INTRODUCTION
According to the World Health Organization (WHO) (Cancer WHO, 2019; Lang-Schwarz et al., 2019), cancer today is one of the major causes of death among the world’s population; in 2018, the number of deaths was 9.6 million people. Recently, the new methods of treating oncological diseases have appeared (nanotherapy, neutron capture, and low-intensity electroresonance therapy), and besides, classical methods are used — chemotherapy, surgery, and radiation therapy. However, all of the above methods are accompanied by a number of side effects, which also negatively affect the patient’s health. Therefore, the development of the cancer drugs from the plant materials and their use in the clinical practice are still today an urgent task (Desai et al., 2008; Sawadogo et al., 2012; Wang et al., 2019). More than 60% of drugs based on the natural products or herbal raw material are currently used for the treatment of cancer (Majolo et al., 2019; Solowey et al., 2014). The recent scientific studies show that various food plants, medicinal plants, and spices contain the primary and secondary metabolites that help in the treatment of cancer (Asowata-Ayodele et al., 2016; Deng et al., 2002). A study of the pharmacological activity of the plant extracts and their biologically active components, namely, piperine, curcumin, thymoquinone, crocin, capsaicin, oenothein B (Li et al., 2018; Zheng et al., 2016), polysaccharides (Ma et al., 2019), polyphenols (Wang et al., 2019), and other bioactive metabolites (Bobach et al., 2014), has demonstrated their anticancer, antibacterial, anti-inflammatory, antioxidant, and immunomodulatory effects. The presence of the above activities ensures the effectiveness of the prevention and treatment of different cancer types such as lung, stomach, skin, breast, cervix, liver, and prostate cancers. Currently, more than 3,000 plants worldwide have anticancer properties. From the scientific viewpoint, the idea of the anticancer herbal raw materials searching is not new, but for the correct user, the detailed phytochemical and pharmacological studies are always necessary. Thus, the present research results undoubtedly provide new knowledge about the anticancer effect of different dry extracts of plants from Iridaceae family (Crocus, Iris, Gladiolus, and Juno genus) of Ukrainian flora.
The diverse study of Iridaceae plants indicates their potential as anticancer agents. The phytochemical research of Iridaceae plants has shown the presence of the phenolic compounds (isoflavones, flavones, flavanones, xanthones, and hydroxycinnamic acids) (Mykhailenko et al., 2019; Singab et al., 2016) as well as polysaccharides and amino acids (Mykhailenko et al., 2020), which possess the presence of the anti-proliferative activity (Alireza et al., 2016; Asif et al., 2013; Gohari et al., 2013; Xie et al., 2014). Different plant extracts and biological active compounds of Iridaceae members have shown different pharmacological activities such as diuretic, anti-inflammatory, antiradical (Jadouali et al., 2019), hepatoprotective, antidepressant, and hypolipidemic activities (Basgedik et al., 2014; Munyemana et al., 2013; Shinwari and Rao, 2018; Singab et al., 2016) as well as immunomodulating, antimutagenic (Kuete et al., 2013; Samarghandian and Borji, 2014), antibacterial (Ayoub et al., 2014), and antitumor activities (Mykhailenko et al., 2019; Wollenweber et al., 2003; Xie et al., 2014). To the best of our knowledge, those plants have not been studied for anticancer activity on leukemia and carcinoma cells in vitro. There are a large number of articles on the antitumor potential of the individual components of Crocus sativus, such as crocin, crocetin, safranal, and picrocrocin; besides, publications on the proliferative activity of an extract from C. sativus stigma (saffron) are presented (Samarghandian and Borji, 2014). However, the effect of C. sativus extracts (stigma, leaves, leaves, and flowers) on the presented in vitro cell lines (MCF-7, HepG2, HCT116, HeLa, HL-60, and HaCaT) was not evaluated. A study of the pharmacological potential of Gladiolus and Juno plants has never been done. The present study is aimed to preliminary evaluate the anticancer potential activities against six cancer cell lines of the different tissue origin and against non-tumor cells in vitro.
MATERIALS AND METHODS
Plant material
Juno bucharica (Foster) Vved. leaves (a voucher specimen CWU0056539) and Gladiolus hybrid Zefir leaves (a voucher specimen CWU0056538) as well as Iris hungarica Waldst. et Kit. rhizomes (a voucher specimen CWU0056534) were harvested from the collection of botanical gardens of V.N. Karazin Kharkiv National University (Kharkiv, Ukraine) in May 2017. C. sativus stigmas, flowers, leaves, and corms were collected from the plantation in the village Lyubymivka (Ukraine) in November 2017 (a voucher specimen CWU0056541–CWU005654). All voucher specimens were verified by Dr. Gamulya and saved at the Herbarium of V.M. Karazin Kharkiv National University, Kharkiv, Ukraine. Fresh plant material was air-dried and crushed.
Preparation of extracts
The ground plant material of Juno leaves, Gladiolus leaves, Crocus leaves, flowers, and corms, and Iris rhizome (100 g each) was extracted with distilled water (1 l), on a water bath for 2 hours for three times. These extracts were cooled and filtered. The extracts were first concentrated using Rotavapor and then completely dried in drying cabinet and preserved at 4°C until further use. Crocus stigma powder (5 g) was poured with hot distilled water (500 ml, 80°C) and infused in a dark place for 24 hours. The resulting extract was drained, and the rest of the raw material was refilled with 500 ml of distilled water and left to maceration for another 24 hours at 4°C. Maceration repeated one more time in the same conditions. The obtained extracts were combined, filtered, and dried in a rotary evaporation apparatus at 80°C. The powders were stored in airtight containers at 4°C for further use.
MTT assay
The antineoplastic activity of plant extracts was established by the MTT test (EZ4U, Biomedica, Austria) (Finiuk et al., 2017) on different tissue origin cell lines. For the experiment, the cells were plated in 96-well plates of 100 μl at the concentrations of 5,000 cells/well (substrate-dependent cells) or 10,000–15,000 cells/well (suspension cells) overnight. Then, aliquots of 100 μl of experimental extracts (0, 1, 10, and 100 μg/ml) as well as the reference compound doxorubicin (0, 1, and 10 μg/ml) in culture medium were added to the cells and incubated for the next 72 hours. Furthermore, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT reagent) was appended to the cells following the manufacture recommendations (Biomedica, Austria). The results of the reaction were determined by an Absorbance Reader Bio Tek ELx800 (BioTek Instruments, Inc., Winooski, VT) at 490 and 630 nm. The relative number of living cells (parts per unit) was determined by the ratio of optical densities measured for the wells with tested compounds to the wells with control (non-treated) cells. The half-maximal inhibitory concentration (IC50) of tested extracts was calculated as the extract concentration that reduced the cell viability to 50%. Doxorubicin (Actavis, Romania) was chosen as a reference drug as a positive control. The extracts were added to the cultured cells at the indicated concentrations (0–100 μg/ml) and then were treated for 72 hours. The results were analyzed and illustrated using GraphPad Prism 6 software (GraphPad Software., San Jose, CA). The obtained data are presented as the average ± standard deviation (SD) of three replications in two parallels (n = 6).
Cell culture
Collection sample of HL-60 human myeloid leukemia cells (acute promyelocytic leukemia) was obtained from the Institute of Cancer Research at Vienna Medical University (Vienna, Austria). HepG2 human hepatocarcinoma cells (liver hepatocellular carcinoma), HCT116 human colon carcinoma cells (human colorectal carcinoma), and the collected sample of HaCaT human cell (normal human epidermal keratinocytes) were received from the Institute of Molecular Biology and Genetics, National Academy of Sciences of Ukraine (Kyiv, Ukraine). Collection samples of HeLa human cervix adenocarcinoma cells (cervical adenocarcinoma) and MCF-7 human breast adenocarcinoma cells (Michigan Cancer Foundation-7) were received from the R.E. Kavetsky Institute of Experimental Pathology, Oncology, and Radiobiology (Kyiv, Ukraine). All cells were grown in RPMI-1640 or Dulbecco’s Modified Eagle’s Medium culture supplemented with 10% fetal bovine serum (all from BioWest, France). Cells were cultivated at 37°C in an atmosphere of 5% CO2.
RESULTS AND DISCUSSION
An in vitro screening was performed by the MTT assay of the anticancer activity of Iridaceae dry extracts toward to the following cell lines: MCF-7 human breast adenocarcinoma cells, HCT116 human colon adenocarcinoma cells, HeLa cervical adenocarcinoma cells, HepG2 hepatocellular carcinoma cells, HL-60 acute promyelocytic leukemia cells, and HaCaT normal human epidermal keratinocytes cells was performed by the MTT assay. The results of the analysis were expressed as IC50 and shown in Table 1. The Iridaceae extracts have shown different antiproliferative activities. Compared to the doxorubicin, reference drug, the activity of those extracts was lower; however, some extracts had a micromolar value of the IC50 (Fig. 1).
The obtained data showed that the human acute promyelocytic leukemia cells of HL-60 line were the most sensitive to the action of all the studied plant extracts. The dry Gladiolus leaf extracts (Table 1 and Fig. 1) had the highest toxicity toward leukemia cells with the IC50 value of 0.5 μg/ml. Juno leaf extracts and Iris rhizome extract demonstrated similar anticancer activity with the IC50 of 3.5 and 3.6 μg/ml, respectively. The IC50 value for Crocus corms extract was 7.4 μg/ml. The extracts of Crocus stigma, flowers, and leaves did not attain the IC50 value even at 100 μg/ml. Previously, the antitumor effect of crocin from Crocus stigma on human leukemia cells of HL-60 was studied but not for the whole extract and not to myeloid leukemia cell line (Sun et al., 2013). Thus, the potential anticancer activity of Iridaceae extracts on leukemia cells of HL-60 line can be placed in the following row: Gladiolus leaves > Juno leaves = Iris rhizome > Crocus corms.
Human colon adenocarcinoma cells of HCT116 line were also sensitive to the activity of plant extracts. The IC50 for Gladiolus leaf extract was 9.4 μg/ml, for Iris rhizome was 42.3 μg/ml, for Crocus corms was 74.6 μg/ml, and for Crocus stigma was 94.8 μg/ml. It was reported about the effect of saffron extract (0.2–1 mg/ml) in HCT116 colorectal cancer cells (Bukhari et al., 2018), but this work includes only Crocus stigma extract. According to the results of the study, the IC50 values for stigma extract ranged between 0.58 and 0.98 mg/ml. It should be noted that the composition of the active components of the raw material has a direct effect on the activity of the extracts (Mykhailenko et al., 2020). Moreover, the component composition may vary depending on the location of the plant cultivation (Ramakrishna and Ravishankar, 2011). This is probably why saffron stigma extract from Ukraine has a slightly higher activity than the extract from Kashmir (Bukhari et al., 2018). The extracts of Juno leaves and Crocus flowers and leaves did not attain the IC50 value even at 100 μg/ml (Table 1 and Fig. 1). In this case, the most active sample was also Gladiolus leaf extract.
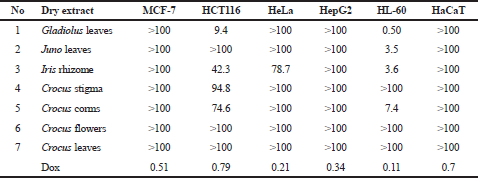 | Table 1. Antiproliferative activity of tested extracts, IC50, μg/ml. [Click here to view] |
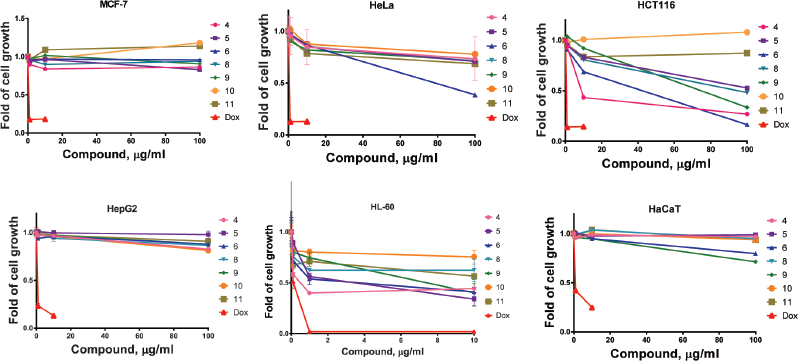 | Figure 1. Cytotoxicity of all tested dry extracts toward different cell lines. After a total experimental time (72 hours), cell vitality was detected by the MTT assay. 1—Gladiolus leaf extract; 2—Juno leaf extract; 3—Iris rhizome extract; 4—Crocus stigma extract; 5—Crocus corm extract; 6—Crocus flower extract; 7—Crocus leaf extract. [Click here to view] |
Iris rhizome extract demonstrated toxicity toward human cervical adenocarcinoma of HeLa line with the IC50 of 78.7 μg/ml. Other studied plant extracts were not toxic to HeLa cells (Table 1 and Fig. 1). MCF-7 human breast adenocarcinoma cells and HepG2 human hepatocellular carcinoma cells showed the weakest sensitivity to the extracts of the studied plants. The tested extracts did not have an IC50 value of 100 μg/ml. Doxorubicin showed a higher cytotoxic effect toward all studied cell lines if compared to the activity of plant extracts.
It should be noted that the studied plants’ extracts were not toxic to normal human epidermal keratinocytes of HaCaT line. The maximal inhibition of the growth of HaCaT cells equaled 28.6% at the action of Crocus corms extract and minimal at the action of Juno leaf extract (Table 1 and Fig. 1). The IC50 value of doxorubicin for HaCaT cells was 0.7 μg/ml. Thus, Gladiolus leaf and Iris rhizome extracts demonstrated the highest cytotoxic effect toward the HL-60 human acute promyelocytic leukemia cells and HCT116 colon adenocarcinoma cells. Previously, for C. sativus flower acetone extract (50–200 μg/ml), the concentration-dependent activity on HaCaT cells was established (Verjee et al., 2017). However, the acetone flower extract has a more toxic solvent than the aqueous extract. In the following studies, we need to increase the dose of Iridaceae plant extract and repeat the experiment, as other authors also take a pre-high concentration of plant extract.
In the current investigation, several species of Iridaceae genus plants have been tested for their anticancer and cytotoxic activity. Kuete et al. (2013) evaluated the cytotoxicity of the methanol extract of Gladiolus quartinianus (40 g/ml) against CCRF-CEM leukemia cells, which exhibited the lowest IC50 values below 30 μg/ml. Besides, the induction of apoptosis in CCRF-CEM cells with the loss of mitochondrial membrane potential for G. quartinianus extract was found. The methanol extract of Iris kashmiriana rhizome (400 mg/ml) showed a potent cytotoxic effect against A549 human epithelial cancer cell lines and Caco-2 epithelial cell lines with IC50 of 128 and 237 mg/ml, respectively (Asif et al., 2013). Sánchez-Vioque et al. (2016) studied the antiproliferative effect of ethanol extracts from C. sativus corms, flowers, and leaves on human colon adenocarcinoma cells (Caco-2). From their results, the extract of the flower and leaves (ED5 0.42 mg/ml) greatly inhibited the activity of Caco-2 cells. In other investigations, C. sativus stigma aqueous extract inhibited cancer progression dose dependently in doses of 100, 150, and 175 mg/kg (Bathaie et al., 2013). Previously, Parizadeh et al. (2011) isolated a glycoconjugate from saffron corms, for which cytotoxic activity in relation to the HepG-2 hepatocellular carcinoma cell line and Hep-2 laryngeal carcinoma cell line was also established. Another study found the cytotoxic effects of crocin on HeLa and MCF-7 cell lines (Mousavi et al., 2011). However, the presented studies relate mainly to the individual compounds from C. sativus and its stigma.
For the first time, the anticancer activity of J. bucharica leaves, G. hybrid Zefir leaves, I. hungarica rhizomes as well as C. sativus raw material extracts was investigated on MCF-7 human breast cancer cell line, HepG2 human hepatocellular carcinoma cell line, HCT116 human colon adenocarcinoma cell line, HeLa cervical adenocarcinoma cell line, HL-60 acute promyelocytic leukemia cells line, and HaCaT normal human epidermal keratinocyte cell line. Thus, the results show the potential of Iridaceae plants for the treatment of cancer, but further research is still needed.
CONCLUSION
In folk and clinical medicine of different countries, plants have always played an important role in the treatment and prevention of cancer and other diseases. Moreover, natural components from plants at the level of synthetic compounds are used in the creation of new drugs. Plants of Iridaceae family (J. bucharica, G. hybrid Zefir, I. hungarica, and C. sativus) are herbaceous plants traditionally used in folk medicines of European countries as antitumor, anti-inflammatory, anticonvulsant, analgesic, antioxidative activity, antipyretic, etc. Gladiolus leaf extract showed particularly strong anticancer properties in vitro toward human leukemia and colon adenocarcinoma cells in MTT assay. Iris rhizome dry extracts have shown lower activity on human cervical adenocarcinoma cells. These plant extracts have demonstrated low toxicity toward normal human epidermal keratinocytes of HaCaT line. The studies on establishing the action mechanism of the investigated extracts will be continued. These herbal extracts have shown their potential anticancer activity in vitro studies that can be used for further drug creation for the prevention and treatment of leukemia, colon, and cervix cancers. This is the first investigation about the anticancer activity of Iridaceae plant extracts.
ACKNOWLEDGMENT
The authors would like to gratitude to Dr. Orlova (Botanical garden of the Kharkiv National University named after V.N. Karazin, Kharkiv, Ukraine) for help in determining the systematic belonging of plants.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
FINANCIAL SUPPORT
None.
REFERENCES
Alireza M, Kurosh D, Banafshe H. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J Nutr Intermed Metab, 2016; 3:23–32. CrossRef
Asif A, Sajad HW, Taseem AM, Shoiab B, Asrar HW, Javid MI, Qazi PH, Qadri RA. Investigating the pharmacological potential of Iris kashmiriana in limiting growth of epithelial tumors. Pharmacognosy J, 2013; 5:170–5. CrossRef
Asowata-Ayodele AM, Afolayan AJ, Otunola GA. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. S Afr J Bot, 2016; 104:69–75. CrossRef
Ayoub IM, El-Shazly M, Lu MC, Singab ANB. Antimicrobial and cytotoxic activities of the crude extracts of Dietes bicolor leaves, flowers and rhizomes. S Afr J Bot, 2014; 95:97–101. CrossRef
Basgedik B, Ugurb A, Sarac N. Antimicrobial, antioxidant, and antimutagenic activities of Gladiolus illyricus. J Pharm Pharmacogn Res, 2014; 2:93–9.
Bathaie SZ, Miri H, Mohagheghi MA, Mokhtari-Dizaji M, Shahbazfar AA, Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the wistar albino rat. Iran J Basic Med Sci, 2013; 16:27.
Bobach C, Schurwanz J, Franke K, Denkert A, Sung TV, Kuster R, Mutiso PC, Seliger B, Wessjohann LA. Multiple readout assay for hormonal (androgenic and antiandrogenic) and cytotoxic activity of plant and fungal extracts based on differential prostate cancer cell line behavior. J Ethnopharmacol, 2014; 155:721–30. CrossRef
Bukhari SI, Din I, Grewal S, Dhar MK. Antiproliferative effect of saffron and its constituents on different cancerous cell lines. Pharmacogn Res, 2018; 10:291–5. CrossRef
Cancer. WHO, Geneva, Switzerland, 2019. Available via https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed 19 November 2019).
Deng Y, Guo Z, Zeng Z, Wang Z. Studies on the pharmacological effects of saffron (Crocus sativus L.) a review. Zhongguo Zhong Yao Za Zhi, 2002; 27:565–8.
Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK. Medicinal plants and cancer chemoprevention. Curr Drug Metabol, 2008; 9:581–91. CrossRef
Finiuk N, Boiko N, Klyuchivska O, Kobylinska L, Kril I, Zimenkovsky B, Lesyk R, Stoika R. 4-Thiazolidinone derivative Les-3833 effectively inhibits viability of human melanoma cells through activating apoptotic mechanisms. Croat Med J, 2017; 58:129–39. CrossRef
Gohari AR, Saeidnia S, Mahmoodabadi MK. An overview on saffron, phytochemicals, and medicinal properties. Pharmacognosy Rev, 2013; 7:61–6. CrossRef
Jadouali SM, Rachid HA, Majourhat MK, Abdelatif ZB, Faouzi LA. Chemical characterization and antioxidant compounds of flower parts of Moroccan Crocus sativus L. J Saudi Society Agricult Sci, 2019; 18:476–80. CrossRef
Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-resistant tumor cells. Evid Based Compl Alt Med, 2013; 2013: Article ID 285903, 10. CrossRef
Lang-Schwarz C, Melcher B, Haumaier F, Schneider-Fuchs A, Lang-Schwarz K, Krugmann J, Vieth M, Sterlacci W. Budding, tumor-infiltrating lymphocytes, gland formation: scoring leads to new prognostic groups in World Health Organization low-grade colorectal cancer with impact on survival. Hum Pathol, 2019; 89:81–9. CrossRef
Li H. Krstin S, Wang S, Wink M. Capsaicin and piperine can overcome multidrug resistance in cancer cells to doxorubicin. Molecules, 2018; 2:557–68. CrossRef
Ma L, Xu GB, Tang X, Zhang C, Zhao W, Wang J, Chen H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J Funct Foods, 2019; 64:103677. CrossRef
Majolo F. Delwing LK de Oliveira B, Marmitt DJ, Bustamante-Filho IC, Goettert MI. Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochem Lett, 2019; 31:196–207. CrossRef
Mousavi SH, Moallem SA, Mehri S, Shahsavand S, Nassirli H, Malaekeh-Nikouei B. Improvement of cytotoxic and apoptogenic properties of crocin in cancer cell lines by its nanoliposomal form. Pharm Biol, 2011; 49:1039–45. CrossRef
Munyemana F, Mondego AP, Cumbane P. Qualitative phytochemical screening and antimicrobial activity evaluation of the bulb extracts of Gladiolus psittacinus Hook (Iridaceae). Int Net Env Manag Conflicts, 2013; 2:14–31.
Mykhailenko O, Ivanauskas L, Bezruk I, Lesyk R, Georgiyants V. Comparative investigation of amino acids content in the dry extracts of Juno bucharica, Gladiolus hybrid Zefir, Iris hungarica, Iris variegata and Crocus sativus raw materials of Ukrainian flora. Sci Pharm, 2020, 88:1–13. CrossRef
Mykhailenko O, Kovalyov V, Goryacha O, Ivanauskas L, Georgiyants V. Biologically active compounds and pharmacological activities of species of the genus Crocus: a review. Phytochemistry, 2019; 162:56–89. CrossRef
Parizadeh MR, Gharib FG, Abbaspour AR, Afshar JT, Ghayour-Mobarhan M. Effects of aqueous saffron extract on nitric oxide production by two human carcinoma cell lines: Hepatocellular carcinoma (HepG2) and laryngeal carcinoma (Hep2). Avicenna J Phytomed, 2011; 1:43–50.
Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav, 2011; 6:1720–31. CrossRef
Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res, 2014; 6:99–107. CrossRef
Sánchez-Vioque R, Santana-Méridas O, Polissiou M, Vioque J, Astraka K, Alaizd M, Herraiz-Peñalvera D, Tarantilis PA, Girón-Calle J. Polyphenol composition and in vitro antiproliferative effect of corm, tepal and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2). J Funct Foods, 2016; 24:18–25. CrossRef
Sawadogo WR, Schumacher M, Teiten MH, Dicato M, Diederich M. Traditional west African pharmacopeia, plants and derived compounds for cancer therapy. Biochem Pharmacol, 2012; 84:1225–40. CrossRef
Shinwari KJ, Rao PS. Thermal-assisted high hydrostatic pressure extraction of nutraceuticals from saffron (Crocus sativus): Process optimization and cytotoxicity evaluation against cancer cells. Innov Food Sci Emerg Technol, 2018; 48:296–303. CrossRef
Singab ANB, Ayoub IM, El-Shazly M, Korinek M, Wu TY, Cheng YB, Chang FR, Wu YC. Shedding the light on Iridaceae: ethnobotany, phytochemistry and biological activity. Ind Crop Prod, 2016; 92:308–35. CrossRef
Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. Sci World J, 2014; 2014:721402–14 CrossRef
Sun Y, Xu HJ, Zhao YX, Wang LZ, Sun LR, Wang Z, Su XF. Crocin exhibits antitumor effects on human leukemia HL-60 cells in vitro and in vivo. Evid Based Complement Alternat Med, 2013; 3:690164. CrossRef
Verjee S, Garo E, Pelaez S, Fertig O, Hamburger M, Butterweck V. Saffron flower extract promotes scratch wound closure of keratinocytes and enhances VEGF production. Planta Med, 2017; 83:1176–83. CrossRef
Wang X, Chen Y, Fang Z. In-vitro photothermal therapy using plant extract polyphenols functionalized graphene sheets for treatment of lung cancer. J Photochem Photobioi B: Biology, 2019; 204:111587–601. CrossRef
Wollenweber E, Stevens JF, Klimo K, Knauft J, Frank N, Gerhauser C. Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica. Planta Med, 2003; 69:15–20. CrossRef
Xie GY, Zhu Y, Shu P, Qin XY, Wu G, Wang Q, Qin MJ. Phenolic metabolite profiles and antioxidants assay of three Iridaceae medicinal plants for traditional Chinese medicine “She-gan” by on-line HPLC–DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS. J Pharmaceut Biomel Anal, 2014, 98:40–51. CrossRef
Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for prevention and treatment of cancers. Nutrients, 2016; 8:495–530. CrossRef