INTRODUCTION
Chitin, a homopolysaccharide structure arranged over N-acetyl-D-glucosamine molecules connected by the β (1→4) glycosidic bonds, is the second largest compound after cellulose. This colorless, crystalline, or amorphous powder is insoluble in water, organic solvents, dilute acids, and bases (Mathur and Narang, 1990).
Chitin occurs in three different polymorphisms isomers (α, β, and, γ) where N-acetyl glycosyl is a crystallographic unit that is common in all forms (Agboh and Qin, 1997). The intermolecular bonds in chitin are arranged like sheets. The bond that presents in one sheet possesses the same orientation “sense”; for example, in β-chitin, the sheets along the c-axis point in the same direction and the arrangement between the sheets is also parallel. In α-chitin, the sheets along the c-axis face the opposite direction (antiparallel) to that of the β-chitin. In γ-chitin, every third sheet has an opposite direction compared to those of the previous two sheets (Aranaz et al., 2009; Roy et al., 2017).
Compared to β-chitin, the α-chitin form is more widely available in nature. Cuttlefish bone is an example of a source of β-chitin (Jung et al., 2018), but the β-chitin form will change into the α-chitin form when it undergoes an excessive deacetylation process using alkaline and acidic solvents (Akpan, 2018). In nature, α-chitin occupies the most amount compared to other polymorphic forms (Maruthiah and Palavesam, 2017). α-Chitin can be found in the sponge (Tarusin et al., 2017), crab (Ifuku et al., 2009), shrimp (Goodrich and Winter, 2007), (Aranaz et al., 2009), insect cuticles (Wu et al., 2020), fungi (Hassainia et al., 2018), and sea snail (Mohan et al., 2019). Of these various sources, most of the industries prefer using crustaceans subphylum in the manufacture of α-chitin. Crustaceans are available in an abundant amount, as recorded globally in 2017 that almost 15.2 million tons of crustaceans had been produced (FAO, 2020). Parts of the shells, claws, heads, and other wasted parts of the crustaceans can reach 70% of the total weight of these sea creatures. Little can be used as animal feed and fertilizer, while the majority of the remainder is discarded (Ordóñez-Del Pazo et al., 2014). This huge amount of waste could affect the global sea pollution; thus, a further process should be undertaken knowing that chitin contained in crustacean waste has a higher amount (approximately 20%–30%) than other species (Vani et al., 2013).
There are two important steps in chitin extraction, for example, deproteination and demineralization. Sometimes an additional step needs to be carried out, that is, decolorization. Currently, the chitin extraction process is growing. Chitin extraction was first carried out using alkali and acids; however, lately, many researchers prefer to employ chemical, enzymatic, microbiological (Bajaj et al., 2015), and natural deep eutectic solvents (NADES) and several other methods.
The existence of chitin is now most preferable because it is biocompatible, biodegradable, easily absorbed in tissues, and nontoxic to both humans and the environment. Its functions are very broad which include in pharmacy, biomedical food, textile, packaging, agriculture, and others (Aranaz et al., 2009; Jollès and Muzzarelli, 1999; Roy et al., 2017). To the best of our knowledge, no existing article has reviewed the pharmacological activities of α-chitin sourced from the subphylum crustaceans. Therefore, this article aims to focus on the up-to-date extraction methods and the pharmacological activities of α-chitin from crustaceans.
METHODS
Articles were obtained from the PubMed database by inputting strategies, population (P) (crustacean); intervention (I) (preparation; extraction); control (C) (α-chitin; chitin); outcome (O) (chitin yield, demineralization, deproteination, and pharmacology effect); MeSH: (“Chitin/analysis” [MeSH] or “Chitin/biosynthesis” [MeSH] or “Chitin/pharmacology” [MeSH] and “Crustaceans” [MeSH]) NOT “Chitosan” [MeSH]. Chitin derivatives and chitin other than the crude form, for example, chitin nanofibers, chitin nanocrystal, nanochitin, and other forms of chitin, which have undergone further processes besides demineralization, deproteination, and decolorization, were excluded from the search. The search was carried out on all articles published with the above keywords until April 2020.
Extraction process
Every sea species that contains chitin is always associated with organic and inorganic substances, which affect its amount. Processes and conditions during extraction also affect the amount of chitin produced (Sorokulova et al., 2009). Deproteination and demineralization steps are considered as critical, due to its role in removing the protein, minerals, lipids, and pigments (Hamdi et al., 2017). Some extraction processes prioritize the demineralization process. However, it is not uncommon that deproteination takes precedence in eliminating some minerals by breaking up the calcium–protein–chitin complex in skeletal tissues during fermentation (Zhang et al., 2012). Before extraction, the shell should be boiled to make it easier to clean the shell from the remaining meat. The rest of the meat must be removed immediately to avoid the occurrence of the odor (Xu et al., 2008). The boiling process is also intended to reduce protease activity with the aim of chitin purification (Flores-albino et al., 2012). Figure 1 shows the process of all extractions.
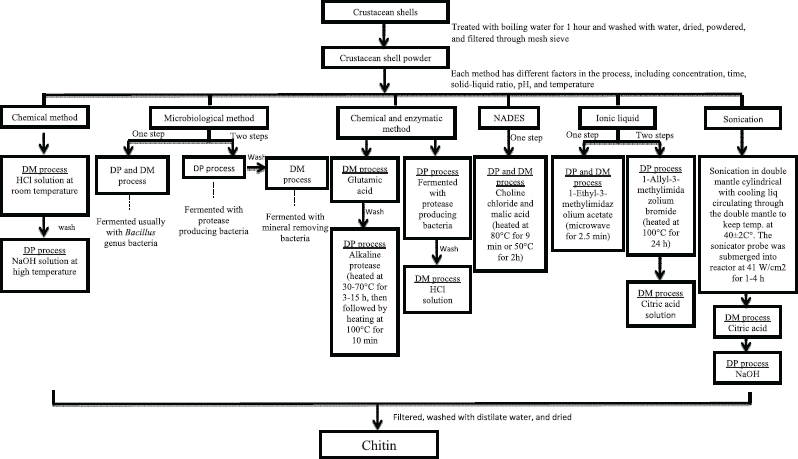 | Figure 1. Chitin extractions with various methods. *DM = demineralization; DP = deproteination. [Click here to view] |
Chemical extraction method
Chemical extraction generally employs alkaline and acidic solvents at high temperatures during the deproteination process (Younes et al., 2014). In general, the conditions during the extraction process will greatly affect the molecular weight and the degree of chitin acetylation. The disproportionate time, pH, and temperature during demineralization will produce chitin with a lower molecular weight. Chitin is a sensitive acid compound and can be degraded through several pathways. Hydrolytic depolymerization, heat degradation, and deacetylation are some of the pathways that will cause the physiological properties of the final product to be inconsistent (Ghorbel-Bellaaj et al., 2013; Sorokulova et al., 2009; Younes et al., 2012). Extraction using alkalis and acids produces waste that contains a lot of chloride, sodium, and calcium ions that are difficult to degrade, which will be a problem for the environment (Ding et al., 2020; Ghorbel-Bellaaj et al., 2013).
The deproteination process produces hydrolyzed protein waste, a hydrolysis solution of protein that is rich in amino acids, peptides, and chitooligosaccharides (Rinaudo, 2006). Hydrolyzed protein from chemical extraction deproteination cannot be used because it contains dangerous alkali solvents (Younes et al., 2014). Another disadvantage is that it consumes energy and produces large amounts of corrosive alkaline acids and can damage the environment (Ghorbel-bellaaj et al., 2013). However, chemical extraction methods produce a consistent amount of chitin and short time extraction (Kaya et al., 2014).
Microbiological extraction method
Given the various limitations of chemical extraction, the researchers keep looking for a safer, environment-friendly, green extraction that could produce more chitin in higher quality. One of the environment-friendly biotechnology methods is the fermentation using lactic acid bacteria (LAB). During fermentation, the bacteria will convert carbon sources, such as glucose or molasses, into lactic acid and pH will decrease due to the production of lactic acid. Crustacean shells have calcium carbonate and will react with lactic acid to form calcium lactate (Mao et al., 2013). The acid produced will be responsible for the demineralization process.
The presence of glucose or other carbon sources becomes a critical point that will determine the level of efficiency of the demineralization process (Ghorbel-Bellaaj et al., 2012). Although high glucose concentrations will accelerate the fermentation cycle, fermentation will be inhibited and pH will increase when the sugar concentration exceeds 15% (Zhang et al., 2012). Glucose at a concentration of 5% can induce protease production even up to four times more than the medium that does not use glucose (Ghorbel-Bellaaj et al., 2012).
The use of protease enzymes in the chitin extraction process will facilitate the removal of protein and calcium carbonate. Bacteria were tested first before use to see the ability to produce proteases. The test was continued to see the activity of proteolysis, for example, by looking at the proteolytic zone of casein agar (Harkin et al., 2015). The use of protease or lactic acid produced by bacteria to extract chitin is simple and inexpensive (Ghorbel-bellaaj et al., 2013).
The deproteination and demineralization can be either done simultaneously or carried out separately into two steps (Dun et al., 2019). Two-step fermentation has the advantage of acquiring chitin with a high grade of purity. LAB and protease-producing microbials have different optimal growth conditions so that it is preferably carried out in two steps. However, the two-step fermentation process requires a longer time and higher costs (Xu et al., 2008). The fermentation process that includes deproteinizing or demineralizing can be carried out in one step. However, the efficiency of deproteination is difficult to achieve; therefore, optimization of the factors that influence the process of demineralization and deproteination is needed (Oh et al., 2007).
 | Table 1. Various reports on α-chitin extraction using the chemical method. [Click here to view] |
Many factors will influence the demineralization and deproteination processes, such as carbon source and glucose concentration, inoculum amount, pH, and fermentation time (Arbia et al., 2013) so that several methods of approach for optimization studies in extracting chitin can be used, such as the Box–Behnken design or the Plackett–Burman design. These methods are also useful for understanding interactions between various physicochemical parameters using a minimum number of experiments. The Plackett–Burman design aims to select important factors from a large number of variables. From many important factors, there will then be tested statistics where this will be useful in designing experiments, building models, and evaluating the effects of different factors to find the optimal conditions for getting chitin (de Coninck et al., 2000; Ghorbel-Bellaaj et al., 2011).
 | Table 2. α-Chitin extraction using microbial fermentation with demineralization and deproteination yield. [Click here to view] |
In general, an enzymatic process using bacteria is carried out by the fermentation process. High protease activity shows the ability to hydrolyze protein more and more (Sedaghat et al., 2016). Most of the microbes used are free of chitinolytic activity which prevents the reduction of chitin quality during deproteination (Bajaj et al., 2015). Deproteination cannot reach 100%; this is because the enzyme does not get access to penetrate some of the protected proteins in the innermost layer, and ultimately proteolysis will not occur (Wang et al., 2006). The result of obtaining chitin by this method is chitin which has molecular weight and crystalline which is higher than chemically prepared chitin (Pacheco et al., 2011). The other disadvantage of microbial extraction is that it takes a long time. In general, the advantages of chitin extraction using the microbial extraction method are as follows:
- It is homogeneous and prevents deacetylation caused by strong alkali acid, resulting in high-quality products (Ramírez-Coutiño et al., 2006; Zhang et al., 2012).
- It is an ecofriendly and green method (Sedaghat et al., 2016).
- It helps in obtaining protein hydrolysate (amino acid and polypeptide) as a byproduct of the deproteination process (Hajji et al., 2015).
Chemical and enzymatic extraction method
Extraction using the chemical and enzymatic method is a method that has two steps in extraction. The combination of chemicals and enzymes is done to get a shorter extraction time. In general, the principle of this method is to replace a microbial in one of the extraction steps with a chemical compound (Table 4).
 | Table 3. α-Chitin extraction using microbial fermentation with chitin yield. [Click here to view] |
Natural deep eutectic solvent
NADES is obtained from an adequate mixture of hydrogen bond acceptor and donor which will enables their bonding through the interaction of hydrogen bonds forming eutectic with a low melting point (Abbott et al., 2004). The advantage of NADES is that it is a nontoxic and biodegradable solvent, where being environmentally friendly which will be an advantage compared to alkaline, acidic, and ionic liquid (IL) solvents (Huang et al., 2018). Besides, NADES can be used in extraction media and as a solvent in several biopolymers, including starch, cellulose, and lignin (Francisco et al., 2012). A mixture of choline halide (chloride/bromide)urea, choline chloride–thiourea, chlorocholine chlorideurea, and betaine hydrochlorideurea is a type of NADES suitable for dissolving α-chitin. Dissolution from biopolymers can be carried out using heating under the microwave, conventional heating, or heating by ultrasonication (Sharma et al., 2013). This is appropriate with the data extraction process presented in Table 5.
 | Table 4. α-Chitin extraction using chemical and enzymatic combination. [Click here to view] |
 | Table 5. α-Chitin extraction using NADES. [Click here to view] |
 | Table 6. α-Chitin extraction using IL. [Click here to view] |
Ionic liquid
The IL is a salt with a low boiling point that will form a liquid at temperatures below the water boiling point, which is useful as a solvent for cellulose or other polysaccharides (Zakrzewska et al., 2010). The advantages of the IL method are that it is more economic, efficient, and ecofriendly (Zhu et al., 2017). However, this method also has disadvantages, such as high cost and toxicity (Sharma et al., 2013), besides handling IL by untrained people is also dangerous (Bajaj et al., 2015). Dissolution using IL solvents will damage the hydrogen bonds in the “reassemble” chains into a new arrangement, thus forming amorphous chitin (Shamshina and Rogers, 2020).
Sonication
It is known that the use of high-intensity ultrasound to extract several polysaccharides requires a short time and little solvent so that it will save production costs (Wang and Wang, 2004). However, the addition of sonication to the chitin extraction process is not very useful in the demineralization step; even chitin can be damaged due to some of the material being dissolved and rinsed with reagents due to depolymerization (Kjartansson et al., 2006a). Besides, chitin yields are low due to extensive perforation of the shell (Kjartansson et al., 2006b).
The addition of sonication in extraction will be very useful if there is an incomplete deproteination process. Furthermore, the addition of sonication will also trigger changes in the crystalline chitin form so that it will be easier if it will be reacted chemically (Kjartansson et al., 2006a). Finally, the use of high-intensity ultrasound will be very useful in accelerating the extraction with a low degree of crystalline, if needed (Kjartansson et al., 2006b).
Pilot-scale chitin production
Chitin production on a pilot scale has been carried out in several experiments (Table 8). Chitin produced at the pilot scale is not very different from chitin production at the laboratory scale. Extraction by bacterial fermentation method is suitable for pilot-scale chitin production; this can be seen in all pilot-scale tests using the microbial method.
 | Table 7. α-Chitin extraction using sonication. [Click here to view] |
 | Table 8. Pilot scale of α-chitin production. [Click here to view] |
Pharmacology activities
Pharmacological studies of crude α-chitin are very rare. Table 9 shows some of the best references we can find. In general, pharmacological activities of α-chitin showed its potential to be anticancer and anti-inflammatory and to accelerate wound healing (Anandan et al., 2004; Bae et al., 2013; Teng et al., 2001). Chitin was tested pharmacologically to Hep2 (human larynx carcinoma cell line), RD (human embryo rhabdomyosarcoma cell line), and THP-1 (human monocytic leukemia cell line). Although the cytotoxic effect is not too large, its anticancer potential can be enhanced by changes in the low molecular weight of chitin (Bouhenna et al., 2015; Salah et al., 2013).
 | Table 9. Pharmacology activities of α-chitin. [Click here to view] |
CONCLUSION
There are several methods to extract α-chitin from crustaceans, that is, chemical, microbiological, chemical–enzymatic combination, using NADES, IL solvent, and sonication. The best α-chitin extraction method from crustaceans is the chemical l–enzymatic combination method. This method was able to provide more efficient extraction time and is environmentally friendly with quality parameters, such as very good and consistent deproteination and demineralization. In addition, the chitin yield was better than that of other methods. Followed by successive recommended methods were the microbiological methods, NADES, chemical methods, and IL, respectively. α-Chitin has proven to possess anticancer and anti-inflammatory potential and could accelerate wound healing. The mechanism of action of α-chitin anti-inflammatory activity is still interesting to be further explored.
CONFLICTS OF INTEREST
All the authors declare that they have no conflicts of interest for this work.
FUNDING
None.
REFERENCES
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc, 2004; 126(29):9142–7. CrossRef
Agboh OC, Qin Y. Chitin and chitosan fibers. Polym Adv Technol, 1997; 8:355–65. CrossRef
Akpan EI, Gbenebor OP, Adeosun SO. Synthesis and characterisation of chitin from periwinkle (Tympanotonus fusatus (L.)) and snail (Lissachatina fulica (Bowdich)) shells. Int J Biol Macromol, 2018; 106:1080–88. CrossRef
Anandan R, Nair PGV, Mathew S. Anti-ulcerogenic effect of chitin and chitosan on mucosal antioxidant defence system in HCl-ethanol-induced ulcer in rats. J Pharm Pharmacol, 2004; 56(2):265–9. CrossRef
Aranaz I, Mengíbar M, Harris R, Paños I, Miralles B, Acosta N, Heras Á. Functional characterization of chitin and chitosan. Curr Chem Biol, 2009; 3:203–30. CrossRef
Arbia W, Adour L, Amrane A, Lounici H. Optimization of medium composition for enhanced chitin extraction from Parapenaeus longirostris by Lactobacillus helveticus using response surface methodology. Food Hydrocoll, 2013; 3:392–403. CrossRef
Bae, MJ, Shin HS, Kim EK, Kim J, Shon DH. Oral administration of chitin and chitosan prevents peanut-induced anaphylaxis in a murine food allergy model. Int J Biol Macromol, 2013; 61:164–8. CrossRef
Bajaj M, Freiberg A, Winter J, Xu Y, Gallert C. Pilot-scale chitin extraction from shrimp shell waste by deproteination and decalcification with bacterial enrichment cultures. Appl Microbiol Biotechnol, 2015; 99(22):9835–46. CrossRef
Bernabé P, Becherán L, Cabrera-barjas G, Nesic A, Alburquenque C, Tapia CV, Ríos PDL. Chilean crab (Aegla cholchol) as a new source of chitin and chitosan with antifungal properties against Candida spp. Int J Biol Macromol, 2020; 149:962–74. CrossRef
Berton P,Shamshina JL, Ostadjoo S, King CA, Rogers RD. Enzymatic hydrolysis of ionic liquid-extracted chitin. Carbohydr Polym, 2018; 199:228–35. CrossRef
Bouhenna M, Salah R, Bakour R, Drouiche N, Abdi N, Grib H, Mameri N. Effects of chitin and its derivatives on human cancer cells lines. Environ Sci Pollut Res Int, 2015; 22(20):15579–86. CrossRef
Cremades O, Ponce E, Corpas R, Gutierrez J, Jover M, Alvarez-Ossorio M, Bautista Palomas JD. Processing of crawfish (Procambarus clarkii) for the preparation of carotenoproteins and chitin. J Agric Food Chem, 2001; 49:5468–72. CrossRef
de Coninck J, Bouquelet S, Dumortier V, Duyme F, Verdier-Denantes I. Industrial media and fermentation processes for improved growth and protease production by Tetrahymena thermophila BIII. J Ind Microbiol Biotechnol, 2000; 24(4):285–90. CrossRef
Ding H, Lv L, Wang Z, Liu L. Study on the “Glutamic Acid-Enzymolysis” process for extracting chitin from crab shell waste and its by-product recovery. Appl Biochem Biotechnol, 2020; 190(3):1074–91. CrossRef
Duan S, Li L, Zhuang Z, Wu W, Hong S, Zhou J. Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydr Polym, 2012; 89(4):1283–8. CrossRef
Dun Y, Li Y, Xu J, Hu Y, Zhang C, Liang Y, Zhao S. Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int J Biol Macromol, 2019; 123:420–6. CrossRef
FAO. 2020. FAO global aquaculture production volume and value statistics. Available via: http://fao.org/figis/servlet/tabselector (Accessed 8 May 2020).
Flores-Albino B, Arias L, Gómez J, Castillo A, GimenoM, Shirai K. Chitin and L(+)-lactic acid production from crab (Callinectes bellicosus) wastes by fermentation of Lactobacillus sp. B2 using sugar cane molasses as carbon source. Bioprocess Biosyst Eng, 2012; 35(7):1193–200. CrossRef
Francisco M, van den Bruinhorst A, Kroon MC. New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing. Green Chem, 2012; 14(8):2153–7. CrossRef
Gamal RF, El-Tayeb TS, Raffat EI, Ibrahim HMM, Bashandy AS. Optimization of chitin yield from shrimp shell waste by Bacillus subtilis and impact of gamma irradiation on production of low molecular weight chitosan. Int J Biol Macromol, 2016; 91:598–608. CrossRef
Ghorbel-Bellaaj O, Hajji S, Younes I, Chaabouni M. Optimization of chitin extraction from shrimp waste with Bacillus pumilus A1 using response surface methodology. Int J Biol Macromol, 2013; 61:243–50. CrossRef
Ghorbel-Bellaaj O, Hmidet N, Jellouli K, Younes I, Maâlej H, Hachicha R, Nasri M. Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett-Burman and response surface methodology approaches. Int J Biol Macromol, 2011a; 48(4):596–602. CrossRef
Ghorbel-Bellaaj O, Jellouli K, Younes I, Manni L, Ouled Salem M, Nasri, M. A solvent-stable metalloprotease produced by Pseudomonas aeruginosa A2 grown on shrimp shell waste and its application in chitin extraction. Appl Biochem Biotechnol, 2011b; 164(4):410–25. CrossRef
Ghorbel-Bellaaj O, Younes I, Maâlej H, Hajji S, Nasri M. Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol, 2012; 51(5):1196–201. CrossRef
Goodrich JD, Winter WT. α-chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules, 2007; 8(1):252–7. CrossRef
Hajji S, Ghorbel-Bellaaj O, Younes I, Jellouli K, Nasri M. Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int J Biol Macromol, 2015; 79:167–73. CrossRef
Hamdi M, Hammami A, Hajji S, Jridi M, Nasri R. Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int J Biol Macromol, 2017; 101:455–63. CrossRef
Harkin C, Brück WM, Lynch C. Isolation & identification of bacteria for the treatment of brown crab (Cancer pagurus) waste to produce chitinous material. J Appl Microbiol, 2015; 118(4):954–65. CrossRef
Hassainia A, Satha H, Boufi S. Chitin from agaricus bisporus: extraction and characterization. Int J Biol Macromol, 2018; 117:1334–42. CrossRef
Huang WC, Zhao D, Guo N, Xue C, Mao X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J Agric Food Chem, 2018; 66(45):11897–901. CrossRef
Ifuku S, Nogi M, Abe K, Yoshioka M, Morimoto M, Saimoto H, Yano H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules, 2009; 10(6):1584–8. CrossRef
Jabeur F, Mechri S, Kriaa M, Gharbi I, Bejaoui N, Sadok S, Jaouadi B. Statistical experimental design optimization of microbial proteases production under co-culture conditions for chitin recovery from speckled shrimp metapenaeus monoceros by-product. Biomed Res Int, 2020; 2020:1–10. CrossRef
Jollès P, Muzzarelli RA. Chitin and chitinases vol.87. Birkhäuser Verlag.EXS (Basel), Basel, Switzerland, .
Jung HS, Kim MH, Shin JY, Park SR, Jung JY, Park WH. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohydr Polym, 2018; 193: 205–11. CrossRef
Jung WJ, Jo GH, Kuk JH, Kim KY, Park RD. Extraction of chitin from red crab shell waste by cofermentation with Lactobacillus paracasei subsp. tolerans KCTC-3074 and serratia marcescens FS-3. Appl Microbiol Biotechnol, 2006; 71(2):234–7. CrossRef
Jung WJ, Jo GH, Kuk JH, Kim YJ, Oh KT, Park RD. Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei KCTC-3074 and Serratia marcescens FS-3. Carbohydr Polym, 2007; 68(4):746–50. CrossRef
Kaya M, Karaarslan M, Baran T, Can E, Ekemen G, Bitim B, Duman F. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula). Nat Prod Res, 2014; 28(23):2186–90. CrossRef
Kjartansson GT, Zivanovic S, Kristbergsson K, Weiss J. Sonication-assisted extraction of chitin from North Atlantic Shrimps (Pandalus borealis). J Agric Food Chem, 2006a; 54(16):5894–902.
Kjartansson GT, Zivanovic S, Kristbergsson K, Weiss J. Sonication-assisted extraction of chitin from shells of fresh water prawns (Macrobrachium rosenbergii). J Agric Food Chem, 2006b; 54(9):3317–23. CrossRef
Manni L, Ghorbel-Bellaaj O, Jellouli K, Younes I, Nasri M. Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl Biochem Biotechnol, 2010; 162(2):345–57. CrossRef
Mao X, Zhang J, Kan F, Gao Y, Lan J, Zhang X, Lin H. Antioxidant production and chitin recovery from shrimp head fermentation with Streptococcus thermophilus. Food Sci Biotechnol, 2013; 22:1023–32. CrossRef
Maruthiah T, Palavesam A. Characterization of haloalkalophilic organic solvent tolerant protease for chitin extraction from shrimp shell waste. Int J Biol Macromol, 2017; 97. CrossRef
Mathur NK, Narang CK. Chitin and chitosan, versatile polysaccharides from marine animals. J Chem Educ, 1990; 67(11):938–42. CrossRef
Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Rajeevgandhi C, Seedevi P. Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int J Biol Macromol, 2019; 126:555–60. CrossRef
Oh KT, Kim YJ, NguyenVN, Jung WJ, Park RD. Demineralization of crab shell waste by Pseudomonas aeruginosa F722. Process Biochem, 2007; 42(7):1069–74. CrossRef
Ordóñez-Del Pazo T, Antelo LT, Franco-Uría A, Pérez-Martín RI, Sotelo CG, Alonso AA. Fish discards management in selected Spanish and Portuguese métiers: identification and potential valorisation. Trends Food Sci Technol, 2014; 36(1):29–43. CrossRef
Pacheco N, Garnica-Gonzalez M, Gimeno M, Bárzana E, Trombotto S, David L, Shirai K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules, 2011; 12(9):3285–90. CrossRef
Percot A, Viton C, Domard A. Optimization of chitin extraction from shrimp shells. Biomacromolecules, 2003; 4(1):12–8. CrossRef
Ramírez-Coutiño L, Marín-Cervantes M del C, Huerta S, Revah S, Shirai K. Enzymatic hydrolysis of chitin in the production of oligosaccharides using Lecanicillium fungicola chitinases. Process Biochem, 2006; 41(5):1106–10. CrossRef
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci, 2006; 31(7):603–32. CrossRef
Roy JC, Salaün F, Giraud S, Ferri A. Solubility of chitin: solvents, solution behaviors and their related mechanisms. In Solubility of polysaccharides an. pp 109–26, 2017. CrossRef
Salah R, Michaud P, Mati F, Harrat Z, Lounici H, Abdi N, Mameri N. Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. Int J Biol Macromol, 2013; 52:333–9. CrossRef
Sedaghat F, Yousefzadi M, Toiserkani H, Najafipour S. Chitin from Penaeus merguiensis via microbial fermentation processing and antioxidant activity. Int J Biol Macromol, 2016; 82:279–83. CrossRef
Setoguchi T, Kato T, Yamamoto K, Kadokawa J. Facile production of chitin from crab shells using ionic liquid and citric acid. Int J Biol Macromol, 2012; 50(3):861–4. CrossRef
Shamshina J, Rogers R. Are ionic liquids enabling technology? Startup to Scale-Up to Find Out. pp 69–85, 2020. CrossRef
Sharma M, Mukesh C, Mondal D, Prasad K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv, 2013; 3. CrossRef
Sorokulova I, Krumnow A, Globa L, Vodyanoy V. Efficient decomposition of shrimp shell waste using Bacillus cereus and Exiguobacterium acetylicum. J Ind Microbiol Biotechnol, 2009; 36:1123–6. CrossRef
Tarusin DN, Bazhenov VV, Schütz K, Brüggemeier S, Gossla E, AkkineniAR, Ehrlich H. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: biocompatibility and cryopreservation. Int J Biol Macromol, 2017; 104:1955–65. CrossRef
Teng WL, Khor E, Tan TK, Lim LY, Tan SC. Concurrent production of chitin from shrimp shells and fungi. Carbohydr Res, 2001; 332(3):305–16. CrossRef
Thang C, Ngoc T, Nguyen VB, Phuong T, Vo K. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int J Biol Macromol, 2019; 131:706–15. CrossRef
Vani R, Amalan Stanley S. Studies on the extraction of chitin and chitosan from different aquatic organisms. Adv Bio Tech, 2013; 12:12–5.
Wang L, Wang YJ. Application of high-intensity ultrasound and surfactants in rice starch isolation. Cereal Chem, 2004; 81(1):140–4. CrossRef
Wang SL, Kao TY, Wang CL, Yen YH, Chern MK, Chen YH. A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzyme Microb Technol, 2006; 39(4):724–31. CrossRef
Wang Y, Chang Y, Yu L, Zhang C, Xu X, Xue Y, Xue C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr Polym, 2013; 92(1):90–7. CrossRef
Wu Q, Mushi NE, Berglund LA. High-strength nanostructured films based on well-preserved α-chitin nanofibrils disintegrated from insect cuticles. Biomacromolecules, 2020; 21(2):604–12. CrossRef
Xu Y, Gallert C, Winter J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl Microbiol Biotechnol, 2008; 79(4):687–97. CrossRef
Younes I, Ghorbel-Bellaaj O, Nasri R, Chaabouni M, Rinaudo M, Nasri M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem, 2012; 47(12):2032–9. CrossRef
Younes I, Hajji S, Frachet V, Rinaudo M, Jellouli K, Nasri M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int J Biol Macromol, 2014; 69:489–98. CrossRef
Zakrzewska ME, Bogel-Åukasik E, Bogel-Åukasik R. Solubility of carbohydrates in ionic liquids. Energy Fuels, 2010; 24(2):737–45. CrossRef
Zhang H, Jin Y, Deng Y, Wang D, Zhao Y. Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr Res, 2012; 362:13–20. CrossRef
Zhu P, Gu Z, Hong S, Lian H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr Polym, 2017; 177:217–23. CrossRef