytochemical screening using various extracts from C. ternatea leaves showed the presence of flavonoids, alkaloids, saponins, glycosides, and carbohydrates. Several studies have reported optimum extraction of the primary phytochemicals in the MCTL extract, which is in agreement with our study (Shan et al, 2005; Patel and Patel, 2011).
Phenolic content represented in terms of gallic acid equivalents demonstrated that among the three extracts, the highest concentration was seen in the MCTL extract of C. ternatea (MCTL = 22.63%), followed by the ACTL extract (ACTL = 20.92%) and CCTL extract (CCTL = 16.83%). The TPC of different extracts is summarized in Table 1. The antioxidant potential of the extracts was assessed by the FRAP and DPPH assays. In the present study, with respect to the FRAP assay, optimal reduction of ferric ion was observed in MCTL (R2 = 0.9838), that was followed by CCTL (R² = 0.9764) and ACTL (R² = 0.9789). The antioxidant activity of these extracts is on par with that of the standard drug (ascorbic acid) in a dose-dependent manner. The antioxidant potential of most plant extracts is not a new concept. However, any plant extract with antioxidant property proves beneficiary because it not only possesses the actual disease-preventing activity but also protects from other secondary damages caused by the condition due to the generation of ROS (Ramu et al., 2014). Therefore, in agreement with previous studies, C. ternatea leaf extracts showed good antioxidant activity, which can be proven to be an adjunct to the able diuretic property of the extract (Benzie and Strain, 1999).
The DPPH-reducing ability was represented as IC50, which was determined by evaluating the substrate concentration required for 50% loss of the DPPH activity. Diverse concentrations of extracts (MCTL, CCTL, and ACTL) demonstrated a dose-dependent antioxidant activity, with the MCTL showing the highest (IC50: 35.5 μg/ml), when compared to that of CCTL (IC50: 45.3μg/ml) and ACTL (IC50: 41.1 μg/ml). The antioxidant activity of the standard in this study (IC50: 21.01 μg/ml) was compared to that of the extracts, with MCTL showing optimum among the three extracts. The antioxidant activity with both assays (FRAP and DPPH) could be summarized as vitamin C>MCTL>ACTL>CCTL. The free radical scavenging capacity and antioxidant activities are summarized in Table 2.
 | Table 1. TPC of different extracts. [Click here to view] |
Lipschitz’s method was used to carry out the diuretic activity. Based on the results in Patel and Patel’s (2011) study, it was suggested that values ranging >1.50 showed significant diuretic activity, whereas 1.00–1.50 were moderate, 0.72–0.99 showed mild, and <0.72 demonstrated no diuretic activity. The results from our study showed that all the extracts revealed diuretic properties in a dose-dependent manner and were comparable with that of the standard drug (furosemide). Optimum diuretic activity was observed in MCTL at a maximum dose of 450 mg/kg (1.70), followed by ACTL (1.68) and CCTL (1.49) in a dose-dependent manner, as against the standard drug (1.99). The mean urine volume and electrolyte concentrations (Na+ and K+) were elevated in a dose-dependent manner in all the extracts tested, but only moderate excretion of CL− ions was seen. The diuretic activity of all the three extracts is summarized in Table 3. The diuretic activity was maximum in the MCTL extract over that of either ACTL and CCTL extracts, as well as the standard drug (MCTL>ACTL>CCTL).
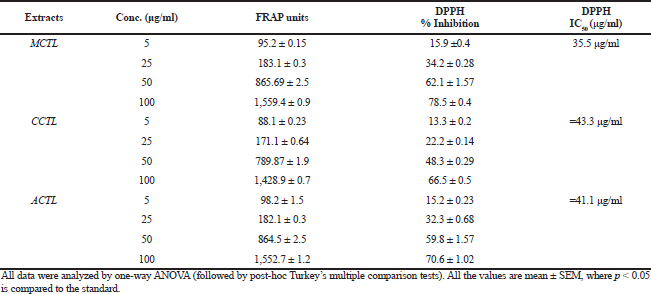 | Table 2. Free radical scavenging and antioxidant activity by FRAP and DPPH assays. [Click here to view] |
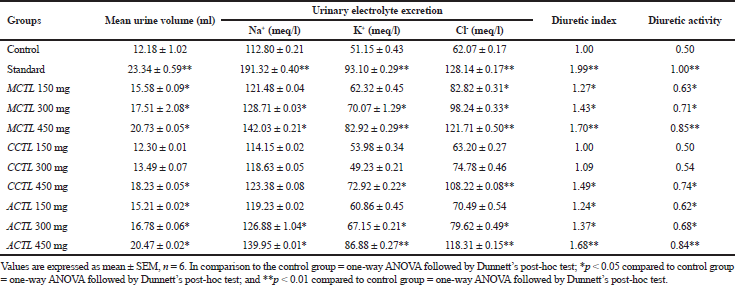 | Table 3. Diuretic activity of MCTL, CCTL, and ACTL extracts of Clitoria ternatea leaves. [Click here to view] |
The urolithiatic abilities of the polyherbal combination, cystone, led to its use as a standard drug in our study. The extracts obtained from various solvents, ranging from polar to non-polar, ensured the extraction of compounds with diversified solubility. In the present study, the three extracts showed significant inhibition at >800 μg/ml. At 1,000 μg/ml, the three extracts showed an appreciable percent inhibition. CaOx crystal nucleation studies revealed that the percent inhibition exerted by MCTL (73.2 ± 2.1), CCTL (63.5 ± 1.1), and ACTL (48.2 ± 3.4) was optimal at the longest duration (3 h) and was on par with the standard drug cystone (8.352 ± 2.2). Inhibition using CCTL did not vary greatly with respect to time, but ACTL was optimal at 1 hour. Also, MCTL, which was on par with the standard drug cystone, was optimal at 3 hours (Table 4).
The effect of different solvent extracts on calcium oxalate crystal aggregation was also evaluated. The optimal activity was observed with the MCTL treatment for 3 hours but was relatively lower than the standard drug cystone. Nevertheless, the inhibition exerted by the MCTL was 48.3 ± 3.1, which was remarkable compared to all the solvent extracts (Table 4).
Furthermore, the CaOx crystals were analyzed using microphotography studies, which revealed that the MCTL extract resulted in the formation of more calcium oxalate dehydrate crystals than COM crystals. Such a formation is beneficial because calcium oxalate monohydrate crystals render greater damage to the epithelial cells of the renal system. The inhibition of nucleation and aggregation shown by the MCTL treatment, therefore, proves beneficiary as possessing antiurolithiatic activity.Diuretic activity is primarily important in conditions such as hypertension, liver cirrhosis, and certain types of edema; therefore, it is advantageous as a pre-treatment before testing with various fluids as screening agents. In our study, the diuretic activity of the extracts was on par with the standard drug furosemide at the highest concentration of the MCTL. This was accompanied by the urinary excretion of electrolytes. Previous studies have reported that greater excretion of sodium over that of potassium is an indicator of safety profile of diuretic agents (Maghrani et al., 2005; Sripanidkulchai et al., 2001). In our study, the excretion of sodium was more than that of the other two electrolytes (potassium and chlorine), thereby agreeing with the previous findings. Increased excretion of these electrolytes is mainly due to the inhibitory role of the extract on carbonic anhydrase. Therefore, our study suggests that one of the possible diuretic potential operates by inhibition of carbonic anhydrase, which is in agreement with the report of Vogel (2007).
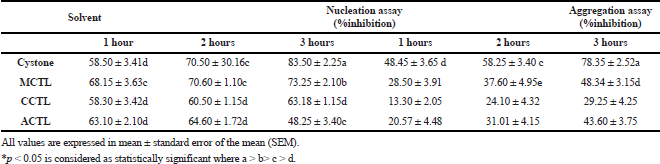 | Table 4. Effect of MCTL, CCTL, and ACTL extracts of Clitoria ternatea on nucleation and aggregation of calcium oxalate crystals at 1,000 μg/ml. [Click here to view] |
CONCLUSION
The extracts possessed remarkable diuretic properties with the MCTL extract showing optimal activity among the three solvent extracts. This may be due to the greater ability of the extraction of saponins, flavonoids, and phenolic acids by MCTL when compared to other solvents. The extract also showed potential antiurolithiatic activity observed by the inhibition of nucleation and aggregation of CaOx crystals. In addition, potential antioxidant activity exerted by the extract on the FRAP and DPPH upholds the beneficiary potential of the C. ternatea leaf extract in several diseases, including hypertension and edema.
ACKNOWLEDGMENT
We express our sincere gratitude to all the faculty members and staffs of the Department of Pharmacology, Biochemistry, JSS Medical College, JSS Academy of Higher Education and Research (JSS AHER) Mysuru 570015, Karnataka, India, for their time and corporation in completing this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
ETHICAL APPROVAL
This study was approved by the Institutional Animal Ethics Committee of JSS College of Pharmacy, JSS AHER, Mysuru, India. (JSSMC/Pharma/P5-260/2017).
REFERENCES
Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med, 2013; 73(12):1058–64. CrossRef
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med, 2012; 367(24):2296–304. CrossRef
Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol, 1999; 299:5–27. CrossRef
De Castro ML, Garcıa-Ayuso LE. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta, 1998; 369(1–2):1–0. CrossRef
Gupta GK, Chahal J, Bhatia M. Clitoria ternatea (L.): old and new aspects. J Pharm Res, 2010; 3(3):2610–4.
Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev, 2012; 70(5):257–65. CrossRef
Harborne JB. Phytochemicals methods. Chapman and Hall Ltd., London, UK, pp 49-188, 1973.
Lipschitz WL, Hadidian Z, Kerpcsar A. Bioassay of diuretics. J Pharmacol Exp Ther, 1943; 79(2):97–110.
Maghrani M, Zeggwagh NA, Haloui M, Eddouks M. Acute diuretic effect of aqueous extract of Retama raetam in normal rats. J Ethnopharmacol, 2005; 99(1):31–5. CrossRef
Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The ayurvedic medicine Clitoria ternatea—from traditional use to scientific assessment. J Ethnopharmacol, 2008; 120(3):291–301. CrossRef
Patel RM, Patel NJ. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res, 2011; 1:52–68.
Piala JJ, Madissoo H, Rubin B. Diuretic activity of roots of Clitoria ternatea L. in dogs. Experientia, 1962; 18(2):89. CrossRef
Ramu R, Shirahatti PS, Zameer F, Ranganatha LV, Prasad MN. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S Afr J Bot, 2014; 95:54–63. CrossRef
Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem, 2005; 53(20):7749–59. CrossRef
Somasekharan S, Tanis J, Forbush B. Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1). J Biol Chem, 2012; 287(21):17308–17. CrossRef
Sripanidkulchai B, Wongpanich V, Laupattarakasem P, Suwansaksri J, Jirakulsomchok D. Diuretic effects of selected Thai indigenous medicinal plants in rats. J Ethnopharmacol, 2001; 75(2–3): 185–90. CrossRef
Vogel HG. Diuretic and saluretic activity. Drug discovery and Evaluation Pharmacological assays. Springer-Verlag, Berlin, Germany, pp 173–5, 3, 2007.
Vogel HG, Vogel WH, Scholkens BA, Sandow J, Muller G, Vogel WF. Drug discovery and evaluation: pharmacological assays. 3rd edition, Berlin, Germany, Springer, 2008. CrossRef
Zimman A, Joslin WS, Lyon ML, Meier J, Waterhouse AL. Maceration variables affecting phenolic composition in commercial-scale Cabernet Sauvignon winemaking trials. Am J Enol Vitic, 2002; 53(2):93–8.