INTRODUCTION
Piper nigrum (black pepper; Piperaceae family) and its active component piperine as shown in Figure 1 have been used in Indonesian traditional medicine to alleviate pain in the neck and throat. The medicine is usually prepared by mixing a teaspoon of honey with a few amounts of black pepper in a half cup of warm water.
A recent study reported that piperine, a nitrogenous substance contained in the fruit of this plant, at doses of 10 and 15 mg/kg body weight (BW) exerted anti-inflammatory effect after 30 minutes (and lasted in 60 minutes) on carrageenan-induced paw inflammation in rats, whereas the hexane and ethanol extracts of P. nigrum produced a similar activity at 10 mg/kg BW but lasted for longer time (Tasleem et al., 2014). The dichloromethane fraction of P. nigrum (dose of 100 mg and 200 mg/kg BW) exhibits anti-inflammatory activity by reducing the production of IL-1β, IL-6, and TNF-α in the cerebral cortical and hippocampal tissues of rats (Wang et al., 2017). Piperine inhibits the activation and translocation of NF-kappaB. This pungent compound blocks the phosphorylation and degradation of IkappaBα by attenuating TNF-α induced IkappaB kinase activity in endothelial cells (Kumar et al., 2007). Moreover, this compound significantly showed an anti-inflammatory effect by inhibiting the expression of IL6 and MMP13 and reduced the production of PGE2 in a dose-dependent manner (10–100 μg/ml) (Bang et al., 2009).
Piperine is a very weak base, which can be hydrolyzed by acid or base to volatile basic piperine (piperidine; C5H11N) and piperic acid (C12H10O4). However, this compound is slightly soluble or practically insoluble in water, which impacts its bioaccessibility. This insolubility of piperine underlies the development of piperine-contained preparations, which includes nanoformulations and encapsulations in lipid bodies (Gorgani et al., 2017). Similarly, another method, e.g., complex formation, is widely used to increase both the water solubility and the stability of hydrophobic drugs. The complexing compounds that are often used in this technique are α-, b-, g-cyclodextrin (contain 6, 7, or 8 dextrose molecules bound in a 1,4 configuration to form rings of various diameters) (Patel et al., 2017). A recent study reported that a co-ground mixture of piperine and β-cyclodextrin (molar ratio of 1:1) revealed a significant increase (16 times) of dissolved piperine at 15 minutes of dissolution test compared to that of pure piperine (Ezawa et al., 2016). Nonetheless, there is a lack of such a study on the inclusion of piperine in carrageenan, and thus, this work studied the bioaccessibility of the carrageenan-complexed piperine in Wistar rats and assayed its anti-inflammatory activity on the edema-induced paw of the rats.
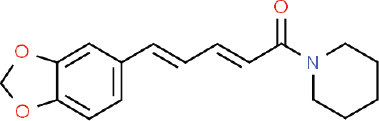 | Figure 1. 2D structure of piperine (C17H19NO3; the IUPAC name 1-(5-[1,3-benzodioxol-5-yl]-1-oxo-2,4-pentadienyl) piperidine; ChemSpider ID 553590). [Click here to view] |
MATERIALS AND METHODS
Materials
The piperine (isolated from P. nigrum) and carrageenan-complexed piperine (prepared by mixing isolated piperine with kappa-carrageenan paste) were provided by the colleagues at the Indonesian School of Pharmacy. The pure standard piperine was purchased from Tokyo Chemical Industry (TCI CAS RN 94-62-2; Product No. P0460). All other chemicals, e.g., sterile bidistilled water (Ikapharmindo, Indonesia), methanol high performance liquid chromatography (HPLC) grade (Fulltime), k-carrageenan (Sigma Aldrich), and other chemicals were of analytical grade and used without further purification.
Animals
A total of 65 healthy Wistar white male rats, age 2–3 months, weight 200–250 g, were purchased from D45 White Rats Experimental Animal Supplier, West Java, Indonesia, and were identified its strain at the Laboratory of Taxonomy, Department of Biology, Faculty Mathematics and Natural Sciences, Universitas Padjadjaran, Indonesia (Document No. 199/10/HB/2018). The rats were acclimatized for 7 days before treatment and housed in cages (30 cm × 24 cm × 10 cm) consisting of three animals per cage under standard laboratory conditions (26oC 2oC; 12 hours light/dark cycles). The rats were given free access to food and drink. All the experimental protocols conducted on rats were performed by following the internationally accepted principles for laboratory animal use and care and were approved by the Universitas Padjadjaran Research Ethics Committee (No. 1426/UN6.KEP/EC/2018).
Instruments
UV-visible spectrophotometer (Shimadzu UV-1800), RP-HPLC (Waters 1525 Binary HPLC) with C18 column (Phenomenex), digital plethysmometer (PLM-01 Plus), and digital analytical balance (Mettler Toledo Dragon 204) were used.
Determination of λmax of piperine and validation of the HPLC analytical method
Accurately weighed 10 mg of standard piperine/isolated piperine/carrageenan-complexed piperine was dissolved in methanol analytical grade to obtain 1,000 μg/ml. All piperine solutions were measured in methanol with a UV spectrophotometer scanning from 200 to 380 nm to obtain the λmax (Fig. 2). The solutions were diluted to serial concentrations and were injected into the C18 HPLC column (mobile phase: methanol–water 70:30; flow rate 10 minutes/minute) to calculate the standard curves. The validation of the analytical method was performed by employing the method proposed by Upadhyay et al. (2013).
Determination of Cmax and Tmax of carrageenan-complexed piperine in Wistar rats
The procedure employed was based on that of Shao et al. (2015) with a few modifications. All rats have fasted for 18 hours (with only free access to water) before drug administration. The rats were randomly divided into: (1) group I (n = 15; three rats for each time of sampling) was treated with piperine isolated from P. nigrum dose of 100 mg/kg BW, (2) group II (n = 15; three rats for each time of sampling) was treated with carrageenan-complexed piperine dose of 393 mg/kg BW (equivalent to piperine 100 mg/kg BW), and (3) group III (n = 15; three rats for each time of sampling) was treated with standard piperine dose of 100 mg/kg BW. All test drugs, e.g., piperine isolate, piperine standard, and carrageenan-complexed piperine, were administered to the rats by using oral gavage feeder. The blood samples (0.3 ml) were collected into the heparinized tubes from rats in each group at 0, 15, 30, 60, and 90 minutes. The blood was centrifuged for 15 minutes at 5,000 rpm (2,884 rcf; rotor diameter = 86 mm), and the supernatant was added with methanol and vortex-homogenized for 1 minute to precipitate the proteins. Finally, an aliquot of 20 μl of the methanolic plasma was injected into the C18 HPLC column (mobile phase: methanol–water 70:30; detection at 340 nm; and flow rate of 10 minutes/minute) (Shao et al., 2015). The maximum plasma concentration (Cmax) and the time to reach maximum plasma concentration (Tmax) were compiled from the blood data. The area under the drug concentration versus time curve was calculated from 0 to 90 minutes using the linear trapezoidal method.
Anti-inflammatory activity of carrageenan-complexed piperine in inflammatory-induced Wistar rats
The anti-inflammatory activity assay was carried out by applying a method proposed by Bang et al. (2009). All rats have fasted for 18 hours (with only free access to water) before drug administration. The rats were randomly divided into: (1) group I (n = 4), the negative control was treated with Arabic gum suspension 2%; (2) group II (n = 4), the positive control was treated with acetosal dose of 36 mg/kg BW; (3) group III (n = 4) was treated with piperine isolated from P. nigrum dose of 100 mg/kg BW; (4) group IV (n = 4) was treated with carrageenan-complexed piperine dose of 393 mg/kg BW (equivalent to piperine 100 mg/kg BW); and (5) group V (n = 4) was treated with piperine dose of 100 mg/kg BW. After 1 hour, carrageenan 1% was injected subcutaneously on the sole of the rat’s left foot. The measurement of edema was carried out at 0th-, 1st-, 2nd-, 3rd-, 4th-, 5th-, and 6th-hour post-inflammatory inducing by using a digital plethysmometer (PLM-01 Plus).
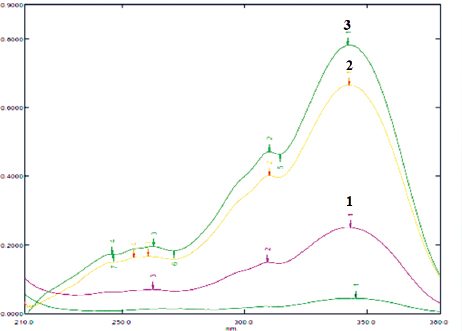 | Figure 2. The absorption spectra of carrageenan-complexed piperine (1), piperine isolated from P. nigrum (2), and standard piperine (3), in methanol analytical grade. The three samples show maxima at 342 nm. [Click here to view] |
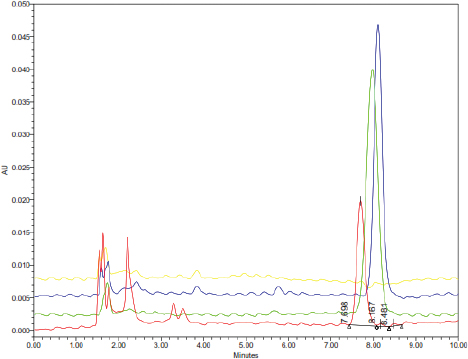 | Figure 3. The HPLC chromatograms of rat’s blood plasma (blank; yellow), carrageenan-complexed piperine (red; tR = 7.698 minutes), piperine isolated from P. nigrum (green; tR = 8.167 minutes), and standard piperine (blue; tR = 8.481 minutes), in methanol HPLC grade. [Click here to view] |
RESULTS AND DISCUSSION
The carrageenan-complexed piperine shows the maximum absorbance at 342 nm (Fig. 2) similar to that of piperine isolated from P. nigrum and standard piperine. This result corresponds with a previous study in Indian polyherbal formulations (Singh et al., 2011).
The carrageenan-complexed piperine is eluted at a shorter time (Fig. 3) than the isolate and the standard, due to its more polar property. This polar property is contributed by the kappa-carrageenan that possesses one sulfate substituent at C4 on the β-linked D-galactose residues. Kappa-carrageenan contains 25%–30% of sulfate ester and 28%–25% of anhydrogalactose units (Barbeyron et al., 2000). A previous study reported that an increase of piperine solubility was observed after this substance was co-ground with b-cyclodextrin in a molar ratio of 1:1. This more polar property was attributed to the interaction between the aromatic ring of piperine (which is lipophilic) and the cavity of β-cyclodextrin (Ezawa et al., 2016).
Validation of the HPLC analytical method revealed that the calibration curve for carrageenan-complexed piperine was linear over the range of 0.05–0.1 μg/ml in plasma (y = 728,755x + 3,683.4; r = 0.9947) with the lower limit of detection of 0.01315 and the lower limit of quantification of 0.04384 μg/ml. Meanwhile, the method proved a good accuracy (the extraction recovery was 100.00%) for carrageenan-complexed piperine.
Carrageenan-complexed piperine, given as a single oral dose of 393 mg/kg BW, could be quantified in the rat’s plasma. The maximum concentration (Cmax) was 0.34 μg/ml at 30 minutes, which is better than that of the isolated piperine from P. nigrum (Cmax = 0.12 μg/ml at 60 minutes). The standard piperine indicated the best bioaccessibility (Cmax = 0.48 μg/ml 30 minutes). However, the inclusion of piperine in the carrageenan complex resulted in faster elimination as proven by its plasma concentration at 60th minute compared to the other piperine (Fig. 4).
Despite its low solubility in water, the pharmacokinetics of a single oral dose of piperine (given as 100 mg of Benjakul tablet of Thai medicine) in 20 healthy Thai subjects revealed that Cmax = 467 ng/ml was reached at 1 hour (Jumpa-ngern et al., 2013). An intravenous administration of piperine lipid nanoparticles, positively charged stearyl amine, and pegylated lipid nanospheres (LN-P-PEG) of piperine in BALB/c mice showed that LN-P-PEG of piperine possessed the best pharmacokinetic parameters (Veerareddy and Vobalaboina, 2008).
The carrageenan-complexed piperine significantly reduces the edema on the inflammatory-induced paw rats (Fig. 5) compared to that of the negative control (p = 0.017). The anti-inflammatory activity of carrageenan-complexed piperine at a dose of 393 mg/kg BW (equivalent to 100 mg piperine) is not significantly different from that of the acetosal dose of 45 mg/kg BW in rats (p = 0.315).
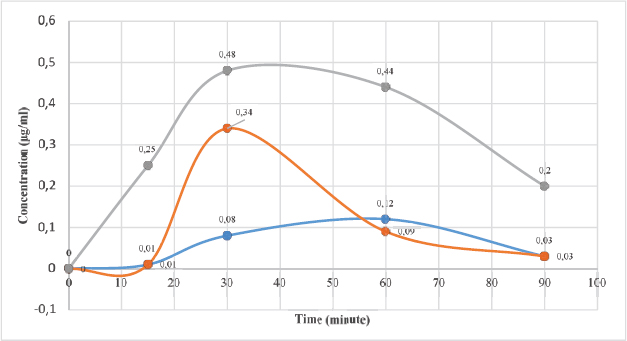 | Figure 4. The curve of piperine concentration in rat’s blood plasma plotted against time. Carrageenan-complexed piperine (red; Cmax = 0.34 μg/ml at 30 minutes), isolated piperine from P. nigrum (blue; Cmax = 0.12 μg/ml at 60 minutes), and standard piperine (gray; Cmax = 0.48 μg/ml at 30 minutes). [Click here to view] |
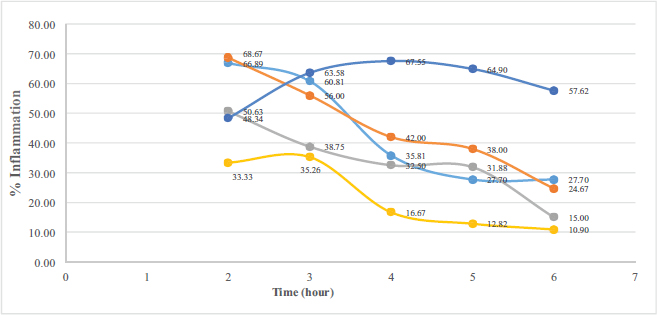 | Figure 5. The anti-inflammatory activity calculated as the percentage of inflammation against time. Carrageenan-complexed piperine is represented as a gray curve, the isolated piperine is the orange curve, and standard piperine is the light blue curve; acetosal: the positive control is the yellow curve; negative control is the dark blue curve. [Click here to view] |
CONCLUSION
The inclusion of piperine in the carrageenan complex given as a single oral dose of 393 mg/kg BW could be quantified in the rat’s plasma. The maximum concentration (Cmax) was 0.34 μg/ml at 30 minutes, which is better than that of the isolated piperine from P. nigrum (Cmax = 0.12 μg/ml at 60 minutes). This carrageenan-complexed piperine gave a faster elimination as proven by its lowest plasma concentration at 60th minute compared to the other piperines. The anti-inflammatory activity of carrageenan-complexed piperine at a dose of 393 mg/kg BW (equivalent to 100 mg piperine) is not significantly different from that of the acetosal dose of 45 mg/kg BW in rats.
ACKNOWLEDGMENT
The authors would like to thank the Indonesian School of Pharmacy (Sekolah Tinggi Farmasi Indonesia) for funding the publication fee of this article.
REFERENCES
Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther, 2009; 11(2):1–9; doi:10.1186/ar2662 CrossRef
Barbeyron T, Michel G, Potin P, Henrissat B, Kloareg B. Iota-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J Biol Chem, 2000; 275(45):35499–505; doi:10.1074/jbc.M003404200 CrossRef
Ezawa T, Inoue Y, Tunvichien S, Suzuki R, Kanamoto I. Changes in the physicochemical properties of piperine/β-cyclodextrin due to the formation of inclusion complexes. Int J Med Chem, 2016; 2016, Article ID 8723139:9; doi:10.1155/2016/8723139 CrossRef
Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. (2017). Piperine-The bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf, 2017; 16(1):124–40; doi:10.1111/1541-4337.12246 CrossRef
Jumpa-ngern P, Kietinun S, Sakpakdeejaroen I, Cheomung A, Na-Bangchang K. Pharmacokinetics of piperine following single dose administration of benjakul formulation in healthy Thai subjects. Afr J Pharm Pharmaco, 2013; 7(10):560–6; doi:10.5897/AJPP2013.3469 CrossRef
Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, Ghosh B. Piperine inhibits TNF-alpha induced adhesion of neutrophils to endothelial monolayer through suppression of NF-kappaB and IkappaB kinase activation. Eur J Pharmacol, 2007; 575(1-3):177–86; doi:10.1016/j.ejphar.2007.07.056 CrossRef
Patel A. Solubility enhancement technologies and research emerged. IJPBA, 2017; 8(2):1–11. Available via https://pdfs.semanticscholar.org/cb77/9a3d0cea10fc8c9fbd66a66920e30a5c4747.pdf
Shao B, Cui C, Ji H, Tang J, Wang Z, Liu H, Qin M, Li X, Wu L. Enhanced oral bioavailability of piperine by self-emulsifying drug delivery systems: in vitro, in vivo and in situ intestinal permeability studies. Drug Deliv, 2015; 22(6):740–7; doi:10.3109/10717544.2014.898109 CrossRef
Singh NK, Kumar P, Gupta DK, Singh S, Singh VK. UV-spectrophotometric method development for estimation of piperine in Chitrakadi Vati. Der Pharmacia Lettre, 2011; 3(3):178–82. Available via https://pdfs.semanticscholar.org/defc/30d84a9d7b1d14f89948fcc13d4ad1b5f46d.pdf
Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac J Trop Med, 2014; 7S1:S461–8; doi:10.1016/S1995-7645(14)60275-3 CrossRef
Upadhyay V, Sharma N, Joshi HM, Malik A, Mishra M, Singh BP, Tripathi S. Development and validation of rapid RP- HPLC method for estimation of piperine in Piper nigrum L. IJHM, 2013; 1(14):6–9.
Veerareddy PR, Vobalaboina V. Pharmacokinetics and tissue distribution of piperine lipid nanospheres. Pharmazie, 2008; 63:352–55.
Wang B, Zhang Y, Huang J, Dong L, Li T, Fu X. Anti-inflammatory activity and chemical composition of dichloromethane extract from Piper nigrum and P. longum on permanent focal cerebral ischemia injury in rats. Rev Bras Farmacog, 2017; 27(3):369–74; doi:10.1016/j.bjp.2017.02.003. CrossRef