INTRODUCTION
Cellular oxidative stress arising as a result of excessive free radicals or depletion of antioxidants is well recognized to cause cell damage (Golbidi et al., 2018). Melanocytes are specialized skin cells producing melanin pigments that act as antioxidants to minimize and neutralize free radicals, resulting in abnormal hyperpigmentation including actinic damage, age spots, and melasma (Cals-Grierson and Ormerod, 2004; Slominski et al., 2004). Free radicals are known to assist in the proliferation of melanocyte and the melanogenesis-related tyrosinase enzyme (Friedmann and Gilchrest, 1987; Mastore et al., 2005). Tyrosinase is a rate-limiting enzyme involved in melanin synthesis. Recently, an application of antioxidants has been proposed, which can reduce the melanocyte proliferation and melanogenesis by targeting on redox balance (Yamakoshi et al., 2003), since modulation of free radicals in melanin-producing cells has been shown to suppress melanin production through bioactivity of cellular tyrosinase-related oxidants.
Tetra-oxygenated stilbenes of oxyresveratrol found in white mulberry and Artocarpus lakoocha Roxb. (Kim et al., 2010; Maneechai et al., 2009) demonstrated the high antioxidant activity (Kim, 2007; Yokozawa and Kim, 2007), whereas the OH group of four-substituted resorcinol of oxyresveratrol was shown to be mostly related to tyrosinase inhibition (Kim and Uyama, 2005). Oxyresveratrol is an effective whitening agent in cosmetics. Hence, this active ingredient is potentially used to control hyperpigmentation disorders as pharmaceutical purposes. Therefore, the effects of oxyresveratrol on melanogenesis through bioactivities of cellular tyrosinase-related oxidative stress in B16 melanoma cells were investigated in this study.
MATERIALS AND METHODS
Oxyresveratrol sample
OxyResvenox, the commercial standard of oxyresveratrol (Sabinsa Co. Ltd., Germany), was used as the oxyresveratrol sample. The sample solution was freshly prepared by dissolving in 40% ethanol solution and filtrating through 0.45-μm polytetrafluoroethylene filter nylon. The sample was subsequently diluted with complete Dulbecco’s Modified Eagle’s Medium (DMEM) before using in the experiment.
2,2'-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activity
ABTS assay was performed according to the previous study (Shang et al., 2018). Briefly, various concentrations of the sample (200 μl) or Trolox as a standard were separately mixed and allowed to react with freshly prepared ABTS working solution (1.8 ml) for 30 minutes, and then, the absorbance was measured at 734 nm (Spectrophotometer Model UV-2650, Labomed, Los Angeles, CA). The scavenging activity was calculated and expressed as % ABTS radical scavenging activity by the following equation:
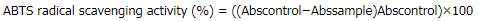
where Abscontrol was the absorbance of control and Abssample was the absorbance of sample at different concentrations. The assay was carried out in triplicate.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging activity was assayed by slightly modified method (Shang et al., 2018). Briefly, various concentrations of the sample (200 μl) or Vitamin C standard were allowed to react with fresh DPPH working solution (1.8 ml) for 30 minutes. The reaction was immediately measured at 540 nm by a spectrophotometer (UV-2650, Labomed, Angeles, CA). The scavenging activity was expressed as % DPPH radical scavenging activity by following equation:
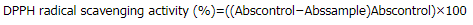
where Abscontrol was the absorbance of control and Abssample was the absorbance of sample at different concentrations. The assay was done in triplicate.
B16 melanoma cells
B16 melanoma cells (RIKEN Cell Bank, Tsukuba, Japan) were maintained in DMEM supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml of penicillin, 100 mg/ml of streptomycin, and 3.7 mg/ml of NaHCO3 at 37°C with 5% CO2.
Oxyresveratrol on B16 cell viability
B16 cell viability was investigated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described by modification (Seo et al., 2018). Briefly, 3.0 × 104 cells of B16 were seeded in the 96-well microculture plates and cultured for 24 hours. The cultured medium was then discarded, and the cells were individually cultured with various concentrations of each sample in conditioned medium (200 μl). After 24 hours of incubation, the cultured medium was substituted with 100 μl of medium containing 10 μl of 5 mg/ml MTT stock solution. The medium was discarded after additional incubation for 2 hours, and 100 μl of absolute dimethyl sulfoxide was subsequently added to each well to solubilize the formazan crystal. The color was immediately read at a wavelength of 570 nm. The result was expressed as a percentage of cell viability by comparing 100% of the untreated control.
Melanin content
The effect of oxyresveratrol on melanin biosynthesis was assayed using a modified method (Seo et al., 2018). In brief, 3.0 × 105 cells of B16 were seeded in 24-well plates and incubated in DMEM for 24 hours. The cells were pretreated with 50 nM of α-melanocyte-stimulating hormone (α-MSH) for 24 hours. The cells treated with 2 μM of H2O2 for 1 hour were incubated in fresh DMEM for 6 hours to induce cellular oxidative stress. Oxidative stress-induced B16 cells were treated with 10 and 12.5 μg/ml of oxyresveratrol in DMEM or 50 mM of sodium L-lactate (SLL), positive control, and incubated for 48 hours. To determine the melanin content, B16 cells were washed with phosphate-buffered saline (PBS) pH 7.4, lysed with 100 μl of 1 N NaOH, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride (PMSF), and incubated at 60°C for 1 hour. The lysate was optically measured at a wavelength of 405 nm. The results were expressed as a percentage of melanogenesis, 100% for untreated cells.
Fontana–Masson staining
The melanin production and cellular morphology of B16 melanoma cells were stained with Fontana–Masson staining and obtained under microscopy (Vasilevska et al., 2016). In brief, 5.0 × 105 cells of B16 were cultured in DMEM (6-well plates) for 24 hours. The cells were pretreated with α-MSH (50 nM) for 24 hours. The cells treated with 2 μM of H2O2 for 1 hour were then incubated in fresh DMEM for 6 hours and incubated with DMEM containing oxyresveratrol or 50 mM of SLL, positive control, for 48 hours. The cells were fixed with absolute ethanol and rehydrated with distilled water. Cells were subsequently incubated with an ammoniacal silver solution for 24 hours and gently rinsed with distilled water. Sodium thiosulfate solution was added for 5 minutes and counterstained with Mayer’s Carmalum stain for 10 minutes. The melanin production and cellular morphology were obtained by a light microscopy (400× of magnification). Melanin-producing cells were calculated and expressed as a percentage of total cells (counted in 1,000 cells).
Cellular tyrosinase activity
Tyrosinase activity was evaluated by a modified method (Lin et al., 2011). B16 cells (3.0 × 105 cells) were seeded in 24-well plates and incubated for 24 hours. The cells were pretreated with 50 nM of α-MSH for 24 hours. Cells treated with 2 μM of H2O2 for 1 hour were incubated in fresh DMEM for 6 hours and then incubated with DMEM containing oxyresveratrol for 48 hours. The cells were then lysed by a freeze-thaw technique using lysing buffer (PBS, pH 6.8 containing 0.1% Triton X-100 and 0.1 mM PMSF). The lysate was clarified by centrifugation at 12,000 g, 4°C for 30 minutes. The 80 μl of supernatant was transferred to 96-well plates and incubated with 20 μl of 20 mM L-DOPA for 60 minutes. The reaction was detected at a wavelength of 492 nm. The results were calculated and expressed as a percentage of tyrosinase activity (100% for untreated cells). About 50 mM of SLL was used as a positive control.
Cellular oxidants
Cellular oxidants were assayed by using a modified method (Aimvijarn et al., 2018). B16 cells (3.0 × 105 cells) were seeded in 96-well plates and incubated at 37°C, 5% CO2 for 24 hours. The cells were pretreated with 50 nM of α-MSH for 24 hours. The cells treated with 2 μM of H2O2 for 1 hours were incubated in fresh DMEM for 6 hours and then incubated with DMEM containing oxyresveratrol for 24 hours. Cultured media were subsequently replaced by 10 μM of dichloro-dihydro-fluorescein diacetate (DCFH-DA) in media and incubated at 37°C, 5% CO2 for 1 hour.
Dichlorofluorescein (DCF) fluorescence was instantly evaluated for cellular oxidants using a fluorescence microplate reader (1420 Victor 2, Wallac, ID) at excitation/emission wavelengths of 485/535 nm. N-acetyl cysteine at 5 mM was used as a positive antioxidant. The results were expressed as 100% control for untreated cells.
Statistical analysis
Data were expressed as mean ± standard deviation. The correlation was tested by the Pearson correlation test. The differences of means among comparative groups were determined by one-way analysis of variance (ANOVA) and independent t-test. Significant differences were considered at the level of p ≤ 0.05.
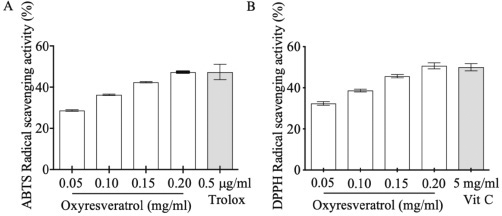 | Figure 1. The antioxidant activity of oxyresveratrol by ABTS (A) and DPPH (B) assays. The results were expressed as mean ± SD. [Click here to view] |
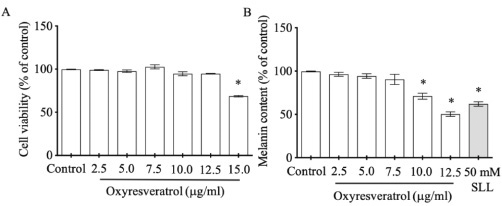 | Figure 2. The effects of oxyresveratrol at various concentrations on B16 cell viability (A) and melanogenesis (B). SLL at 50 mM was used as a melanogenesis inhibitor. The results were expressed as mean ± SD. * Statistical significance between each treatment group and control at p < 0.001. [Click here to view] |
RESULTS AND DISCUSSION
The generation of reactive oxygen species, especially H2O2, results in oxidative stress in epidermal melanocytes that promote the overproduction of melanin pigments (Panich et al., 2012). Here, we demonstrated that oxyresveratrol plays a crucial role in free radical scavenging activity in vitro examined by DPPH and ABTS assays at 28.735 mg ± 0.547 mg Vit C/g sample and 2.312 mg ± 0.422 mg Trolox/g sample, respectively. Both assays also showed gradually increasing antioxidant scavenging activities in a dose-dependent manner in Fig. 1. These results concurred with the previous studies that oxyresveratrol, a polyphenol in the family of stilbenes, is a free radical scavenger (Sun et al., 2010; Yokozawa and Kim, 2007).
Oxidative stress increases melanin production through tyrosinase enzyme pathway (Chang, 2009). In this study, we investigated noncytotoxic dose of oxyresveratrol to apply further in melanogenesis experiment. B16 cells treated with various doses of oxyresveratrol at 2.5, 5.0, 7.5, 10, and 12.5 μg/ml did not impact on cell viability when compared with control (Fig. 2A). Interestingly, at doses 10 and 12.5 μg/ml, oxyresveratrol suppressed melanogenesis significantly at 71% ± 4.900% and 51% ± 3.682%, respectively, compared with 100% control (Fig. 2B). These results were supported by the previous report that oxyresveratrol contained the moieties of 4-resorcinal and 5-resocinal, which act as a melanin inhibitor through tyrosinase function (Chang, 2009).
Hydrogen peroxide demonstrated the induction of both melanin content and tyrosinase activity. H2O2-induced oxidative stress enhanced melanogenesis at 140% ± 5.933% (p < 0.001) compared with control (Fig. 3A). We found that oxyresveratrol at the doses of 10 and 12.5 μg/ml showed melanogenesis inhibition in a dose-dependent manner at 39% ± 8.419% (p < 0.001) and 64% ± 10.536% (p < 0.001), respectively, compared with the H2O2-treated group. In addition, H2O2 (2 μM) also induced tyrosinase activity at 119% ± 4.252% compared with control (p < 0.001) as showed in Fig. 3B. Oxyresveratrol at the doses of 10 and 12.5 μg/ml inhibited tyrosinase activity significantly in a dose-dependent manner (p < 0.001). The effect of oxyresveratrol on tyrosinase activity was greatly associated with melanogenesis (r = 0.948, p < 0.05). These results concurred with the previous report that H2O2-induced oxidative stress induced the melanin production through the pathway of tyrosinase enzyme (Kim and Lee, 2013). Tyrosinase enzyme catalyzes the oxidation of l-tyrosine and l-3,4-dihydroxyphenylalanine (DOPA) to DOPA quinone, resulting in the formation of the brownish-black eumelanin. The excessive of eumelanin production is shown in hyperpigmentation (Khemis et al., 2007; Kim and Uyama, 2005). Interestingly, the phytopolyphenol compounds found in oxyresveratrol can inhibit tyrosinase-related melanogenesis through the antioxidant defense system and also inhibit melanogenesis associated with melanin production (Lorenz et al., 2003; Panich et al., 2012, Park et al., 2014). The cellular oxidants modulated by oxyresveratrol may thus play a crucial role in inhibiting melanogenesis through tyrosinase activity-related cellular oxidants. We also studied the effect of oxyresveratrol on cellular oxidants. Induction of cellular oxidants was achieved by the 2 μM H2O2-treated group at 173% ± 1.247% (p < 0.001) when compared with the untreated group (100% cellular oxidative stress). Oxyresveratrol treatment at the doses of 10 and 12.5 μg/ml showed a statistical significance in decreasing cellular oxidants in a dose-dependent manner at 87% ± 2.055% (p < 0.001) and 72% ± 1.700% (p < 0.001), respectively, compared with H2O2-induced B16 cells (Fig. 3C). The effect of oxyresveratrol on cellular oxidants was considerably associated with melanogenesis (r = 0.948, p < 0.05) and tyrosinase activity (r = 0.965, p < 0.05).
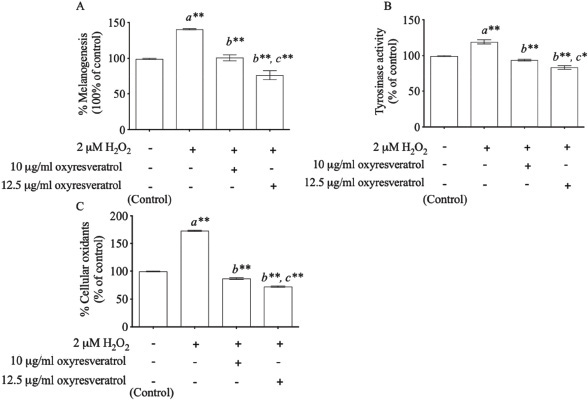 | Figure 3. The effects of oxyresveratrol at the doses of 10 and 12.5 μg/ml on melanogenesis (A), tyrosinase activity (B), and cellular oxidants (C) in H2O2-induced B16 cells. The results were expressed as mean ± SD. * and ** represent the significant difference at p < 0.05 and p < 0.001 versus untreated group (a), H2O2-induced group (b), and H2O2-induced combined with 10 μg/ml oxyresveratrol-treated group (c), respectively. [Click here to view] |
In addition, staining melanin pigments in B16 cells by the Fontana–Masson Stain Kit gave the percentage of melanin-containing cells at 45% ± 4.497% in the control group (Fig. 4A), and it increased to 82% ± 2.625% after treatment with 2 μM of H2O2 (Fig. 4B). Oxyresveratrol at the doses of 10 and 12.5 μg/ml showed a significant suppression of melanin accumulation in a dose-dependent manner at 69% ± 2.944% (p < 0.05) (Fig. 4C) and 44% ± 4.989% (p < 0.001) (Fig. 4D), respectively, compared with the H2O2-treated group. Oxyresveratrol might be a potential candidate for further development of pharmaceutical products to combat antihyperpigmentary disorders.
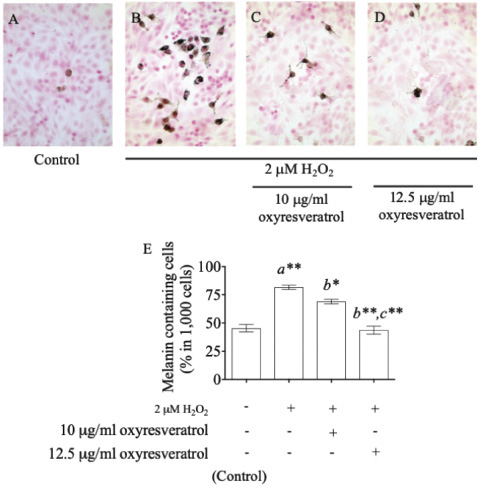 | Figure 4. The effect of oxyresveratrol at the doses of 10 and 12.5 μg/ml on melanin accumulation in H2O2-induced B16 cells. Fontana–Masson staining for melanin accumulation of untreated cells (A), 2 μM H2O2-induced B16 cells (B), 2 μM H2O2 induction with 10 μg/ml (C), and 12.5 μg/ml (D) of oxyresveratrol. The percentages of melanin-containing cells were visualized by a light microscope at magnification 400×, and all the results were expressed in graph with mean ± SD (E). Statistical significance was evaluated by ANOVA test. * and ** represent the significant difference at p < 0.05 and p < 0.001 versus untreated group (a), H2O2-induced group (b), and H2O2-induced combined with 10 μg/ml oxyresveratrol-treated group (c), respectively. [Click here to view] |
CONCLUSION
H2O2-induced oxidative stress promoted a melanin production by inducing tyrosinase-related cellular oxidative stress. Oxyresveratrol was shown to inhibit melanogenesis through the bioactivity of tyrosinase-associated oxidative stress in B16 cells.
ACKNOWLEDGMENTS
This research was financially supported by the Department of Pathobiology, Faculty of Science, Mahidol University. The authors would like to thank Dr. Nasapon Povichit, Detox (Thailand) Co. Ltd., for providing standard oxyresveratrol.
CONFLICT OF INTEREST
No conflict of interest associated with this work.
REFERENCES
Aimvijarn P, Palipoch S, Okada S, Suwannalert P. Thai water lily extract induces B16 melanoma cell apoptosis and inhibits cellular invasion through the role of cellular oxidants. Asian Pac J Cancer Prev, 2018; 19:149–53.
Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide, 2004; 10:179–93. CrossRef
Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci, 2009; 10:2440–75. CrossRef
Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol, 1987; 133:88–94. CrossRef
Golbidi S, Li H, Laher I. Oxidative stress: a unifying mechanism for cell damage induced by noise, (water-pipe) smoking, and emotional stress-therapeutic strategies targeting redox imbalance. Antioxid Redox Signal, 2018; 28:741–59. CrossRef
Khemis A, Kaiafa A, Queille-Roussel C, Duteil L, Ortonne JP. Evaluation of efficacy and safety of rucinol serum in patients with melasma: a randomized controlled trial. Br J Dermatol, 2007; 156:997–1004. CrossRef
Kim HE, Lee SG. Induction of ATP synthase β by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int J Biochem Cell Biol, 2013; 45:1217–22. CrossRef
Kim JK, Kim M, Cho SG, Kim MK, Kim SW, Lim YH. Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition. J Ind Microbiol Biotechnol, 2010; 37:631–7. CrossRef
Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci, 2005; 62:1707–23. CrossRef
Kim YJ. Antimelanogenic and antioxidant properties of gallic acid. Biol Pharm Bull, 2007; 30:1052–5. CrossRef
Lin CH, Ding HY, Kuo SY, Chin LW, Wu JY, Chang TS. Evaluation of in vitro and in vivo depigmenting activity of raspberry ketone from Rheum officinale. Int J Mol Sci, 2011; 12:4819–35. CrossRef
Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide, 2003; 9:64–76. CrossRef
Maneechai S, Likhitwitayawuid K, Sritularak B, Palanuvej C, Ruangrungsi N, Sirisa-Ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and “Puag-Haad”. Med Princ Pract, 2009; 18:223–7. CrossRef
Mastore M, Kohler L, Nappi AJ. Production and utilization of hydrogen peroxide associated with melanogenesis and tyrosinase-mediated oxidations of DOPA and dopamine. FEBS J, 2005; 272:2407–15. CrossRef
Panich U, Onkoksoong T, Limsaengurai S, Akarasereenont P, Wongkajornsilp A. UVA-induced melanogenesis and modulation of glutathione redox system in different melanoma cell lines: the protective effect of gallic acid. J Photochem Photobiol B, 2012; 108:16–22. CrossRef
Park J, Park, JH, Suh HJ, Lee IC, Koh J, Boo YC. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res, 2014; 306:475–87. CrossRef
Seo JO, Yumnam S, Jeong KW, Kim SY. Finasteride inhibits melanogenesis through regulation of the adenylate cyclase in melanocytes and melanoma cells. Arch Pharm Res, 2018; 41:324–32. CrossRef
Shang HM, Zhou HZ, Yang JY, Li R, Song H, Wu HX. In vitro and in vivo antioxidant activities of inulin. PLoS One, 2018; 13:e0192273. CrossRef
Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev, 2004; 84:1155–228. CrossRef
Sun HY, Xiao CF, Cai YC, Chen Y, Wei W, Liu XK, Lv ZL, Zou Y. Efficient synthesis of natural polyphenolic stilbenes: resveratrol, piceatannol and oxyresveratrol. Chem Pharm Bull (Tokyo), 2010; 58:1492–6. CrossRef
Vasilevska J, De Souza GA, Stensland M, Skrastina D, Zhulenvovs D, Paplausks R, Kurena B, Kozlovska T, Zajakina A. Comparative protein profiling of B16 mouse melanoma cells susceptible and non-susceptible to alphavirus infection: Effect of the tumor microenvironment. Cancer Biol Ther, 2016; 17:1035–50. CrossRef
Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res, 2003; 16:629–38. CrossRef
Yokozawa T, Kim YJ. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol Pharm Bull, 2007; 30:2007–11. CrossRef