INTRODUCTION
Geldanamycin was the first benzoquinone ansamycin antibiotic that exhibited anticancer activity by inhibiting kinase folding by the Hsp90 chaperone complex in a wide range of cancers (Whitesell et al., 1994). The blockage of Hsp90 function induced the proteasome-dependent degradation of cancer relevant target proteins, known as client proteins (Mimnaugh et al., 1996). Despite its anticancer potential in vitro, the clinical use of geldanamycin has not been considered due to several limitations (Supko et al., 1995). First, it exhibited severe hepatotoxicity and nephrotoxicity at therapeutic recommended doses in animal models, limiting effective doses, and thus was unacceptable as a therapeutic profile. This toxicity seemed to be caused by metabolisms of the benzoquinone moiety. Second, geldanamycin is metabolically unstable and poorly soluble in water. Accordingly, variants of geldanamycin have been developed, most notably by altering the quinone ring structure, which have led to improvements in tolerance, potency, metabolic stability, and water solubility (Le Brazidec et al., 2004). Although, the synthesized series of geldanamycin derivatives to make new types of Hsp90 inhibitor with weak toxicity and high efficiency have been attempting (Jurczyszyn et al., 2014; Lee, 2018; Lin et al., 2015; Vasilevskaya et al., 2003). However, there was a limit number of water solubility of these geldanamycin derivatives.
Tryptamine was derived by the decarboxylation of tryptophan. Tryptamine has been used in the past as a neurotransmitter and neuromodulator, vasoconstrictor, and vasodilator, and as antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory agent (Kousara et al., 2017). Its modification has led to many compounds of pharmacological importance. Recently, tryptamine-based compounds have been synthesized as anticancer agents (Guo et al., 2019; Malik et al., 2019; Wang et al., 2016). It has been an effective tool for improving water solubility, biological potency, and pharmacokinetic properties of many natural products (Kousara et al., 2017). According to these effects, the invention of tryptamine-geldanamycin hybrids has been designed. The C17 methoxyl of the geldanamycin molecule can allow for various nucleophiles to be introduced. Thus, geldanamycin from the beginning has been a popular template for semi-synthetic analogs (Lin et al., 2015; Modi et al., 2011; Supko et al., 1995; Tian et al., 2004; Wrona et al., 2010). In this study, two new geldanamycin derivatives were synthesized in form tryptamine-geldanamycin hybrids by substituting with tryptamine and 5-methoxytryptamine that consist of heterocyclic and strong polar groups in the 17-position to improve the pharmacokinetic properties. To our knowledge, this is the first report to describe the screening of tryptamine-geldanamycin hybrids as anticancer agents. The aim of the present study was to evaluate the anticancer activity of the synthesized tryptamine-geldanamycin hybrids against some cancer cell lines: [human breast carcinoma cells (MCF-7); human cervical carcinoma cells (HeLa); and human hepatocellular carcinoma cells (HepG2)] and their water solubility was also determined.
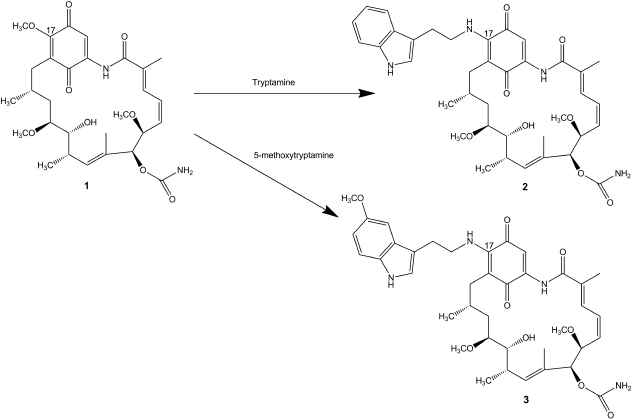 | Figure 1. Geldanamycin chemical structure (1) and structures and synthesis of two derivatives; 17-(tryptamine)-17-demethoxygeldanamycin (2) and 17-(5′-methoxytryptamine)-17-demethoxygeldanamycin (3). [Click here to view] |
MATERIALS AND METHODS
Isolation and cultivation of endophytic actinomycete
Actinomycete strain W14 was isolated from the rhizome tissue of Zingiber zerumbet (L.) Smith, collected from Chanthaburi province, Thailand. The samples were used to isolate the endophytic actinomycetes by surface-sterilization technique and validation of surface sterilization was performed as described in the previous studies (Coombs and Franco, 2003; Taechowisan et al., 2017). Strain W14 was selected and identified using morphological, cultural, physiological, and biochemical characteristics, chemotaxonomy and 16S rDNA sequencing (Taechowisan and Lumyong, 2003; Taechowisan et al., 2019). This strain was grown on ISP-2 agar at 30°C for 14 days, and then the culture medium was cut into small pieces that were extracted with ethyl acetate (3 × 500 ml). This organic solvent was taken to dryness under rotary evaporation. The solid was separated by column chromatography using silica gel 60 (Merck, 0.040–0.063 mm), and 30%, 50%, 75%, and 100% of ethyl acetate in hexane as the eluent to give 17 main fractions (F1–F17). Fraction F13 (30.3 mg) gave a very prominent single spot of pure compound on thin-layer chromatography (TLC) and was used to investigate on nuclear magnetic resonance (NMR) spectroscopy. The spectral data revealed this compound to be geldanamycin (C29H40N2O9) (1).
Synthesis of geldanamycin derivatives
All procedures and experiments described were geladanamycin derivative synthesis.
Geladanamycin derivatives; 17-(tryptamine)-17-demethoxygeldanamycin (C38H48N4O8) (2) and 17-(5′-methoxytryptamine)-17-demethoxygeldanamycin (C39H50N4O9) (3) were synthesized from geldanamycin (Fig. 1). To a solution of geldanamycin (0.84 g, 100 mmol) in dichloromethane (15 ml) at 25°C, tryptamine (0.29 g, 150 mmol) (Sigma-Aldrich) or 5′-methoxytryptamine (0.36 g, 150 mmol) (Sigma-Aldrich) was added. The reaction mixture was stirred at 25°C for 30 minutes before the addition of saturated aqueous CaCl2 (5 ml). The organic layer was removed and washed with saturated CaCl2 solution (3 × 5 ml) and dried (Na2SO4). The mixture was filtered over Celite, rinsed with ethyl acetate, and concentrated under reduced pressure to give a dark purple solid. Purification by flash chromatography (silica, 60% ethyl acetate/hexanes) affords compound 2 (1.01 g, 97 mmol, 96.8%) or compound 3 (1.03 g, 95 mmol, 94.7%).
Water solubility test
The water solubility of geldanamycin and its derivatives was carried out by UV-spectroscopic assay in 96-well plates and measured using a SpectraMax Plus plate reader (Molecular Devices Sunnyvale, CA) as described by Hoelke et al. (2009). Briefly, the compounds were solubilized in water at various concentrations and incubated on a shaker deck at 30°C for 30 minutes. The samples were filtered and 200 μl of the samples were transferred to 96-well plates. The absorbance was measured at 450 nm for compound 1 and at 570 nm for compounds 2 and 3. The calibration of UV-spectroscopic measurements for solubility of each compound was plotted against the concentrations. The stable part of the optical density change was ascribed to water solubility of the compounds. A check of the photometric linearity of the UV plate reader using solutions of K2Cr2O7 at variable concentrations led to a linear range of the instrument from 0.003 to 3.5 absorption units for wavelengths 350 nm.
MTT assay for cytotoxicity activity
A normal cell line [African green monkey kidney cells (Vero)] and three cancer cell lines (MCF-7; HeLa; HepG2) were obtained from the Korean Cell Line Bank (Seoul, Korea). These cells were grown at 37°C in Dulbecco's modified eagle's medium (DMEM) supplement with 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycein sulfate (100 μg/ml) in a humidified atmosphere of 5% CO2. Cytotoxicity studies were performed on a 96-well plate. The cells were mechanically scraped and plated 2 × 105 per well containing 100 μl of DMEM medium with 10% FBS and incubated overnight. The purified compound was dissolved in dimethylsulfoxide for stock solution. Cells were incubated with purified compound at increasing concentrations (1.5625, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μl/ml) in FBS-free medium for 24 hours. The dimethyl sulfoxide (DMSO) concentrations in all assays did not exceed 0.1%. Cells were washed once before adding 50 μl FBS-free medium containing 5 mg/ml 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). After 4 hours of inoculation at 37°C, the medium was discarded and the formazan blue, which formed in the cells, was dissolved in 50 μl DMSO. The optical density was measured at 570 nm. Consecutively, measurements for concentration required for 50% (IC50) inhibition were noted. Cell viability percent was calculated using the formula given below:
The graph was plotted with the Y-axis showing the percentage of viability of cells and X-axis showing the compound concentration.
The therapeutic index was calculated as the ratio of IC50 of normal cells to IC50 of cancer cells.
Statistical analysis
Values are expressed as means ± standard deviation (SD) of three experiments. The SPSS v.16.0 software (SPSS Inc., Chicago, IL) was used for data analysis. Comparisons between two groups were analyzed using the two-tailed Dunnett t-tests treated compound 1 as a control group. A p-value <0.05 is considered as statistically significant.
RESULTS
The mass spectral data of geldanamycin and geldanamycin derivatives were carried out by 1H-NMR, 13C-NMR as following.
Compound (1): The infrared (IR) spectrum displayed characteristic absorption bands of NH and OH stretches at n 3,478, 3,440, 3,336, and 3,297cm−1, CH stretch in CH3 and CH2 at n 2,927 cm−1, CH stretch in methyl ether at n 2,853 cm−1, C=O stretch in OCONH2 at n 1,729 cm−1, C=O stretch in a,b-unsaturated amide at n 1,701 cm−1 and C=O stretches in quinone at n 1,675 and 1,652 cm−1 (Fig. 2). The MS gave a [M+Na]+ ion at m/z 583.2571 (Fig. 3a) which corresponded to the molecular formula C29H40N2O9 for the compound, indicating eleven double bond equivalents in the molecule. The structure was fully elucidated by 1H-NMR, 13C-NMR spectroscopy, DEPT-135, and 2D-NMR spectral studies. The spectral data revealed the compound 1 to be geldanamycin. Its 1H-NMR and 13C-NMR spectral data of which were in good agreement with those of geldanamycin (Table 1) previously reported by ÅŒmura et al. (1979) and Qin and Panek (2008).
Compound (2): The MS gave a [M + Na]+ ion at m/z 711.3384 (Fig. 3b) which corresponded to the molecular formula C38H48N4O8. The structure was fully elucidated by 1H-NMR, 13C-NMR spectroscopy, DEPT-135, and 2D NMR spectral studies as shown in Table 2.
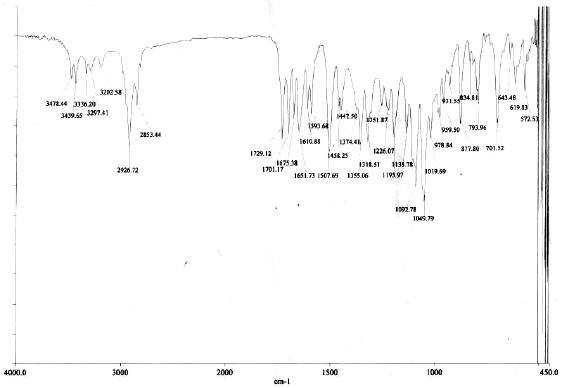 | Figure 2. IR spectrum of compound 1. [Click here to view] |
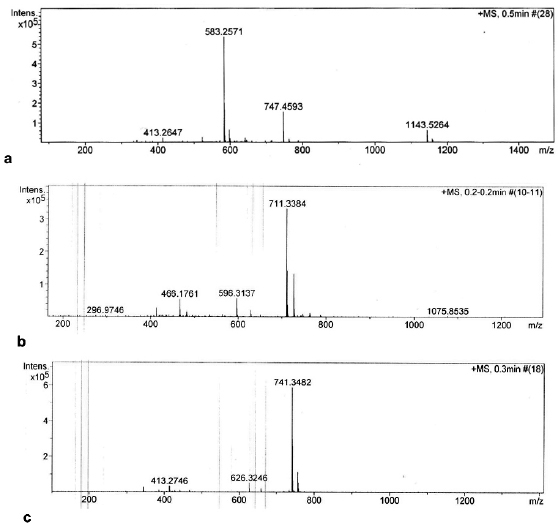 | Figure 3. Mass spectrum of compounds 1 (a), 2 (b) and 3 (c). [Click here to view] |
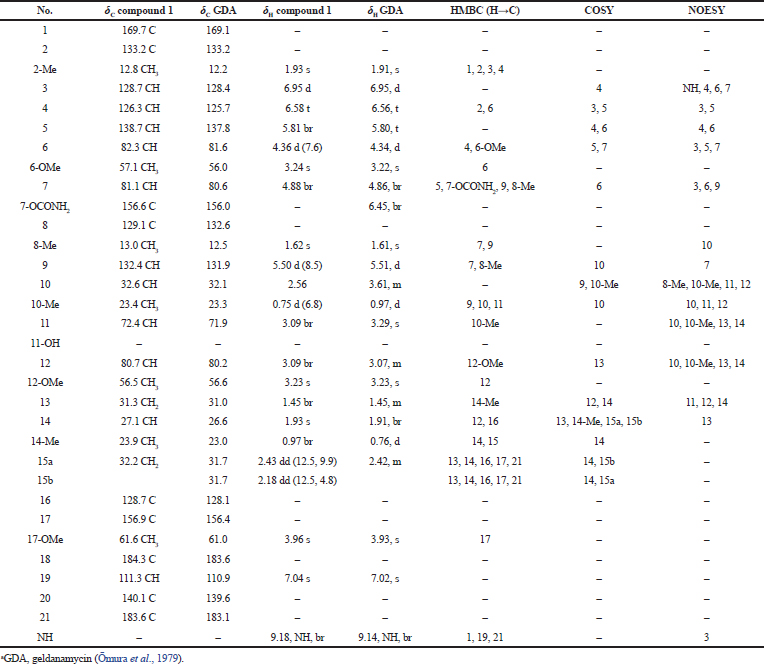 | Table 1. Comparison of the spectral data of the compound 1 and GDAa. [Click here to view] |
Compound (3): The MS gave a [M + Na]+ ion at m/z 741.3482 (Fig. 3c) which corresponded to the molecular formula C39H50N4O9. The structure was fully elucidated by 1H-NMR, 13C-NMR spectroscopy, DEPT-135, and 2D NMR spectral studies as shown in Table 2. The detailed comparison of spectral data of compounds 3 and 1 is shown in Table 3.
The water solubility of geldanamycin (1) was found to be 151.78 μM (Table 4). In contrast, the solubility of its derivatives (2 and 3) in water was 290.69 and 348.18 μM, respectively, about 1.91 and 2.29 times, respectively, higher than that of geldanamycin. These data suggest that the attachment of a tryptamine moiety to geldanamycin at the C17 position greatly enhanced its water solubility.
Geldanamycin and its derivatives were evaluated for cytotoxicity activity against Vero, MCF-7, HeLa, and HepG2 cell lines using the MTT assay. Compounds 2 and 3 exhibited weak cytotoxicity activity toward Vero and HeLa cells with IC50 values of >200.00 μg/ml (Table 5 and Fig. 4). However, these compounds were able to exhibit stronger cytotoxicity activity to MCF-7 and HepG2 than geldanamycin. These data were of interest, as it suggested that two novel geldanamycin derivatives were more toxic to some cancer cells than normal cells. Accordingly, they could be considered potential anticancer drugs in appropriate dosages.
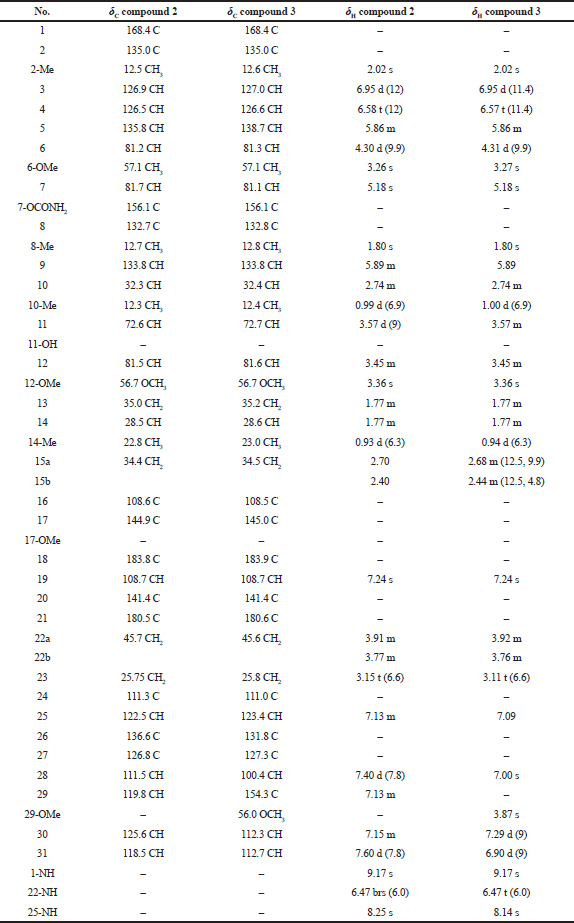 | Table 2. Comparison of the spectral data of the compound 2 and compound 3. [Click here to view] |
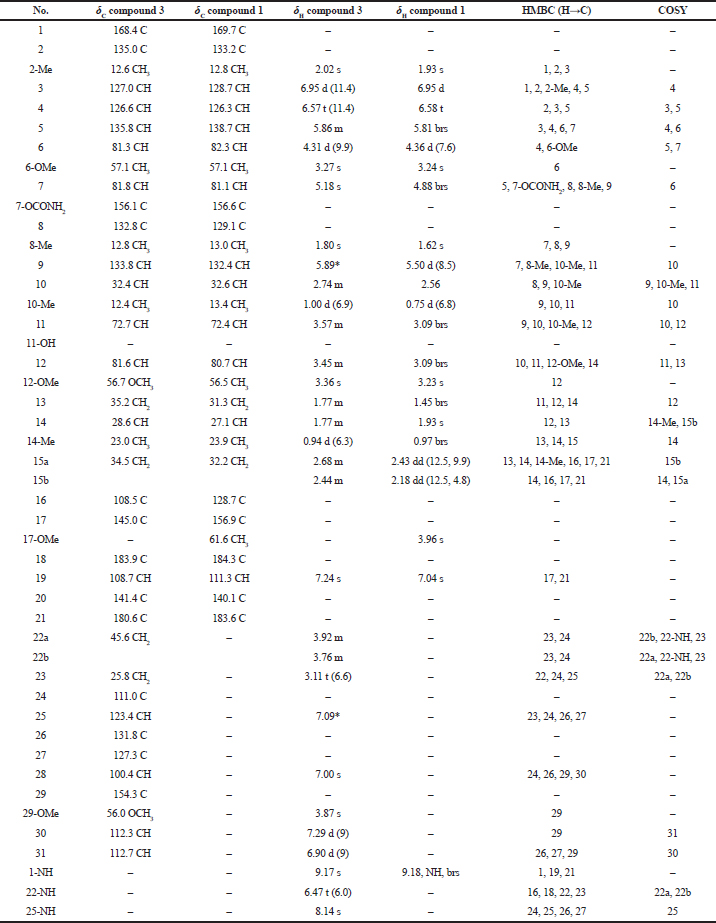 | Table 3. Comparison of the spectral data of the compound 3 and compound 1. [Click here to view] |
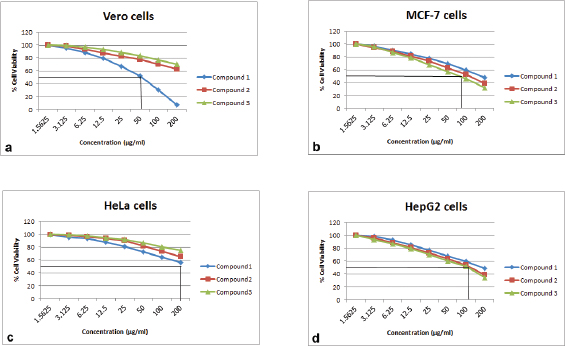 | Figure 4. Cytotoxicity effects of compounds 1(♦), 2(â– ) and 3(â–²) on Vero cells (a), MCF-7 cells (b), HeLa cells (c) and HepG2 cells (d) using an MTT colorimetric assay. [Click here to view] |
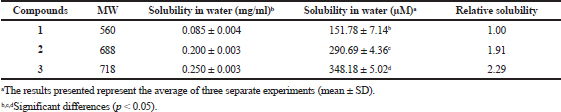 | Table 4. Water solubility of geldanamycin (1) and its derivatives (2 and 3). [Click here to view] |
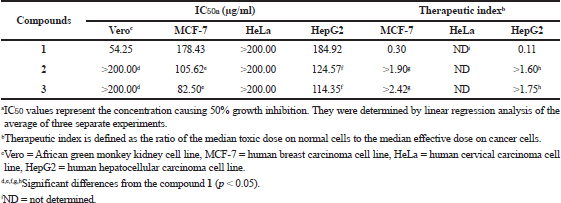 | Table 5. Cytotoxicity activity (IC50) of geldanamycin (1) and its derivatives (2 and 3). [Click here to view] |
DISCUSSION
The benzoquinone ansamycins are an important class of Hsp90 inhibitors that possess potent anticancer activity in preclinical models and may emerge as efficacious therapeutic agents for the treatment of cancer (Isaacs, 2005). More generally, Hsp90 is an attractive therapeutic anticancer target because inhibition of its chaperone activity results in lower cellular levels of multiple client proteins that are critical for cancer-cell survival (Neckers, 2002). Geldanamycin, an ansamycin antibiotic, along with its analogues, had significant anticancer properties (Schnur et al., 1995; Whitesell et al., 1992). These compounds bind specifically to the adinosine triphosphate (ATP) binding site of Hsp90 agents, these disrupted Hsp90 association with client protein (Grenert et al., 1997; Prodromou et al., 1997; Stebbins et al., 1997). Therefore, the prevention of binding of Hsp90 with ATP affected extremely the composition of Hsp90-containing chaperone complexes (An et al., 1997; Obermann et al., 1998; Workman et al., 2002). However, the use of geldanamycin and its derivatives as a chemotherapeutic agent did not proceed owing to its severe hepatotoxicity, metabolic instability, and poor water solubility (Fukuyo et al., 2009; Supko et al., 1995). The improvement of water solubility leads to drug potency and advantageous pharmacological profiles. Therefore, water-soluble geldanamycin derivatives with improved pharmacokinetic and pharmacological properties are necessary (Cheng et al., 2005; Wu et al., 2012). Tryptamine has been an effective tool for improving the water solubility, biological potency, and pharmacokinetic properties of many natural products (Kousara et al., 2017). Recently, tryptamine derivatives have showed moderate to good anticancer activity against HepG2, HeLa, CNE1, and A549 human cancer cell lines with IC50 values of 16.5–18.7 μM (Guo et al., 2019). In this study, geldanamycin exhibited lower solubility in water than its derivatives. It also exhibited more cytotoxicity against normal cells than cancer cells, but no cytotoxicity against HeLa cells. This differs from those reports that geldanamycin was extremely active against KB cells, an ubiquitous keratin-forming HeLa cell line (<0.001 μg/ml) (Deboer et al., 1970), but showed moderate cytotoxicity against MCF-7 and HepG2. According to other reports, geldanamycin was active against various human breast cancer cell lines including: SKBr3 (IC50 value of 37 nM) (Hu et al., 2004); BC (IC50 value of 0.01 μg/ml) (Jongrungruangchok et al., 2006); MDA-MB-231, MCF-7 and T-47D (IC50 value of 100 μM) and human hepatocellular carcinoma cell lines, HepG2, and SMMC7721 (IC50 value of 200 μM) (Zhang et al., 2016). In contrast, cytotoxicity activity of tryptaminated geldanamycins against cancer cells (MCF-7 and HepG2) exhibited greater activity than geldanamycin. This was due to the increased water solubility of geldanamycin derivatives and enhanced anticancer activity of tryptaminated geldanamycins.
CONCLUSION
In summary, the two new tryptaminated-geldanamycins have been synthesized; they showed the water solubility improvement. They also have potent cytotoxic effects on MCF-7; human breast cancer cells, and HepG2 with low cytotoxicity against Vero cells. These results suggest that the tryptaminated-geldanamycin derivatives may be a potential therapeutic candidate for the treatment of some cancers. They could be useful for future drug development.
ACKNOWLEDGMENTS
The authors are grateful to Ms Chanjira Jaramornburapong and Ms Chanakan Winyakul in the Department of Chemistry, Faculty of Science, Silpakorn University, Thailand, for measuring NMR and MS data, respectively. This work was supported by the Faculty of Science, Silpakorn University, Nakhon Pathom, Thailand.
CONFLICT OF INTEREST
Authors declare that they do not have any conflicts of interest.
REFERENCES
An WG, Schnur RC, Neckers L, Blagosklonny MV. Depletion of p185erbB2, Raf-1, and mutant p53 proteins by geldanamycin derivatives correlates with antiproliferative activity. Cancer Chemother Pharm, 1997; 40(1):60–4. CrossRef
Cheng H, Cao X, Xian M, Fang L, Cai TB, Ji JJ, Tunac JB, Sun D, Wang PG. Synthesis and enzyme-specific activation of carbohydrategeldanamycin conjugates with potent anticancer activity. J Med Chem, 2005; 48(2):645–52. CrossRef
Coombs JT, Franco CMM. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol, 2003; 69(9):5603–8. CrossRef
Deboer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. J Antibiot, 1970; 23(9):442–7. CrossRef
Fukuyo Y, Hunt CR, Horikoshi N. Geldanamycin and its anti-cancer activities. Cancer Lett, 2009; 290(1):24–35. CrossRef
Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (Hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates Hsp90 conformation. J Biol Chem, 1997; 272(38):23843–50. CrossRef
Guo Z, Xu Y, Peng Y, Rashid HU, Quan W, Xie P, Wu L, Jiang J, Wang L, Liu X. Design, synthesis and evaluation of novel (S)-tryptamine derivatives containing an allyl group and an aryl sulfonamide unit as anticancer agents. Bioorg Med Chem Lett, 2019; 29(1):1133–7. CrossRef
Hoelke B, Gieringer S, Arlt M, Saal C. Comparison of nephelometric, UV-spectroscopic, and HPLC methods for high-throughput determination of aqueous drug solubility in microtiter plates. Anal Chem, 2009; 81(8):3165–72. CrossRef
Hu Z, Liu Y, Tian ZQ, Ma W, Starks CM, Regentin R, Licari P, Myles DC, Hutchinson CR. Isolation and characterization of novel geldanamycin analogues. J Antibiot, 2004; 57(7):421–8. CrossRef
Isaacs JS. Heat-shock protein 90 inhibitors in antineoplastic therapy: is it all wrapped up? Expert Opin Investig Drugs, 2005; 14(6):569–89. CrossRef
Jongrungruangchok S, Tanasupawat S, Kittakoop P, Bavovada R, Kobayashi H, Kudo T. Identification of Streptomyces and Kitasatospora strains from Thai soils with geldanamycin production strain. Actinomycetologica, 2006; 20(1):10–14. CrossRef
Jurczyszyn A, Zebzda A, Czepiel J, Perucki W, Banzan-Socha S, Cibor D, Owczarek D, Majka M. Geldanamycin and its derivatives inhibit the growth of myeloma cells and reduce the expression of the MET receptor. J Cancer, 2014; 5(6):480–90. CrossRef
Kousara S, Anjuma SN, Jaleela F, Khana J, Naseema S. Biomedical significance of tryptamine: a review. J Pharmacovigil, 2017; 5(5):239. CrossRef
Le Brazidec JY, Kamal A, Busch D, Thao L, Zhang L, Timony G, Grecko R, Trent K, Lough R, Salazar T, Khan S, Burrows F, Boehm MF. Synthesis and biological evaluation of a new class of geldanamycin derivatives as potent inhibitors of Hsp90. J Med Chem, 2004; 47(15):3865–73.
Lee EJ. Cancer chemoprevention effects of geldanamycin and 17-AAG in human oral squamous cell carcinoma. Korean J Clin Lab Sci, 2018; 50(4):462–9. CrossRef
Lin Z, Peng R, Li Z, Wang Y, Lu C, Shen Y, Wang J, Shi G. 17-ABAG, a novel geldanamycin derivative, inhibits LNCaP cell proliferation through heat shock protein 90 inhibition. Int J Mol Med, 2015; 36(2):424–32. CrossRef
Malik MA, Raza MK, Dar OA, Amadudin, Abid M, Wani MY, Al-Bogami AS, Hashmi AA. Probing the antibacterial and anticancer potential of tryptamine based mixed ligand schiff base ruthenium(III) complexes. Bioorg Chem, 2019; 87:773–82. CrossRef
Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem, 1996; 271(37):22796–801. CrossRef
Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res, 2011; 17(15):5132–9. CrossRef
Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med, 2002; 8(4):S55–61. CrossRef
Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of HSP90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol, 1998; 143(4):901–10. CrossRef
ÅŒmura S, Nakagawa A, Sadakane N. Structure of herbimycin a new ansamycin antibiotic. Tetrahedron Lett, 1979; 20(44):4323–6. CrossRef
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell, 1997; 90(1):65–75. CrossRef
Qin HL, Panek JS. Total synthesis of the Hsp90 inhibitor geldanamycin. Organic Lett, 2008; 10(12):2477–9. CrossRef
Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, Miller PE, Pollalk VA, Savage DM, Sloan DE, Pustilnik LR, Moyer MP. ErbB-2 oncogene inhibition by geldanamycin derivatives: synthesis, mechanism of action, and structure-activity relationships. J Med Chem, 1995; 38(19):3806–12. CrossRef
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell, 1997; 89(2):239–50. CrossRef
Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemoth Pharm, 1995; 36(4):305–15. CrossRef
Taechowisan T, Chaisaeng S, Phutdhawong WS. Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07; an endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agri Immunol, 2017; 28(6):1330–46. CrossRef
Taechowisan T, Lumyong S. Activity of endophytic actinomycetes from roots of Zingiber officinale and Alpinia galanga against phytopathogenic fungi. Ann Microbiol, 2003; 53(3): 291–8.
Taechowisan T, Puckdee W, Phutdhawong WS. Streptomyces zerumbet, a Novel species from Zingiber zerumbet (L.) Smith and isolation of its bioactive compounds. Adv Microbiol, 2019; 9(3):194–219. CrossRef
Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem, 2004; 12(20):5317–29. CrossRef
Vasilevskaya IA, Rakitina TV, O’Dwyer PJ. Geldanamycin and its 17-allylamino-17-demethoxy analogue antagonize the action of cisplatin in human colon adenocarcinoma cells: differential caspase activation as a basis for interaction. Cancer Res, 2003; 63(12):3241–6.
Wang X, Su H, Wang W, Chen C, Cao X. Peptidomimetics based on dehydroepiandrosterone scaffold: synthesis, antiproliferation activity, structure-activity relationship, and mechanisms. Sci Rep, 2016; 6:32654. CrossRef
Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA, 1994; 91(18):8324–8. CrossRef
Whitesell L, Shifrin SD, Schwab G, Neckers LM. Benzoquinonoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res, 1992; 52(7):1721–8.
Workman P, Maloney A. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther, 2002; 2(1):3–24. CrossRef
Wrona IE, Gozman A, Taldone T, Chiosis, G, Panek JS. Synthesis of reblastatin, autolytimycin, and non-benzoquinone analogues: potent inhibitors of heat shock protein 90. J Org Chem, 2010; 75(9):2820–35. CrossRef
Wu CZ, Jang JH, Woo MH, Ahn JS, Kim JS, Hong YS. Enzymatic glycosylation of non benzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase. Appl Environ Microbiol, 2012; 78(21):7680–6. CrossRef
Zhang Z, Li HM, Zhou C, Li Q, Ma L, Zhang Z, Sun Y, Wang L, Zhang X, Zhu B, Hong YS, Wu CZ, Liu H. Non-benzoquinone geldanamycin analogs trigger various forms of death in human breast cancer cells. J Exp Clin Cancer Res, 2016; 35(1):149. CrossRef