INTRODUCTION
Rinorea dentata (P Beauv.) Kuntze. is a rare wild plant species, found in the tropics. It belongs to the family of Violaceae, growing majorly in the understory of humid or semi-deciduous forests and particularly abundant in Omo Forest Reserve, Ogun State, Southwestern Nigeria (Ojo, 2004). R. dentata is a shrub or small tree growing as tall as 10-m high dominating the lower forest strata, where exposure to sunlight becomes a challenge (Chuyong et al., 2011). The common names of the plant include “iyokheze” (Edo), “oloborowo” (Yoruba), or “stone plant” (Burkill, 2000) and used locally as a chewing stick for mouth cleaning. R. dentata is an important medicinal plant reported with various ethnomedicinal uses such as against neurodegenerative disorders, malaria, diabetes, headaches, diarrhea, and body inflammation (Attah et al., 2016; Erhenhi and Obadoni, 2015; Jiofack, 2008). However, despite all these indigenous usages, the pharmacological reports on these ethnomedicinal claims are found wanting in the literature. This work was, therefore, designed to explore the pharmacological potentials of this important Nigerian medicinal plant.
Inflammation is an underlying element in the onset of many diseases such as diabetes, cancer, infections, obesity, and cardiovascular and even neurodegenerative diseases such as Alzheimer’s (AD) (Corrado et al., 2010; Crowson et al., 2013). Certain non-steroidal anti-inflammatory drug on administration over a long period exhibits a decreased risk of AD. In fact, the report had found a link between the cholinergic system and inflammation with acetylcholine playing a role in the release of cytokine (Borovikova et al., 2000). Lipid-peroxidizing enzymes (lipoxygenase) required in the bioformation (from arachidonic acid) of leukotriene serve as an intermediary for inflammation and allergic responses. They are also involved in the induction of many diseases related to inflammation (Dobrian et al., 2011) as they (lipoxygenases) trigger the introduction of molecular oxygen to unsaturated fatty acids (UFAs), such as linoleic and arachidonic acids, which are their common substrates (Porta and Rocha-Sosa, 2002). It is worthy of mention that there are four major isoenzymes of lipoxygenases including 5, 8, 12, and 15, with each isoform differing from one another based on the position of oxidation in the UFAs. Despite the major involvement of this enzyme in inflammation, their actions are similarly witnessed in the formation of inflammatory lipid mediators, such as leukotrienes and prostaglandins. Intriguingly, the inhibition of these enzymes is considered as the hallmark measure for disease prevention, particularly those linked to oxidative stress and inflammation and, perhaps, diabetes mellitus (Butterfield et al., 2014; Radmark and Samuelson, 2007).
Diabetes mellitus (DM) is a chronic derangement attributed to partial or complete insulin deficiency arising from elevated glucose level in the blood (hyperglycemia) (Yadav et al., 2008). The ability of any drug substance to salvage postprandial blood sugar levels (BGL) is considered as a very important step in early diabetes mellitus therapy and reduction in protracted vascular problems (Kwon et al., 2008). Persistent starch hydrolysis by α-amylase (α-AL) of the pancreas and the subsequent glucose absorption by α-glucosidases (α-GSDs) found in the intestine lead to a continuous increase in BGL, termed as hyperglycemia in non-insulin-dependent diabetes (NIDD), individuals. Thus, a useful approach for NIDD management is a strong α-GSD and mild α-AL inhibition (Krentz and Bailey, 2005). Interestingly, the prevention of carbohydrate (CHO) absorption in the form of glucose after food uptake is a potent schedule or plan for alleviating postprandial hyperglycemia in patients with DM. It is exciting to also note that substances (inhibitors), which can hinder the activities of these enzymes by delaying CHO digestion, would bring about a reduction in the extent to which glucose is absorbed, consequently preventing post-prandial elevation of plasma glucose (Rabasa-Lhoret and Chiasson, 2004). The common examples of such inhibitors of α-AL and α-GSDs available in the pharmaceutical industry are acarbose, miglitol, and voglibose (Bailey, 2003) known to lower postprandial blood glucose after a starch load. These inhibitors exert their action by inhibiting the final process in CHO digestion [conversion of disaccharide to monosaccharide (glucose)], resulting in the reduced concentration of glucose absorbed into the blood circulation (Joubert et al., 1990). Unfortunately, these drugs are not without side effects including bloating, abdominal distension, and flatulence. Hence, there is the need or search for alternative medicines from plants.
The aim of this study was to investigate the potentials of R. dentata crude extracts and fractions on the activities of 15-lipoxygenase (15-LOX), acetylcholinesterase (AChE), α-AL, and α-GSD with the hope of establishing potential treatments (from natural sources) that could come handy in managing inflammatory-related, diabetes, and Alzheimer’s diseases with the minimal or outright absence of side effects.
MATERIALS AND METHODS
Chemicals
Linoleic acid was purchased from Merck (Germany). Quercetin, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), 15-lipoxygenase from Glycine max, Folin–Ciocalteu reagent, aluminum chloride, trolox, 2, 4, 6-tri(2-pyridyl)-s-triazine, 5,5′-dithio-bis-2-nitrobenzoic acid, eserine, acetylcholine iodide, electric eel AChE (C3389), acarbose, p-nitrophenyl glucopyranoside, α-AL, and α-GSD were also procured from Sigma-Aldrich, South Africa.
Plant material collection, authentication, and extraction
Rinorea dentata whole plant was collected from Omo Forest Reserve, Ogun, Nigeria, on February 10, 2016. The plant species was recognized and authenticated at the Forest Herbarium Ibadan (FHI) with voucher number FHI 111531. Voucher specimens were also deposited in the Department of Pharmacognosy Herbarium, University of Ibadan. The leaves were separated from the stem, air-dried, and powdered. The powdered leaves (1,000 g) and stem (2,000 g) were weighed and macerated in methanol for 4,320 minutes followed by another 1,440 minutes of extraction. The filtered extracts and filtrates were concentrated in vacuo resulting in the crude (leaf and stem) methanol extract.
The crude methanol extract (CME) of R. dentata leaf (RDL) (85.29 g) and R. dentata stem (RDS) (68.38 g) was dissolved in methanol–water (3:1) mixture and subsequently partitioned successively into n-hexane, dichloromethane, ethyl acetate, and n-Butanol fraction (NBF). All fractions obtained were concentrated and preserved at −4°C before assays.
Qualitative phytochemical analysis
The phytochemical analysis was performed on all the extracts and fractions for the presence of alkaloids, anthraquinones, flavonoids, phenolics, tannins, triterpenes, phytosterols, glycosides, and saponins adopting well-established qualitative procedures (Sofowora, 2006; Prashant et al., 2011).
Determination (in vitro) of the study parameters
DPPH antioxidant and ferric reducing antioxidant power (FRAP) assays
The DPPH antioxidant activity of the samples and standard measured by their ability to scavenge the DPPH was evaluated using the method described by Susanti et al. (2007) and Ayoola et al. (2017) . The FRAP of the samples was determined using the method of Musa et al. (2011) and Sonibare et al. (2018) .
Estimation of total phenolic content (TPC) and total flavonoid content (TFC)
The method of Khatoon et al. (2013) and Ayoola et al. (2017) was used to explore the TPC of all the extracts. The TPC expressed as milligram gallic acid equivalent (GAE)/g of extract was calculated from the gallic acid calibration curve (y = 0.0099x + 0.1207, r2 = 0.9228). The colorimetric method involving the use of aluminum chloride was adopted in assessing the TFC of the extracts (Ayoola et al., 2017; Ebrahimzadeh et al., 2009) and was expressed as milligram quercetin equivalent (QE)/g of extract, obtained from standard curves (y = 0.0077x + 0.0884, r2 = 0.9980) prepared with 6.25–200 μg quercetin/ml.
Inhibition of 15-LOX enzyme and anticholinesterase assay
The inhibition of 15-LOX (enzyme) method was used to determine the anti-inflammatory activity of the extracts (Adebayo et al., 2015), whereas AChE inhibition was determined using Ellman’s colorimetric method modified by Elufioye et al. (2010) . Different sample/control concentrations (0.1–1 mg/ml) were tested, whereas quercetin and eserine were used as the positive controls for the anti-inflammatory and anticholinesterase assays, respectively. The results were expressed as inhibitory concentration (IC50), i.e., the concentration of extracts or controls that gave 50% enzyme inhibition.
Alpha-amylase and alpha-glucosidase enzymes inhibition, and kinetics of inhibitory assays
The α-amylase (α-AML) inhibition (McCue and Shetty, 2004) and α-GSD inhibitory potential (Kim et al., 2005) of the extracts and fractions were determined based on cited standard methods. Different extracts or controls (7.81–250 μg/ml) were reacted with α-amylase from the porcine pancreas or α-GCD from Saccharomyces cerevisiae. Acarbose was used as the positive control. Moreover, the mode (kinetics) of α-AML and α-GCD inhibitions was examined by the method described by Ali et al. (2006) . The kinetics of the inhibition of α-AML and α-GCD by R. dentata (RD) was evaluated using Lineweaver and Burk equation (Lineweaver and Burk, 1934).
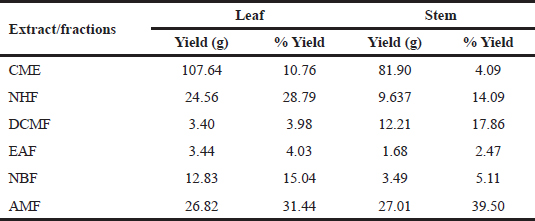 | Table 1. Extraction yield and percentage yield of RDL and stem. [Click here to view] |
Statistical analysis
The experiment or assays were carried out three times, and the results were reported as mean ± standard error of mean (SEM) followed by using a one-way analysis of variance. Duncan’s new multiple range tests were used to compare the means. All statistical analyses were carried out by using GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego, CA. The IC50 values for the anti-inflammatory, α-AL, and α-GSD inhibitory assays were calculated from the linear regression curve.
RESULTS
Extraction and fractionation yield
The leaf samples gave a better yield compared to the stem. The yield of the extracts was calculated and expressed as the weight of fraction/extract relative to the weight of the obtained crude/starting powdered sample. The percentage yield of the leaf ranged from 3.98 to 31.44, whereas the extraction yield for the stem ranged from 2.47% to 39.50%. The aqueous–methanol fraction (AMF) of both the studied parts of the plant gave the highest yields as shown in Table 1 . Polar solvents were shown to produce high extraction yield.
Phytochemical screening
The qualitative phytochemical screening of the crude extract and fractions of the leaf and stem of R. dentata revealed the presence of alkaloids aside from the n-hexane fraction (NHF) of the leaf and NBF as well as NHF (stem). Saponin was present in all the extracts and fractions except CME of the stem. Phenols were present in the AMF, NBF, ethyl acetate fraction (EAF), and dichloromethane fraction (DCMF) of the leaf and EAF and DCMF fractions of the stem. While anthraquinone was absent in all the crude extracts and fractions, triterpenes and steroids were found in AMF of the leaf and stem and CME of the stem. Flavonoids were similarly found in the AMF, NBF, and EAF of the leaf and AMF, CME, and EAF of the stem. Tannins were detected only in the EAF of the leaf and the EAF and DCM of the stem (Table 2).
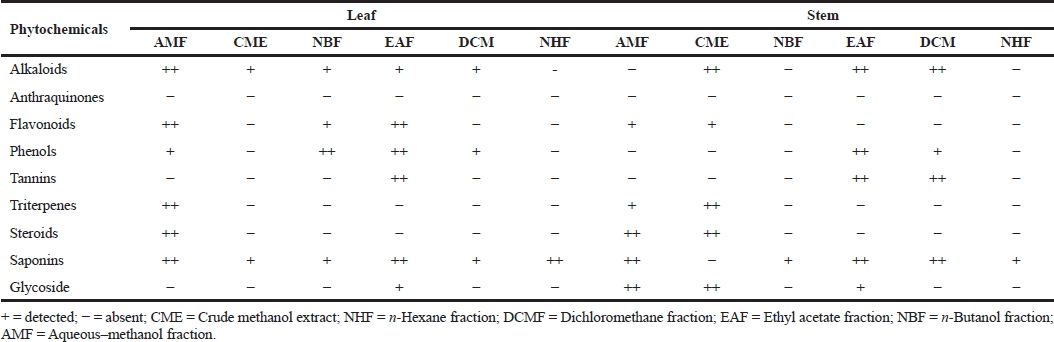 | Table 2. Phytochemical screening of the extract and fractions of RDL and stem. [Click here to view] |
Antioxidant activity
The total phenolic and flavonoid contents, as well as antioxidant activity of both the leaf and stem fractions, are shown in Table 3 . The leaf fractions presented higher total phenolic and TFCs than the stem; however, the EAF of both the leaf (447.41 ± 18.77 mgGAE/g) and stem (228.20 ± 0.17 mgGAE/g) depicted higher TPCs than the other fractions. The DCMF and NHF of both the plant parts had the highest TFCs. Regarding the DPPH radical scavenging activity, the NBF followed by the CME of both the leaf and stem showed the best activity, whereas for the ferric reducing antioxidant power, the EAF of both the leaf and stem reflected the optimum result. Among all the tested fractions, there was a strong correlation between the TPC and the ferric reducing antioxidant power (R = 0.84).
Anti-inflammatory effect
The anti-inflammatory potential of RD leaf and stem was evaluated by adopting the anti-15 LOX model of inhibition. In general, the leaf extract and its corresponding fractions gave a better inhibition of the enzyme than the stem (Table 4). The EAF of the leaf gave a very promising result presenting an IC50 value of 34.14 ± 16.69 μg/ml, which was better than the reference standard, quercetin, with an IC50 value of 54.75 ± 17.07 μg/ml. The DCMF and NHF of both the parts gave the least activity with an IC50 value of 676.11 ± 24.60 and 589.57 ± 103.83 μg/ml (leaf) and 1,298.58 ± 409.86 and 701.52 ± 66.28 μg/ml (stem), respectively. The AMF of the stem gave the best activity with an IC50 value of 96.41 ± 29.75 μg/ml (Table 4).
Anticholinesterase activity
The CME of both the leaf (19.61 ± 10.36 μg/ml) and the stem (99.95 ± 3.67 μg/ml) of RD showed a significant AChE inhibition when individually compared to their respective fractions. The CME (19.61 ± 10.36 μg/ml) and the AMF (26.08 ± 1.70 μg/ml) of RD leaf had a significant and optimum inhibitory activity than the standard, eserine (31.23 ± 7.33 μg/ml), as shown in Table 4 .
Antidiabetic activity
α-Amylase inhibitory assay
In this study, the potential of RD leaf and stem and fractions to inhibit α-AL enzyme was compared with that of acarbose, the positive control. For the leaf, the EAF gave the best inhibitory activity of the enzyme with the lowest IC50 value of 358.40 ± 3.43 μg/ml, followed by the NBF (367.50 ± 8.34 μg/ml) when compared with other fractions and crude extract. There was no significant difference (p < 0.05) between the activity of the EAF and NBF, which were close to that of acarbose (342.00 ± 6.30 μg/ml). CME of the leaf gave the lowest inhibitory activity with an IC50 value of 630.30 ± 4.31 μg/ml (Table 4). However, for the stem, NHF gave the best activity (356.90 ± 4.24 μg/ml) statistically difference (p < 0.05) from other fractions and crude (methanol) extract. This was followed by EAF (405.90 ± 10.41 μg/ml), whereas DCMF revealed the least α-AL inhibitory activity (Table 4). Above all, the leaf gave a better α-AL inhibitory potential than the stem.
α-Glucosidase inhibitory activity
The DCMF of the leaf (204.00 ± 8.12 μg/ml) exhibited the best α-GSD inhibitory potential statistically different (p < 0.05) when compared to other extracts and control. The activities of the NB and CM fractions are not different (p > 0.05) from that of acarbose, the positive control (Table 4). The least inhibitory activity was observed with AMF. The DCMF (222.80 ± 3.54 μg/ml) of the stem presented the best α-GSD inhibitory activity. This is followed by the EAF (363.50.00 ± 8.48 μg/ml), whereas the AMF depicted the least inhibitory activity. The DCMF fraction of both the leaf and stem provided the most effective α-GSD inhibitory activity than other fractions, whereas the AMF gave the least activity for both leaf and stem. In general, the leaf fractions gave a better α-GSD inhibitory activity than the stem.
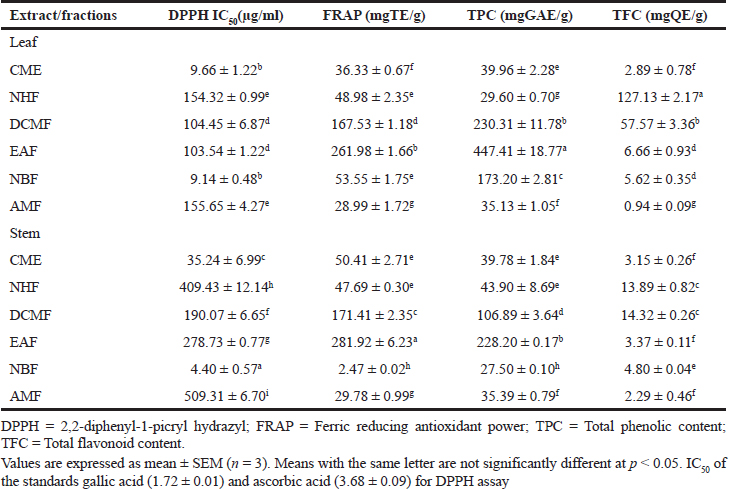 | Table 3. Total phenolic content, TFC and radical scavenging effect of crude and different fractions of RDL and RDS. [Click here to view] |
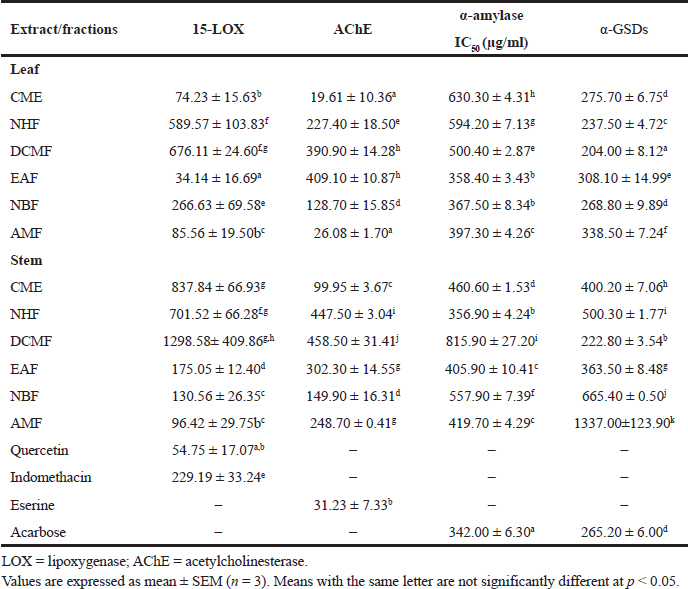 | Table 4. Inhibition of 15-lipoxygenase, AChE, α-amylase, and α-GSD enzymes by crude extracts and fractions of RD leaf and stem. [Click here to view] |
Mode of inhibition of α-AL and α-GSD enzymes
The DCMF of the leaf with the best α-GSD inhibitory activity and moderate α-AL inhibitory activity was chosen for studying the kinetics of enzymes’ inhibition, whereas, in the same manner, the EAF of the stem was chosen for the assay. The modes of α-AL and α-GSD inhibition by DCM (leaf) and EAF of the stem are shown in Figures 1 and 2 , respectively. The Lineweaver–Burk plot revealed a non-competitive inhibition of α-AL revealing a reduction in both Vmax values among the extract and the control from 0.036 to 0.032 mM/minutes with a constant Km value (0.35 mM−1) for both control and extract for the leaf (Fig. 1A). Although the mode of α-AL and α-GSD inhibitions for the stem is uncompetitive (Figs. 2A and B), a constant Vmax values (0.08 mM/minutes) for both extract and control with a decrease in Km values from 0.297 (extract) to 0.140 (control) mM/minutes for α-glucosidase (Fig. 1B) suggest a competitive mode of inhibition by the DCMF of the leaf.
DISCUSSION
In this study, the CME of the leaf and stem of RD were first fractionated using different solvents of increasing polarity to obtain different fractions with different phytochemical profiles. Interestingly, the leaf and stem fractions are rich in phenols, alkaloids, and saponins. Subsequently, the antioxidant, anti-inflammatory, anticholinesterase, and antidiabetic properties of RD leaf and stem fractions were investigated to support the ethnomedicinal claims on the use of the plant in traditional medicine toward the management of neurodegenerative diseases, body inflammation, and diabetes.
EAF was found to contain the highest phenolics, EAF and DCMF (though also high in flavonoids) presented the highest antioxidant activity in the FRAP, and in the DPPH assay, NBF fraction was the best in scavenging the radical followed by the crude extracts of both the plant parts (leaf and stem) going by their lowest IC50 values. Phenolic compounds are reported to have wide pharmacological activities, ranging from their ability to scavenge free radicals (antioxidants) to antidiabetic and anti-inflammatory potentials (Dani et al., 2010). The previous reports had established a relationship between reactive oxygen species and many other diseases such as cancer, diabetes, and neurodegenerative disorder (NDD), particularly AD. In fact, during aging, the antioxidant defense mechanism is compromised or weakened, due to the elevated oxidative stress arising from increased free radical production (Mathew and Subramanian, 2014). The crude extract and AMF of the leaf reflected the most effective AChE inhibition with the IC50 values of 19.61 ± 10.36 and 26.08 ± 1.70 μg/ml, respectively, which is better than that of the standard, eserine (31.23 ± 7.33 μg/ml). This result supports the ethnomedicinal claim on the use of R. dentata (RD) in the management of the (NDD) (Sonibare and Ayoola, 2015). Hence, RD leaf could be a good therapeutic alternative against NDD, particularly AD. The anticholinesterase effect of the plant could be attributed to the presence of detected alkaloids, known to have cholinesterase inhibitory properties. Current drugs used for the management of AD such as rivastigmine are alkaloids (Mukherjee et al., 2007).
The anti-inflammatory properties of RD leaf and stem fractions were assessed based on the inhibition of 15-lipoxygenase enzyme. The enzyme is important in the bioformation of leukotriene while triggering the first process in the breakdown of arachidonic acid to leukotrienes (biologically active). Leukotrienes are known as an effective intermediary of inflammatory and allergic responses. The 15-lipoxygenase pathway inhibited with respect to their pro-inflammatory properties is assumed as the hallmark therapy in the management of numerous inflammation-related diseases (Schneider and Bucar, 2005). The EAF of the leaf gave the best inhibitory activity (34.14 ± 16.69 μg/ml) better than the positive control, quercetin (54.75 ± 17.07 μg/ml). It is worthy of mention that EAF is rich in phenols as shown by the phytochemical screening result (Table 2) and TPC. The presence of phenolic compounds could be suggested to be responsible for its anti-inflammatory property. Muthuraman et al. (2011) and Adebayo et al. (2015) attributed a good anti-inflammatory power to the presence or detection of rich amount of phenols in plant extracts. Hence, this fraction could harbor the bioactive compounds that could pave a way for new anti-inflammatory drug discovery. However, indomethacin, which was used as a control in this experiment, had an insignificant or weak lipoxygenase (LOX) inhibitory activity with an IC50 value of 229.19 ± 33.24 μg/ml. This is as expected because it is a non-selective cyclooxygenase 1 and 2 inhibitor and a non-steroidal anti-inflammatory agent (Hart and Boardman, 1963). This type of poor activity by indomethacin witnessed in this study was also in consonance with the report of Dzoyem and Eloff (2015).
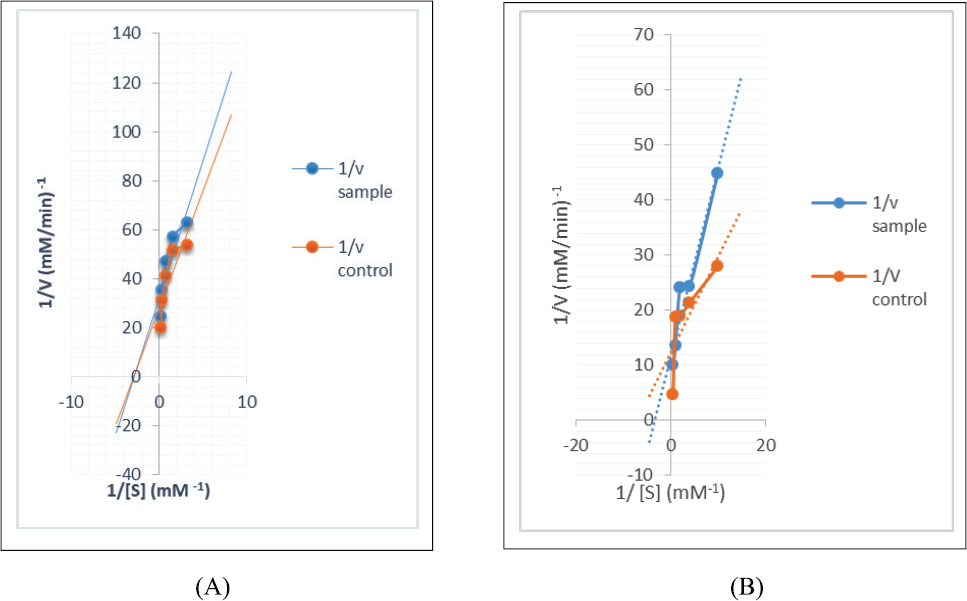 | Figure 1. (A) Non-competitive mode of inhibition of α-amylase by DCM fraction of RDL. (B) Competitive mode of the inhibition of α-GSD by DCM fraction of RDL. [Click here to view] |
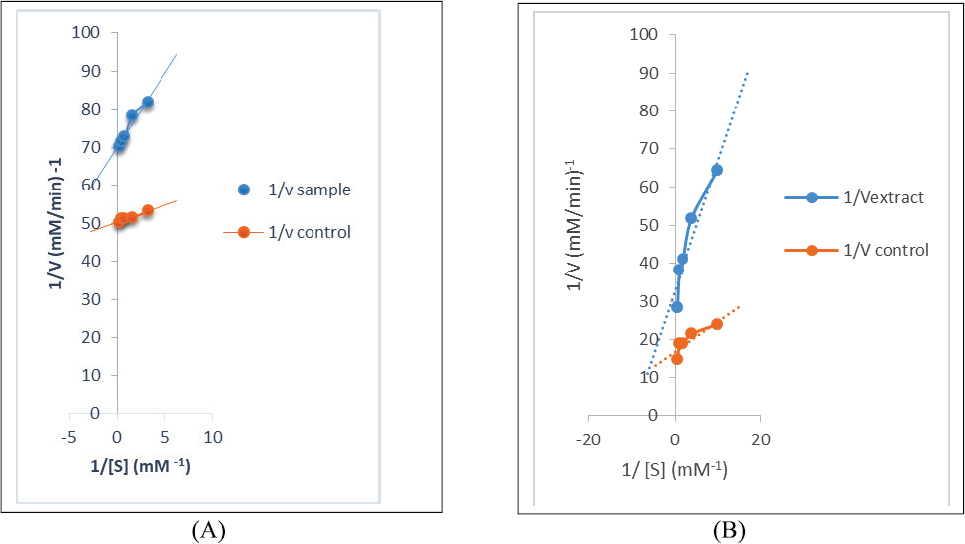 | Figure 2. (A) Uncompetitive mode of the inhibition of α-amylase by EA fraction of RDS. (B) Uncompetitive mode of the inhibition of α-GSD by EA fraction of RDS. [Click here to view] |
Alpha-amylase and α-GSD enzymes are prominent enzymes involved in the persistent hydrolysis of CHOs to glucose (Mayur et al., 2010). The peak treatment in the management of blood glucose during postprandial hyperglycemia for diabetic patients is the inhibition of the two enzymes. The inhibition of α-AL and α-GSD enzymes was used in this study to evaluate the antidiabetic potential of RD leaf and stem crude extracts and fractions. Besides, a good antihyperglycemic substance that could serve as an alternative treatment for synthetic drugs such as acarbose known with side effects, such as gastrointestinal disturbances, should be mild α-AL and strong α-GSD inhibitors. In line with the aforementioned, DCMF (leaf) showed a mild inhibition of α-AL depicting 500.40 ± 2.87 μg/ml going by its half-maximal IC50 value and strong inhibition of α-GSD (204.00 ± 8.12 μg/ml) better than acarbose (IC50, 265.20 ± 6.00 μg/ml). Similarly, EAF (stem) showed a mild inhibition of α-AL (405.90 ± 10.41 μg/ml) and strong inhibition of α-GSD (363.50 ± 8.48 μg/ml). Hence, these fractions reflecting good antidiabetic potentials of the plants were studied for the kinetics of inhibition of the two enzymes.
The kinetics of inhibition of the enzymes depicted by the double-reciprocal plot indicated uncompetitive inhibitions of α-AL and α-GSD enzymes, which is evident from the reduction in Vmax and Km values for the stem. This suggests that the extract bioactives bind to other position close to the enzyme active site forming complexes with available enzyme (FE) or enzyme substrate (ES), thus having an effect on the activity of both FE and ES (Balogun and Ashafa, 2017) and thereby retarding the activities of these enzymes in breaking down CHOs to glucose. Moreover, the non-competitive inhibition of α-AL for the leaf signifies the binding at other sites instead of the active site of the enzymes. However, the competitive α-GSD inhibition of the leaf indicates that the active components of the leaf extract bind at the active site of the enzyme, thus preventing the binding of the substrate, which is indicative of the fact that the extract has the potential at slowing the constant hydrolysis of disaccharides to glucose. Above all, the reports from this study revealed the presence of secondary metabolites such as saponins, phenols, and alkaloids, which could be responsible for the lowering effect of these extracts on α-AL and α-GSD due to their interaction singly or in combination. Kwon et al. (2007) reported that plants’ secondary metabolites are effective inhibitors of α-GSD. Furthermore, Kumar et al. (2011) also reported the inhibition of α-GSD by alkaloids and phenols from medicinal plants while the antihyperglycemic potential of alkaloids and saponins from plants are well documented (El-Barky et al., 2017; Elekofehinti, 2015; Yin et al., 2014).
CONCLUSION
In summary, this study revealed the presence of phytochemicals from the leaf and stem of R. dentata, the antioxidant property, the anti-inflammatory as well as their anticholinesterase and anti-diabetic potentials. Further works are ongoing and centered toward isolating and identifying important anti-inflammatory, anticholinesterase, and antidiabetic compounds from the leaf of R. dentata.
AUTHORS’ CONTRIBUTIONS
I.O. did most of the experiments and wrote the manuscript draft. M.S. supervised antioxidant and anticholinesterase experiments. F.B supervised the antidiabetic experiments and manuscript draft. S.A. performed the anti-inflammatory. A.O.T supervised the antidiabetic and anti-inflammatory experiments. All the authors revised the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the Association of African Universities for the small grant for thesis and dissertation given to Ibukun Oresanya and the African-German Network of Excellence in Science for granting a Mobility Grant (2017) to Ibukun Ayoola sponsored by German Federal Ministry of Education and Research as well as the Alexander von Humboldt Foundation. The authors also acknowledge the Directorate Research and Development of the University of the Free State for the postdoctoral research fellowship granted Drs. FO Balogun and S Adebayo tenable at phytomedicine and phytopharmacology research group, Department of Plant Sciences.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
None.
REFERENCES
Adebayo SA, Dzoyem JP, Shai LJ, Eloff JN. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement Altern Med, 2015; 15:159–69. CrossRef
Ali H, Houghton PJ, Soumyanath A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol, 2006; 107(3):449–55. CrossRef
Attah AF, Hellinger R, Sonibare MA, Moody JO, Arrow Smith S, Wray S, Gruber CW. Ethnobotanical survey of Rinorea dentata (Violaceae) used in South-Western Nigerian ethnomedicine and detection of cyclotides. J Ethnopharmacol, 2016; 179:83–91. CrossRef
Ayoola IO, Gueye B, Sonibare MA, Abberton MT. Antioxidant activity and acetylcholinesterase inhibition of field and in vitro grown Musa L. species. J Food Meas Charact, 2017; 11:488–99. CrossRef
Bailey CJ. New approaches to the pharmacotherapy of diabetes. Textbook of Diabetes. 3rd edition, vol. 2. Blackwell Science Ltd, Hoboken, NJ, pp 1–73, 2003.
Balogun FO, Ashafa AOT. Aqueous root extract of Dicoma anomala (Sond.) extenuates postprandial hyperglycaemia in vitro and its modulation on the activities of carbohydrate-metabolism enzymes in streptozotocin–induced diabetic Wistar rats. S Afr J Bot, 2017; 112:102–12. CrossRef
Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci, 2000; 85:141–7. CrossRef
Burkill HM. The useful plants of West tropical Africa: families S–Z. vol. 5. Royal Botanic Gardens, London, UK, 2000.
Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta, 2014; 1842(9):1693–706. CrossRef
Chuyong GB, Kenfack D, Harms KE, Thomas DW, Condit R, Comita LS. Habitat specificity and diversity of tree species in an African wet tropical forest. Plant Ecol, 2011; 212(8):1363–74. CrossRef
Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, Ferrara F, Novo S. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb, 2010; 17(1):1–11. CrossRef
Crowson CS, Liao KP, Davis JM III, Solomon DH, Matteson EL, Knutson KL, Hlatky MA, Gabriel SE. Rheumatoid arthritis and cardiovascular disease. Am Heart J, 2013; 166:622–8. CrossRef
Dani C, Oliboni LS, Agostini F, Funchal C, Serafini L, Henriques JA, Salvador M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol In Vitro, 2010; 24(1):148–53. CrossRef
Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases progress in lipids. Prog Lipid Res, 2011; 50(1):115–31. CrossRef
Dzoyem JP, Eloff JN. Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J Ethnopharmacol, 2015; 160:194–201. CrossRef
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami B. Antioxidant and Antihemolytic potentials of Physospermum cornobiense (L.) DC. Pharmacologyonline 2009; 1:1318–23.
El-Barky AR, Hussein SA, Eldeen AA, Hafez A, Mohamed TM. Saponins and their potential role in diabetes mellitus. Diabetes Mgt, 2017; 7(1):148–58.
Elekofehinti OO. Saponins: anti-diabetic principles from medicinal plants-A review. Pathophysiology, 2015; 22(2):95–103; doi:10.1016/j.pathophys.2015.02.001 CrossRef
Elufioye TO, Obuotor EM, Sennuga AT, Agbedahunsi JM, Adesanya SA. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some selected Nigerian medicinal plants. Rev Bras Farmacogn, 2010; 20(4):472–7. CrossRef
Erhenhi AH, Obadoni BO. Known medicinal and aphrodisiac plants of Urhonigbe forest reserve, Edo State. Nigeria J Med Plants Stud, 2015; 3(4):101–6.
Hart F, Boardman P. Indomethacin: a new non-steroid anti-inflammatory agent. Br Med J, 1963; 2(5363):965–70. CrossRef
Jiofack T, Fokunang C, Kemeuze V, Fongnzossie E, Tsabang N, Nkuinkeu R, Mapongmetsem PM, Nkongmeneck BA. Ethnobotany and phytopharmacopoea of the South-West ethnoecological region of Cameroon. J Med Plants Res, 2008; 2(8):197–206.
Joubert PH, Venter HL, Foukaridis GN. The effect of miglitol and acarbose after an oral glucose load: a novel hypoglycaemic mechanism. Br J Clin Pharmacol, 1990; 30:391–6. CrossRef
Khatoon M, Islam E, Islam R, Abdur Rahman A, Alam k. Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Res Notes, 2013; 6:121–7. CrossRef
Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI. Inhibitory effects of pine bark extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition, 2005; 21(6):756–61. CrossRef
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65:385–411. CrossRef
Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev, 2011; 5(9):21–9. CrossRef
Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food, 2007; 10(2):266–75. CrossRef
Kwon YI, Apostolidis E, Shetty K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J Food Biochem, 2008; 32:15–31. CrossRef
Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc, 1934; 56:658–66. CrossRef
Mathew M, Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of Ayurvedic medicinal plants used for cognitive disorders. PLoS One, 2014; 9(1):e86804; doi:10.1371/journal.pone.0086804 CrossRef
Mayur B, Sandesh S, Shruti S, Sung-Yum S. Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L. J Med Plant Res, 2010; 4:1547–53.
Mccue P, Shetty K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asian Pac J Clin Nutr, 2004; 13:101–6.
Mukherjee PK, Kumar V, Mal M, Houghton PJ, Acetylcholinesterase inhibitors from plants. Phytomedicine, 2007; 14(4):289–300. CrossRef
Musa KH, Abdullah A, Jusoh K, Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Analytical Methods, 2011; 4(1): 100–7. CrossRef
Muthuraman A, Sood S, Kumar SS. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology, 2011; 19:327–34. CrossRef
Ojo LO. The fate of a tropical rainforest in Nigeria: abeku sector of omo forest reserve. Global Nest J, 2004; 6:116–30. CrossRef
Porta H, Rocha-Sosa M. Plant lipoxygenases, physiological and molecular features. Plant Physiol, 2002; 130:15–21. CrossRef
Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K. Phytochemical screening and extraction: a review. Int J Pharm Sci, 2011; 1(1):98–106.
Rabasa-Lhoret R, Chiasson JL. Alpha-Glucosidase Inhibitors. In: Defronzo RA, Ferrannini E, Keen H, Zimmet P (eds.). International textbook of diabetes mellitus. John Wiley, London, UK, 2004.
Radmark O, Samuelsson B. 5-Lipoxygenase: regulation and possible involvement in atherosclerosis. Prostaglandins and Other Lipid Mediators. J Lipid Res, 2007; 83:162–74. CrossRef
Schneider I, Bucar F. Lipoxygenase inhibitors from natural plant sources. Part 1: medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase [sol] cyclooxygenase. Phytother Res, 2005; 19:81–102. CrossRef
Sofowora A. Medical plants and traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria, pp 191–289, 2006.
Sonibare MA, Ayoola IO, Gueye B, Abberton MT, D'Souza R, Kuhnert N. Leaves metabolomic profiling of Musa acuminata accessions using UPLC – QTOF – MS/MS and their antioxidant activity. J Food Meas Charact, 2018; 12:1093–106. CrossRef
Sonibare MA, Ayoola IO. Medicinal plants used in the treatment of neurodegenerative disorders in some parts of Southwest Nigeria. Afr J Pharm Pharmacol, 2015: 9(38):956–65. CrossRef
Susanti D, Sirat HM, Ahmad F, Ali RM, Aimi N, Kitajima M. Antioxidant and cytotoxic flavonoids from the flowers of Melastomama labathricum L. Food Chem, 2007; 103:710–6. CrossRef
Yadav JP, Saini S, Kalia AN, Dangi AS. Hypoglycemic and hypolipidemic activity of ethanolic extract of Salvadora oleoides in normal and alloxan-induced diabetic rats. Ind J Pharmacol, 2008; 40(1):23–7. CrossRef
Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Human Wellness, 2014; 3:136–74. CrossRef