INTRODUCTION
Stem cells have been recognized as a promising source for cellular therapy in treating various diseases, including cancers, chronic diseases, and tissue repair and regeneration (Biehl and Russel, 2009). To date, hematopoietic stem/progenitor cells (HSPCs) are among the preferred source for stem cell-based cellular therapy due to their unique characteristics of pluripotency and self-renewal that are capable of generating an entire hematopoietic system (Weissman, 2000). HSPC transplantation is a common clinical procedure that utilizes HSPCs derived from principal sources such as bone marrow, mobilized peripheral blood, or cord blood (Yu et al., 2015). In fact, bone marrow transplant has been supplanted by mobilized peripheral blood transplantation to treat both hematologic and nonhematologic disorders.
HSPC transplantation can be carried out either in an autologous or in an allogeneic setting. In an autologous transplant, HSPCs are isolated from recipient’s bone marrow or mobilized peripheral blood, which are often cryopreserve for later use. Meanwhile, allogeneic transplant requires stem cell donation from the matched donor which are often used shortly after collection; however, in certain circumstances, umbilical cord blood (UCB) transplant is performed (Berz et al., 2007). Based on the Malaysian Standard for Stem Cell Transplantation, the desired storage condition for HSPCs is determined by the requirement in the clinical setting; whereby samples to be used within 48 hours should be kept in 20 °C–24°C and for samples to be utilized within 72 hours, storage at 2°C–6°C is recommended. Meanwhile, UCB transplantation often requires extensive storage of the stem cells and relies heavily on the cryopreservation on initial collection prior to clinical usage (Ministry of Health Malaysia, 2009). Hence, the cryopreservation of HSPCs is a crucial component to facilitate therapeutic efficacy concerning the use of HSPCs in cellular therapy and regenerative medicine.
To date, the optimal cryopreservation technique for HSPC storage remains to be defined and standardized. In principle, cryopreservation refers to a process that preserves organelles, cells, tissues, and any biological components by cooling the samples to lower temperatures, such as at −80°C, −150°C, and −196°C, with the aim to maintain the structural integrity and functionality of the cells or tissues (Berz et al., 2007). Several methods have been developed for cryopreservation of HSCPs, namely slow-freezing or known as controlled-rate freezing, followed by the most recent technique known as vitrification or flash freezing. Until now, slow-freezing is the most frequently used method in cryopreservation of HSPCs, but the method has been showed to cause cellular injury, resulting in cell death (Yong et al., 2010). Thus, vitrification or flash freezing begins to be employed in HSPC cryopreservation setting as the technique has been successfully utilized for the preservation of embryos, spermatozoa, and embryonic stem cells (Isachenko et al., 2013; Zhou, 2004).
Although these techniques are being utilized in clinical settings and research laboratories, such protocols, whether for vitrification or conventional slow freezing, remain suboptimal due to the resultant cell loss and oxidative stress-mediated cellular injury imposed by these cryopreservation strategies, compromising the counts and quality of cryopreserved cell products (Djuwantono et al., 2011). Thus, the usage of bio-oxidant as cryopreservative has been recommended to minimize the occurrence of oxidative stress, and subsequent secondary effects associated with cell loss, apoptosis, and cellular injury during the cryopreservation process, with a number of encouraging results, have been reported (Motta et al., 2010).
Recently, the usage of antioxidant supplements such as α-tocopherol and catalase improves the cryopreservation outcome of preserved spermatogonial stem cells and UCB (Cecarini et al., 2007). Research concerning the use of antioxidant as cryoprotectant for HSPCs, particularly with regard to the repopulation capacity outcome of the cryopreserved HSPCs into hematopoietic lineages, still remains limited. Thus, in the present study, the role of N-acetyl cysteine (NAC) supplements during cryopreservation of HSPCs is investigated. NAC is an antioxidant that is commonly used to inhibit ROS-induced cellular damage and apoptosis (Berniakovich et al., 2012). The studies conducted by Hamid et al. (2018) and Yi et al. (2018) demonstrated that NAC supplementation conferred a significant protection against oxidative stress-mediated cellular damage during ex vivo maintenance of cultured HSPCs. However, the role of NAC as cryoadditive for the preservation of HSPCs remains to be explored. Therefore, this research is conducted to investigate the effect of NAC supplementation following different cryopreservation time points toward the cryopreservation outcomes concerning the aspects of cell viability, oxidative stress status, and repopulation capacity of HSPCs into differential lineage-committed progenitors comprising of myeloid, erythroid, and pre-B lymphoid lineages using bone marrow-derived HSPCs.
MATERIALS AND METHODS
N-acetyl cysteine
The NAC was purchased from Sigma-Aldrich (Sigma Grade, ≥ 99 % thin layer chromatography). Briefly, the stock solution was prepared by diluting the NAC powder in a phosphate-buffered saline (PBS) and filtered using a filter with a pore size of 0.22 μM. The working solution was prepared by using serial dilutions before the experiment. All preparation was performed in a laminar flow hood.
Animal handling and experimental design
All procedures involving the use of laboratory animals were reviewed and approved by the UKM Animal Ethics Committee (Ethics Approval Number: FSK/2017/ZARIYANTEY/22-NOV.-2017-JULY-2018-AR-CAT2). Bone marrow-derived HSPCs were isolated from 30 to 35 g weighed male imprinting control region strain mice. After cervical dislocation, the long bones (tibia and femur) were harvested, and a flushing technique was applied to isolate the bone marrow cells (BMCs) from the long bones (Hamid et al., 2014). The harvested cells were filtered through a 40-μM cell strainer, resuspended in dulbecco’s modified eagle medium, and allowed to grow overnight for 24 hours prior to the downstream study. Then, the cells were counted using the trypan blue exclusion method and 1 × 106 cells/ml were allocated per vial for the cryopreservation process that uses a final volume of 1 ml of cryomedium containing the standard cryoprotective agent of 10% dimethyl sulfoxide (DMSO), with or without the NAC supplementation. NAC supplementations were added directly to the cells and were carried out at the concentrations of 0.25 μM, 0.5 μM, and 2.0 Mm before freezing. Meanwhile, the cells without NAC supplementation (0 μM) represent the control group (Hamid et al., 2018; Yi et al., 2018). Cells were then cryopreserved at −80°C (Ashwood-Smith, 1961) using controlled-rate freezing, where the temperature decreases by the following freezing rate’s setting (4°C, −20°C, and finally −80°C) at three time point intervals of 48 hours, 2 weeks, and 4 weeks. Following each cryopreservation time point, cells were thawed and analyzed manually for cell viability using standard trypan blue exclusion test (Hamid et al., 2018; Yi et al., 2018), followed by the determination of oxidative stress status as indicated by the following markers: glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), and protein carbonyl (PC). Meanwhile, the repopulation capacity of HSPCs into lineage-committed progenitors using colony-forming cell (CFC) assay was only evaluated following 4 weeks of cryopreservation.
Evaluation of GSH level and superoxide dismutase activity
In this study, the impact of cryopreservation and NAC supplements on the cellular antioxidants, namely, GSH and superoxide dismutase was studied following 48 hours, 2 weeks, and 4 weeks of cryopreservation. The analyses of GSH and SOD were carried out according to the protocol as described (Hamid et al., 2018; Yi et al., 2018). Briefly, cell lysate was prepared, and total protein was measured using a Bradford assay. The quantification of GSH was achieved using spectrophotometric assay, which involves oxidation of GSH by the sulfhydryl reagent 5,5′-dithio-bis (2-nitrobenzoic acid) to form the yellow derivative 5′-thio-2-nitrobenzoic acid that was measurable at the absorbance of 412 nm. As for SOD, the activity was evaluated based on the reduction of nitroblue tetrazolium by superoxide radicals to blue colored formazan, which can be measured at the spectrophotometric absorbance of 560 nm.
Evaluation of malondialdehyde and protein carbonyl levels
Malondialdehyde and PC are common oxidative stress markers to indicate the presence of lipid peroxidation and protein oxidation, respectively. MDA was determined according to the protocol as described (Esterbauer and Cheeseman, 1990). Briefly, precryopreserved and post-thawed cryopreserved HSPCs were collected and centrifuged in cold PBS, followed by the addition of lysis buffer and subsequent centrifugation at 1,000 rpm for 10 minutes. Supernatants were then collected and reacted with thiobarbituric acid (TBA), of which the mixture was then heated for 60 minutes at 95°C. Then, the mixture was left at room temperature for 10 minutes. Finally, the supernatant containing MDA-TBA complex was placed into a 96-well microtiter plate and measured at the absorbance of 532 nm.
Protein carbonyl was estimated according to the procedure as described (Levine, 1990). Cells were collected through centrifugation at 1,000 rpm for 10 minutes at 4°C, followed by sonication on ice in 2 ml of buffer solution and subsequent centrifugation at 1,000 rpm for 15 minutes at 4°C. Protein lysates were then collected and mixed with 20% TCA, followed by centrifugation for 1,000 rpm for 5 minutes at 4°C. The supernatant was discarded, and the precipitates were mixed with 500 μl of 2,4-dinitrophenyl hydrazine (2,4-DNPH), of which the mixture was then incubated in the dark for 1 hour at 4°C. This was followed by a second wash with 10% TCA as described above. Samples were incubated on ice for 5 minutes and washed again in 1 ml of ethanol:ethyl acetate (1:1; v/v). The final protein pellets were dissolved in 1 ml of 5 M urea and incubated at 37°C for 15 minutes. The mixture was then centrifuged at 1,000 rpm for 5 minutes at 4°C, and the carbonyl contents were then measured at an absorbance of 360 nm.
Repopulation capacity of bone marrow-derived HSPCs into lineage-committed progenitors
The CFC assay was performed to assess the repopulation capacity of bone marrow-derived HSPCs into lineage-committed progenitors following the protocol as described (Yi et al., 2018). The detection of committed progenitors was achieved using semi-solid agar specialized to support the growth of progenitors for myeloid (Methocult™ GF 3534), erythroid (Methocult™ GF 3334), and pre-B lymphoid (Methocult GF 3630) lineages. Briefly, the cells from every experimental group were collected and added into methylcellulose agar at the respective cell density according to lineages, 2 × 105 cells/ml for myeloid, 0.5 × 106 cells/ml for pre-B lymphoid, and 2 × 105 cells/ml for erythroid. Cultures were incubated at 37°C in a 5% CO2 incubator. Colonies were counted on Day 7 of incubation for the determination of pre-B lymphoid and erythroid progenitors and on Day 14 of incubation for the analysis of myeloid progenitors.
Statistical analysis
The results are reported as mean ± standard error mean (SEM) from three independent experiments. The results were analyzed using one-way analysis of variance as p < 0.05 is considered to be statistically significant. All of the results were compared with their precryopreservation baseline control and the post-thawing control (absence of NAC supplementation) groups.
RESULTS
Cell viability
The analysis of HSPC viability post-thawed after cryopreservation storage at a temperature of –80°C for 48 hours, 2 weeks, and 4 weeks was enumerated using the trypan blue exclusion method (Fig. 1). It was noted that cryopreservation process caused a significant reduction (p < 0.05) in the number of viable cells post-thawed for all experimental groups following 48 hours, 2 weeks, and 4 weeks of cryopreservation compared to the number of viable cells in the precryopreserved group (1 × 106 cells). However, the cell recovery and viability post-thawed were found to be influenced by the cryopreservation’s time point, whereby longer time points of cryopreservation conferred greater cell recovery and viability post-thawed compared to the shorter time-points. This is clearly evidenced that the recorded percentage of cell recovery was improved from 23% (48 hours) to 53% (2 weeks) and 76% (4 weeks) compared to the number of cells in the precryopreservation group. Meanwhile, the NAC supplementation was found to be less effective at most of the concentrations applied and cryopreservation time points in improving the cell recovery and viability during cryopreservation process, whereby a significant improvement (p < 0.05) of cell recovery and viability post-thawed was only observed following cryopreservation for 48 hours at selective NAC concentrations (0.50 and 2.00 μM) with respect to control group (without NAC).
GSH level and SOD activity
Based on the presented data on GSH level (Fig. 2) and SOD activity (Fig. 3), no significant difference was observed throughout all experimental groups and cryopreservation time points. It was noted that the level of GSH and SOD activity was not remarkably affected by the cryopreservation process regardless of the time points with respect to the precryopreservation group. Meanwhile, NAC supplementation showed no significant effect on the level of GSH and SOD activity compared to the control group (without NAC) throughout the NAC concentrations and cryopreservation time points.
MDA and PC levels
Cryopreservation process caused a significant reduction in the MDA level at all the time points compared to the MDA level as in the precryopreservation group (Fig. 4). Meanwhile, NAC supplementation was able to cause a further significant (p < 0.05) reduction in the MDA level only at 0.5 μM NAC after 48 hours of cryopreservation compared to the control group. On contrary to the MDA level, no significant difference was observed for the PC level, whereby the level was not affected by the cryopreservation process, NAC concentration, and cryopreservation time points (Fig. 5)
Repopulation capacity into lineage-committed progenitors
The effect of NAC supplementation on the repopulation capacity of post-thawed HSPCs in myeloid, erythroid, and pre-B lymphoid progenitors after 4 weeks of cryopreservation is shown in Figure 6. Overall, there is a general decrease in clonogenic potential into hematopoietic progenitors in all the cryopreserved groups compared to the precryopreservation group. Cryopreservation storage caused a significant (p < 0.05) reduction in the repopulation capacity of post-thawed HSPCs into committed progenitors of myeloid and erythroid progenitors than those of pre-B lymphoid progenitor. Meanwhile, NAC supplementation showed no significant effect on the repopulation capacity of post-thawed HSPCs into lineage-committed progenitors. Although the effect of NAC supplementation was found to be nonremarkable, a trend toward improved maintenance of HSPC repopulation capacity into myeloid and erythroid progenitors was noted when compared to the control group.
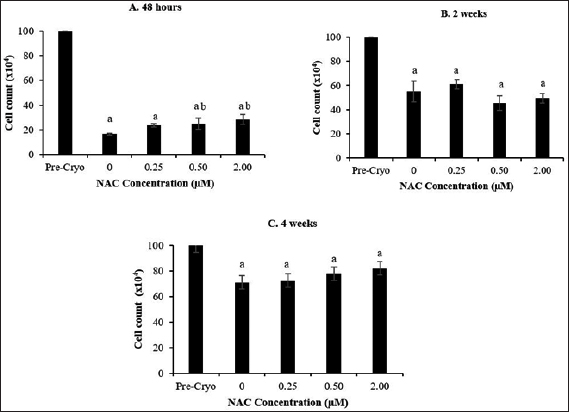 | Figure 1. Effect of NAC supplementation on the viability of HSPCs after 48 hours (A), 2 weeks (B), and 4 weeks (C) of cryopreservation. Each datum is obtained from three different experiments. Data are expressed as mean ± SEM. a Significant different (p < 0.05) against precryopreservation group (Pre-Cryo); b Significant different (p < 0.05) against control group (0 μM). [Click here to view] |
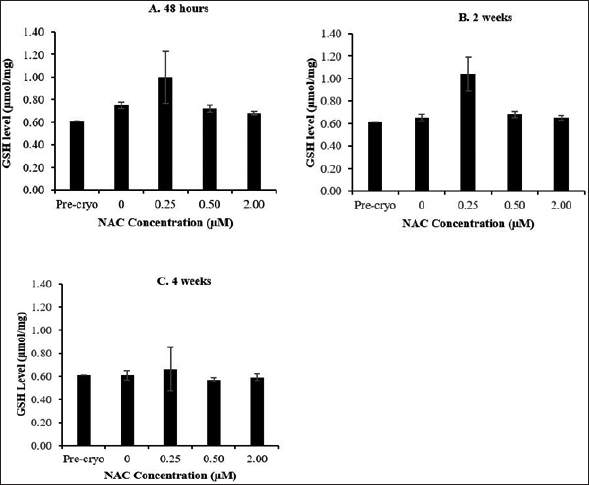 | Figure 2. Effect of NAC supplementation on the GSH level of HSPCs after 48 hours (A), 2 weeks (B), and 4 weeks (C) of cryopreservation. Each datum is obtained from three different experiments. Data are expressed as mean ± standard error of mean (SEM). [Click here to view] |
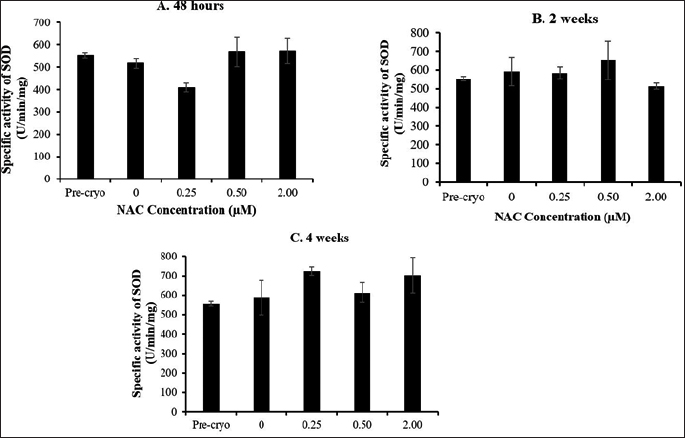 | Figure 3. Effect of NAC supplementation on the SOD activity of HSPCs after 48 hours (A), 2 weeks (B), and 4 weeks (C) of cryopreservation. Each datum is obtained from three different experiments. Data are expressed as mean ± standard error of mean (SEM). [Click here to view] |
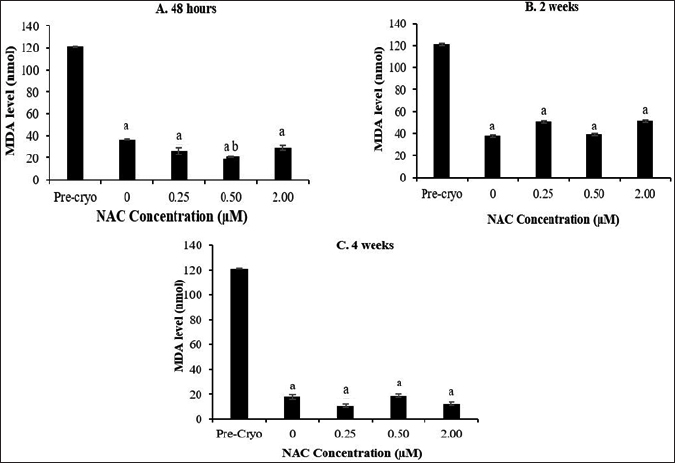 | Figure 4. Effect of NAC supplementation on the MDA level of HSPCs after 48 hours (A), 2 weeks (B), and 4 weeks (C) of cryopreservation. Each datum is obtained from three different experiments. Data are expressed as mean ± standard error of mean (SEM). aSignificant different (p < 0.05) against pre-cryopreservation group (Pre-Cryo); bSignificant different (p < 0.05) against control group (0 μM). [Click here to view] |
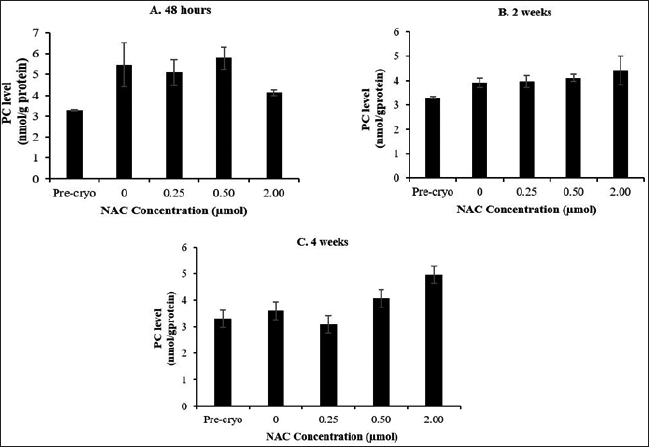 | Figure 5. Effect of NAC supplementation on the protein carbonyl activity of HSPCs after 48 hours (A), 2 weeks (B) and 4 weeks (C) cryopreservation. Each data is obtained from three different experiments. Data is expressed as mean ± standard error of mean (SEM). [Click here to view] |
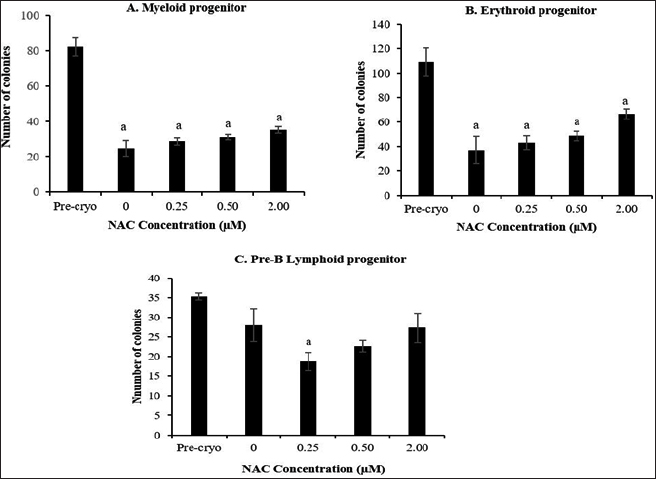 | Figure 6. Effect of NAC supplementation on the repopulation capacity of HSPCs into myeloid (A), erythroid (B), and pre-B lymphoid (C) progenitors after 4 weeks of cryopreservation. Each datum is obtained from three different experiments. Data are expressed as mean ± standard error of mean (SEM). a Significant different (p < 0.05) against precryopreservation group (Pre-Cryo). [Click here to view] |
DISCUSSION
HSPCs are among the preferred and established sources of stem cells being utilized in cell-based therapy as well as in stem cell-related research laboratories. However, the utilization of such cellular products depends heavily on the effective preservation of those cell products before its usage. Thus, cryopreservation of stem cells is crucial to fulfill the therapeutic demand. Despite the available protocols concerning the preservation of HSPCs, finding the optimal cryopreservation technique for HSPC storage remains to be defined and standardized. A number of studies have reported that the cryopreservation process causes cell loss and oxidative stress-mediated cellular injury, compromising the functional outcome of the cryopreserved HSPCs (Djuwantono et al., 2011). Hence, the usage of bio-oxidant supplements in cryopreservation medium offers an alternative solution to overcome these limitations (Motta et al., 2010).
NAC has been used as a nutritional supplement because of its antioxidant property as a scavenger of free radicals, especially oxygen radicals. NAC is a precursor of L-cysteine that results in GSH biosynthesis. Previously, NAC showed a protective effect against oxidative stress-mediated cellular damage during ex vivo maintenance of cultured HSPCs by reducing cell loss, lowering oxidative stress, and maintaining the repopulation capacity of HSPCs into committed progenitors (Hamid et al., 2014, 2018; Yi et al., 2018). However, the cryopreservative role of NAC for the preservation of HSPCs remains unexplored. Thus, the effect of NAC supplementation on the cryopreservation outcome of post-thawed bone marrow-derived HSPCs was evaluated based on the cell viability, oxidative stress status, and repopulation capacity into committed progenitors of myeloid, erythroid, and pre-B lymphoid lineages following the cryopreservation for 48 hours, 2 weeks, and 4 weeks at −80°C.
Cryopreservation at various temperatures has been employed for the storage of bone marrow-derived HSPCs for clinical use at a later point in time (Asghar et al., 2014). This (cryopreservation) is crucial to ensure the availability of cell products at the desired quality. In this study, the analyses showed that the cryopreservation process caused a significant reduction in the number of viable bone marrow-derived HSPCs post-thawed for all experimental groups following 48 hours, 2 weeks, and 4 weeks of cryopreservation compared to the number of viable cells in the precryopreserved group. The effect of freezing on the viability, structural integrity, and functionality of preserved cell products has been documented. Cryopreservation induces ice crystal formation that imposes mechanical stress on the preserved cells, leading to subsequent cell injury and cell death (Muldrew and McGann, 1994). Meanwhile, the generation of osmotic pressure and oxygen-free radicals is another factor which has been implicated as the potential causes of cell loss during freezing (Katkov et al., 1998). Thus, these factors could justify the notable reduction in cell viability of post-thawed bone marrow-derived HSCPs as observed in this study. Furthermore, the accumulated free radicals should be scavenged to avoid the secondary effects associated with oxidative damage-mediated cellular injury such as lipid peroxidation, protein oxidation, and DNA damage. Although the cryopreservation was found to compromise cell viability, the result showed that the cryopreservation outcome of bone marrow-derived HSPCs was influenced by the cryopreservation time point, whereby longer time points of cryopreservation performed greater preservation of bone marrow-derived HSPCs compared to the shorter time-points. This is evidenced from the notable improvement of cell recovery from 23% (48 hours) to 53% (2 weeks) and 76% (4 weeks) compared to the number of cells in precryopreservation group. A similar finding was reported by Matsumoto et al. (2002), who showed that the storage of hematopoietic progenitor cells around the freezing point (−2°C) is more appropriate in short-term storage for a period of less than 72 hours than the usual deep-freezing temperatures (−80°C) to obtain higher cell recovery. The observation that longer time points of cryopreservation conferred a greater cell recovery and viability post-thawed compared to the shorter time points could be due to damage from ice recrystallization during thaw. The studies have shown that the survival of cell postcryopreservation is dependent on the warming rate during thawing after storage and that cell death occurs because large ice crystals grow at the expense of smaller ones. It is very likely that there are more small ice crystals during the short-term storage, and thus, greater cryoinjury was noted.
The role of NAC supplementation on viability maintenance of post-thawed bone marrow-derived HSPCs was found to be less remarkable at most of the concentrations applied and cryopreservation time points. However, a significant improvement of cell recovery and viability post-thawed was observed following cryopreservation for 48 hours at selective NAC concentrations (0.50 and 2.00 μM) with respect to control group (without NAC). Meanwhile, greater bone marrow-derived HSPC preservation was also noted in the presence of NAC following cryopreservation for 4 weeks although the improvement was not statistically significant. To date, most of the reported studies concerning the use of antioxidant as cryopreservative agent are mostly applied for the preservation of embryos, spermatozoa, and embryonic stem cells (Isachenko et al., 2013; Zhou, 2004) with limited reports regarding the preservation of HSPCs which are available. The previous studies had shown a combination of the bioantioxidant catalase and the membrane stabilizer. Trehalose in the conventional freezing mixture affords a better cryoprotection to hematopoietic progenitor cells. The addition of catalase along with low concentrations of dimethyl sulfoxide improved the cryopreservation outcome, and a greater preservation of mice BMCs was achieved in the presence of antioxidant supplements (Motta et al., 2010; Limaye, 1997). In this study, a tendency toward the improved preservation of bone marrow-derived HSPCs was noted following NAC supplementation in 48 hours and 4 weeks of cryopreservation which could owe its potent antioxidant capacity to scavenge ROS and inhibit cellular damage and apoptosis. However, the influence of cryopreservation time points on the cell recovery post-thawed cryopreservation deserves further investigation as no similar study with regard to time points effect has been reported.
Next, the effect of the cryopreservation and NAC supplementation on the oxidative stress status of the post-thawed bone marrow-derived HSPCs was evaluated by focusing on the antioxidant capacity and oxidative damage markers. The GSH level and SOD activity were not significantly affected by the cryopreservation process. No remarkable effects were noted following NAC supplementation although an enhanced antioxidant capacity of post-thawed bone marrow-derived HSPCs was observed in NAC-supplemented group throughout cryopreservation time-points as compared to the non-supplemented control group. It has long been known that the process of cryopreservation plays a crucial role in the decline of viability in cryopreserved cells as it can cause cellular damage and subsequent biochemical changes, leading to ion disturbances and oxidative stress (Alvarez and Storey, 1992; Pérez-Cerezales et al., 2010). Within eukaryotic cells, the antioxidant system is regulated by mitochondria and also cytosol. The cryopreservation process may cause injury to mitochondrial organelles and subsequently deactivate antioxidant pathways (Figueroa et al., 2017). However, when cell viability is compared, the antioxidant capacity seems highly contradictory. Ideally, less viability would most probably also occur with a lower antioxidant capacity post-thawed. Thus, the deactivation of antioxidant pathway during the cryopreservation process could lead to the notable unchanged antioxidant capacity post-thawed which may not be the only justification. Hence, it is possible to say that due to generated free radicals after cryopreservation, a compensatory mechanism may have triggered the increase in antioxidant capacity to mitigate oxidative stress and reduce oxidative damage. Meanwhile, NAC showed no remarkable boosting effects on the antioxidant capacity of post-thawed bone marrow-derived HSPCs. NAC has been used as a nutritional supplement because of its antioxidant property as a scavenger of free radicals, especially oxygen radicals. NAC is a precursor of L-cysteine that results in GSH biosynthesis. If NAC increases the levels of GSH, it is intriguing that the GSH levels remained the same despite increasing supplementation of NAC. This finding is in accordance with the previous report, indicating that antioxidant supplementation caused no significant alteration on the GSH level and SOD activity of post-thawed sperm cells (Bucak et al., 2009).
The presence of intracellular free radicals during cryopreservation may cause cellular damage and disruption of cellular macromolecules through DNA damage, protein oxidation, and lipid peroxidation. In this study, the level of MDA and PC was evaluated as oxidative damage markers. Overall, cryopreservation process caused a significant reduction of the malondialdehyde level (p < 0.05) along with no significant changes of PC level, with NAC caused a further reduction in MDA levels only at 0.5-μM NAC after 48 hours of cryopreservation. The finding implies that the cryopreservation was able to minimize the MDA level but not the protein oxidation and that the NAC showed no remarkable effects on oxidative stress-mediated cryodamage of post-thawed bone marrow-derived HSPCs. A contradictory finding has been reported concerning the oxidative damage status of cell products following cryopreservation. Previously, DMSO showed the ability to inhibit the formation of oxygen-free radicals and prevent the formation of oxidative stresses on cells (Fleck et al., 2000), resulting in a significant decline of MDA levels post-thawed compared to precryopreservation point. On the contrary, the level of MDA was found to be significantly increased after cryopreservation of cord blood (Djuwantono et al., 2011). Meanwhile, the levels of PC were not significantly affected by the cryopreservation process and NAC supplementation. The process of protein oxidation occurs in the cytosol and nucleus of the cell (Cecarini et al., 2007). Thus, at this point, it is noteworthy to suggest that the cryopreservation process at the studied time points up to 4 weeks is unlikely to promote protein oxidation that occurs mainly in cytosol and nucleus. Furthermore, the role of NAC as a potential cryoprotective agent for the cryropreservation of bone marrow-derived HSPCs deserves further investigations.
One of the goals in the present study was to see whether with the cryopreservation and addition of NAC helped to preserve the functionality of bone marrow-derived HSPCs as evaluated by CFC assay. CFC assay revealed a significant decline (p < 0.05) in the repopulation capacity of HSPCs into myeloid–erythroid committed progenitors. On the contrary, the repopulation capacity of HSPCs into pre-B lymphoid progenitor was least affected after 4 weeks of cryopreservation. Meanwhile, a tendency toward improved repopulation capacity of myeloid–erythroid progenitors was noted in the NAC-supplemented group. Reduced cell viability post-thawed as noted in the present study could contribute to the lowered repopulation capacity of post-thawed HSPCs into committed progenitors of myeloid and erythroid compartments. Reduced cell viability post-thawed may affect the number of viable committed progenitor cells, leading to defective repopulation capacity. Despite the affected myeloid–erythroid lineages, the repopulation capacity of HSPCs into pre-B lymphoid progenitor was least affected. CFC assay or also known as clonogenic assay is a sophisticated functional substitute assay. However, this assay is expensive and time-consuming and requires skilled experts that limit its usage in routine standard qualitative assessments of stem cell products prior to transplantation (Berz and Colvin, 2012). The previous studies reported that the use of cryoprotectant solutions containing catalase conferred the optimal preservation of post-thawed hematopoietic progenitor clonogenic potential (myeloid lineage) and their receptors (Motta et al., 2010).
To date, the myeloid and erythroid lineages are the most studied progenitors concerning the cryopreservation effect on the repopulation capacity of HSPCs, while the HSPC commitment into lymphoid progenitor remains unexplored. Hence, it remains unclear if the differential repopulation outcome post-thawed as noted in the functional assay holds the clinical relevance. Meanwhile, it also remains to be explored whether the notable lineage-dependent effect concerning the cryopreservation outcome is associated with cell loss post-thawed or due to the skewed repopulation capacity of HSPCs toward lymphoid lineage, leading to a significant decline in the myeloid–erythroid compartments. Hence, further studies are required to establish the conclusion pertaining to the effects of cryopreservation and NAC supplementation on the HSPC compartments and lineage specification.
CONCLUSION
In conclusion, cryopreservation impaired the viability and repopulating capacity of bone marrow-derived HSPCs. NAC showed a potential to improve the cell viability following shorter time-point cryopreservation. Meanwhile, NAC supplementation showed no remarkable effect on oxidative stress-mediated cryodamage and repopulation capacity of HSPCs despite its notable potency to restore the repopulation capacity of myeloid–erythroid progenitors. Thus, the role of NAC on the cryopreservation of HSPCs deserves further investigation.
ACKNOWLEDGMENT
This study is supported by FRGS (UKM) FRGS/1/2016/SKK13/UKM/03/1. The authors would like to thank the Biotechnology Laboratory, Centre for Health and Applied Sciences, UKM, for providing facilities to conduct this research.
CONFLICTS OF INTERESTS
The authors have no conflicts of interest to declare regarding the publication of this paper.
REFERENCES
Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl, 1992; 45:3232–41.
Asghar W, El Assal R, Shafiee H, Anchan RM, Demirci U. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol J 2014; 9:7895–903. CrossRef
Ashwood-Smith MJ. Preservation of mouse bone marrow at−79. with dimethyl sulphoxide. Nature, 1961; 190:47821204. CrossRef
Berniakovich I, Laricchia-Robbio L, Belmonte JCI. N-acetylcysteine protects induced pluripotent stem cells from in vitro stress: impact on differentiation outcome. Int J Dev Biol, 2012; 56:9729–35. CrossRef
Berz D, Colvin G. Cryopreservation of hematopoietic and non-hematopoietic stem cells - review for the clinician. In Taner D (ed.). New advances in stem cell transplantation, Ankara, Turkey, Ankara University, 2012; doi:10.5772/26448 CrossRef
Berz D, Mccormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Haematol, 2007; 82:6463–72. CrossRef
Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs, 2009; 24:298. CrossRef
Bucak MN, Sariözkan S, Tuncer PB, UlutaÅŸ PA, AkçadaÄŸ HÄ°. Effect of antioxidants on microscopic semen parameters, lipid peroxidation and antioxidant activities in Angora goat semen following cryopreservation. Small Ruminant Res, 2009; 81:290–95. CrossRef
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta, 2007; 1773:293–104. CrossRef
Djuwantono T, Wirakusumah FF, Achmad TH, Sandra F, Halim D, Faried AA. Comparison of cryopreservation methods: Slow-cooling vs rapid-cooling based on cell viability, oxidative stress, apoptosis, and CD34+ enumeration of human umbilical cord blood mononucleated cells. BMC Res Notes, 2011; 4:1371. CrossRef
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol, 1990; 186:407–21. CrossRef
Figueroa E, Valdebenito I, Zepeda AB, et al. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev Aquacult, 2017; 9:176–87. CrossRef
Fleck RA, Benson EE, Bremner DH, Day JG. Studies of free radical-mediated cryoinjury in the unicellular green alga Euglena gracilis using a non-destructive hydroxyl radical assay: a novel approach for developing protistan cryopreservation strategies. Free Radical Res 2000; 32:2157–70. CrossRef
Hamid ZA, Lin WHL, Abdalla BJ, Yuen OB, Latif ES, Mohamed J, Rajab NF, Wah CP, Harto MKAW, Budin SB. The role of Hibiscus sabdariffa L.(Roselle) in maintenance of ex vivo murine bone marrow-derived hematopoietic stem cells. Sci World J, 2014; 2014:1–10. CrossRef
Hamid ZA, Tan HY, Chow PW, Harto KAW, Chan CY, Mohamed J.. The role of N-Acetylcysteine supplementation on the oxidative stress levels, genotoxicity and lineage commitment potential of ex vivo murine haematopoietic stem/progenitor cells. Sultan Qaboos Univ Med J, 2018; 18(2):130; doi: 10.18295/squmj(2018)18.02.002. CrossRef
Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod Biomed Online, 2013; 6:2191–200.
Katkov II, Katkova N, Critser JK, Mazur P. Mouse spermatozoa in high concentrations of glycerol: chemical toxicity vs osmotic shock at normal and reduced oxygen concentrations. Cryobiology, 1998; 37:4325–38. CrossRef
Levine RL, Garland D, Oliver C N, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol, 1990; 186:464–78.
Limaye LS. Bone marrow cryopreservation: improved recovery due to bioantioxidant additives in the freezing solution. Stem Cells, 1997; 15:5353–8.
Matsumoto N, Yoshizawa H, Kagamu H, Abe T Fujita N, Watanabe S. Successful liquid storage of peripheral blood stem cells at subzero non-freezing temperature. Bone Marrow Transpl, 2002; 30:11777–84; doi:10.1038/sj.bmt.1703692 CrossRef
Ministry of Health Malaysia. National standards for stem cell transplantation: collection, processing, storage and infusion of haemopoietic stem cell and therapeutic cells. MOH/P/PAK/349.17(BP), 2009.
Motta JPR, Gomes BE, Bouzas LF, Paraguassu-Braga FH, Porto LC. Evaluations of bioantioxidants in cryopreservation of umbilical cord blood using natural cryoprotectants and low concentrations of dimethylsulfoxide. Cryobiology, 2010; 60:3301–7. CrossRef
Muldrew K, Mcgann LE. The osmotic rupture hypothesis of intracellular freezing injury. Biophys J, 1994; 66:2532–41. CrossRef
Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez M P. Evaluation of DNA damage as a quality marker for rainbow trout sperm cryopreservation and use of LDL as cryoprotectant. Theriogenology, 2010; 74:2282–9. CrossRef
Weissman, IL. Stem cells. Cell, 2000; 100:1157–68. CrossRef
Yi CC, Hamid ZA, Taib IS, Yee TH, Harto MKAW, Wah CP. Effects of N-acetylcysteine supplementation on ex-vivo clonogenicity and oxidative profile of lineage-committed hematopoietic stem/progenitor cells. J Technol, 2018; 80:31–8. CrossRef
Yong KW, Pingguan-Murphy B, Xu F, et al. Phenotypic and Functional Characterization of Long-Term Cryopreserved Human Adipose-derived Stem Cells. Sci Rep, 2010; 5:9596; doi:10.1038/srep09596 CrossRef
Yu C, Liu Y, Ma T, Liu K, Xu S, Zhang Y, Liu H, La Russa M, Xie M, Ding S, Qi LS. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell stem cell, 2015; 16:2142–7. CrossRef
Zhou C Q. Cryopreservation of human embryonic stem cells by vitrification. Chin Med J (Engl), 2004; 117:1050–55.