INTRODUCTION
Geldanamycin is a benzoquinone ansamycin, and it binds specifically to heat shock protein 90 (Hsp90) (Prodromou et al., 1997; Sullivan et al., 1997), resulting in dysfunction and rapid degradation of Hsp90-associated client proteins (Blagosklonny, 2002; Richter and Buckner, 2001). As a specific inhibitor of Hsp90 function, its derivatives showed antitumor activity (Miyata, 2005; Ochel et al., 2001) and has been used for the treatment of various cancers (Biamonte et al., 2010; Jhaveri et al., 2012; Pacey et al., 2006). Intervention with the Hsp90 function is the mode of action of geldanamycin (Roe et al., 1999).
For viral replications, Hsp90 is necessary for the viral protein synthesis (Burch and Weller, 2005; Basha et al., 2005; Connor et al., 2007; Geller et al., 2007; Hu and Seeger, 1996; Hu et al., 1997; Hung et al., 2002; Li et al., 2004; Smith et al., 2010; Shan et al., 2011; Waxman et al., 2001). It has been shown that geldanamycin blocks the replication of the viruses both in vitro and in vivo via inhibition of Hsp90 (Amraiz et al., 2017; Li et al., 2004; 2010; 2012; Luo et al., 2003; Schang et al., 2002; Smith et al., 2010; Wang et al., 2017). However, the therapeutic utilization of this compound has been restricted by low water solubility, metabolic instability, and severe hepatotoxicity (Fukuyo et al., 2009; Supko et al., 1995). Therefore, its derivatives with improved pharmacokinetic profiles have been developed. The synthesized series of geldanamycin derivatives to make new types of Hsp90 inhibitor with weak toxicity and high efficiency have been seeking (Kitson et al., 2013; Li et al., 2010; Lin et al., 2015; Modi et al., 2011; Shan et al., 2011; Supko et al., 1995; Tian et al., 2004; Wrona et al., 2010).
Tryptamine was the product of the decarboxylation of tryptophan. It has been used in the past as a vasodilator, neurotransmitter, antiviral, antibacterial, antifungal, anti-inflammatory, and antioxidant agent (Kousara et al., 2017). Its modification has been conducted to be pharmacologically active compounds. Recently, tryptamine has been synthesized as a novel non nucleosidic compound against hepatitis B virus (Qu et al., 2011). It has been a useful tool for improving the solubility, biological activities, and pharmacological properties of numerous natural products (Kousara et al., 2017). According to these effects, the invention of tryptamine-geldanamycin hybrids has been designed. The C17 methoxyl group of geldanamycin molecule can permit for various nucleophiles to be introduced. Thus, geldanamycin has been a popular template for producing various types of bioactive compounds (Lin et al., 2015; Modi et al., 2011; Supko et al., 1995; Tian et al., 2004; Wrona et al., 2010). Furthermore, the other report showed that some of the 17-substituted geldanamycin derivatives contained stronger activity against hepatitis B virus than geldanamycin with higher LD50 values than that of geldanamycin (Li et al., 2010). It has been reported that influenza virus replication could be inhibited by interfering the Hsp90 function (Chase et al., 2008). However, the activity of geldanamycin and its derivatives against influenza virus has been not reported. Therefore, geldanamycin could inhibit the functions of viral proteins by interfering with the complex formation of Hsp90 and viral proteins. Inhibition of Hsp90 activity also causes the inhibition of viral protein synthesis. Furthermore, it has been reported that geldanamycin could inhibit viral replication by preventing the chaperone-mediated process in viral protein folding and functions (Li et al., 2004).
In this study, novel tryptamine-geldanamycin hybrids were synthesized, their antiviral activity against influenza virus was evaluated based on virus propagation in embryonated chicken eggs and viral absorption by hemagglutination (HA) inhibition test, and their water solubility and cytotoxicity were also determined.
MATERIALS AND METHODS
Isolation and cultivation of the actinomycete
Eighteen actinomycetes were isolated from the various tissues (leaf, pseudostem, rhizome, and root) of Zingiber zerumbet (L.) Smith (Taechowisan et al., 2017). Among the 18 isolates, the isolate W14 was found to be the best producer of antibacterial substances (Taechowisan et al., 2019). This isolate was selected and identified using a polyphasic approach (Taechowisan and Lumyong, 2003; Taechowisan et al., 2019). The strain was cultured on ISP-2 agar plates at 30°C for 14 days and then was extracted with ethyl acetate. The crude extract was fractionated using low-to-high polar solvents (ethyl acetate in hexane) and purified on Thin Layer Chromatography (TLC) (Taechowisan et al., 2019). The purified compound was undertaken to investigate on Nuclear Magnetic Resonance (NMR) spectroscopy. The spectral data of this compound corresponded to be geldanamycin (C29H40N2O9) (1).
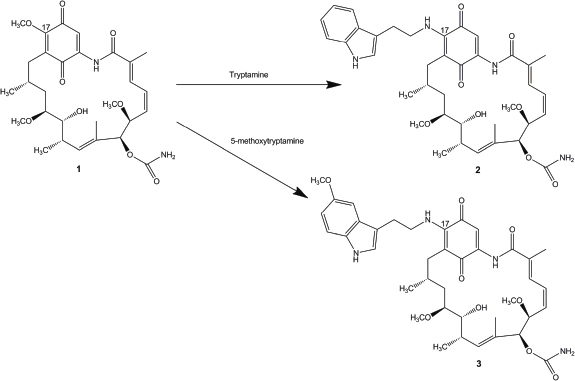 | Figure 1. The semisynthetic route of 17-(tryptamine)-17-demethoxygeldanamycin (2) and 17-(5′-methoxytryptamine)-17-demethoxygeldanamycin (3) from geldanamycin (1). [Click here to view] |
Synthesis and solubility of geldanamycin derivatives
Geldanamycin derivatives were synthesized from geldanamycin (Fig. 1) as described earlier (Taechowisan et al. 2019). The solubility of the novel geldanamycins in water was determined by comparing with geldanamycin.
Viral strain propagation
Influenza viruses A/free-grazing duck/Nakhon Pathom/1/2017 (H5N2) (Taechowisan et al., 2018) were cultivated in embryonated eggs. Viral titer was determined using the HA assay as previously described (Brauer and Chen, 2015).
Virus cultivation inhibition assay
Virus cultivation inhibition assay was carried out by embryonated chicken egg inoculation. About 100 μl of tested compounds (12.5, 25, and 50 μg/ml) was incubated with 100 μl of seed virus (2.86 × 108 virus particles/ml) at 37°C for 30 minutes, and then, 100 μl of the mixture was inoculated into each embryonated chicken egg and incubated at 37°C for 4 days. The allantoic fluid was harvested and then was tested by HA assay (Brauer and Chen, 2015). About 20 μg/ml of heparin (AppliChem, Germany) was used as a positive control.
HA inhibition (HAI) assay
HAI assay was used to evaluate the effect of the compounds in virus adsorption to target cells. The compounds (25 μl) with two-fold serial dilution with Phosphate Buffered Saline (PBS) were mixed with an equal volume of seed influenza virus (400 HAU per 50 μl). After incubation at room temperature for 30 minutes, 50 μl of the solution was mixed with an equal volume of 1% chicken erythrocyte suspension and then was incubated at 4°C for 30 minutes. The highest dilution of the compounds that prevented HA is called the HAI titer. About 20 μg/ml of heparin (AppliChem, Germany) was used as a positive control.
MTT assay for cell viability
The normal cells (LLC-MK2: rhesus monkey kidney epithelial cell line and Vero: African green monkey kidney cell line) were grown at 37°C in Dubecco's Modified Eagle Medium (DMEM) medium supplement with 10% FBS, penicillin (100 units/ml), and streptomycin sulfate (100 μg/ml) in a humidified atmosphere of 5% CO2. Cytotoxicity studies were performed on a 96-well plate. The cells were trypsinized and plated on a 96-well plate (2 × 105 per well) containing DMEM medium with 10% FBS and incubated overnight. Cells were incubated with the compounds at increasing concentrations in FBS-free medium for 24 hours. Cells were washed once, and 50 μl of FBS-free medium containing 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well and incubated in 5% CO2 at 37°C for 4 hours. The medium was replaced with 50 μl of DMSO to dissolve the formazan product. The optical density was measured at 450 nm. The half inhibitory concentration (IC50) was defined as a 50% reduction of the absorbance compared with the control assay.
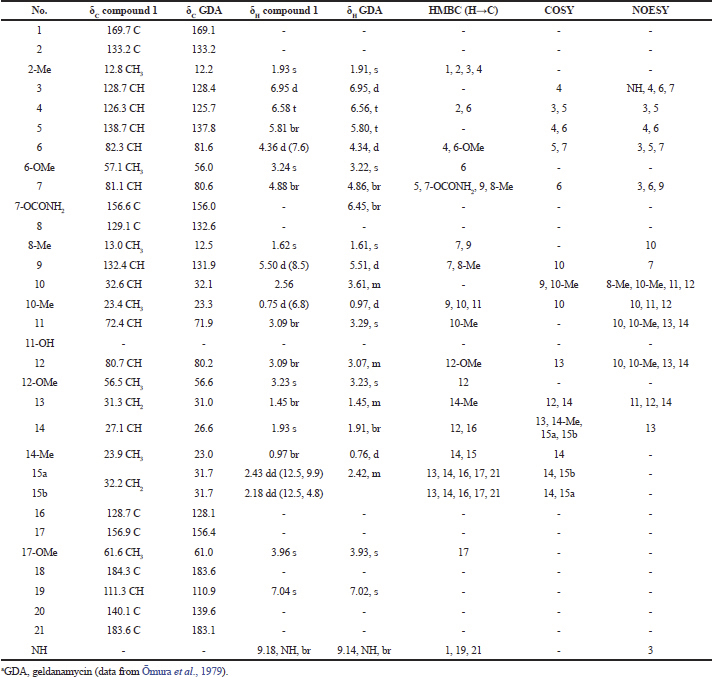 | Table 1. 1H-NMR and 13C-NMR spectral data of compound 1 and GDAa. [Click here to view] |
RESULTS
The characterization of geldanamycins was carried out by 1H-NMR, 13C-NMR, and mass spectral methods as follows.
Compound (1): The mass spectrum showed a [M+Na]+ ion at m/z 583.2571 (molecular formula : C29H40N2O9). The spectral data revealed this compound to be a geldanamycin, which was in agreement with those of geldanamycin (Table 1), previously described by Omura et al. (1979) and Qin and Panek (2008).
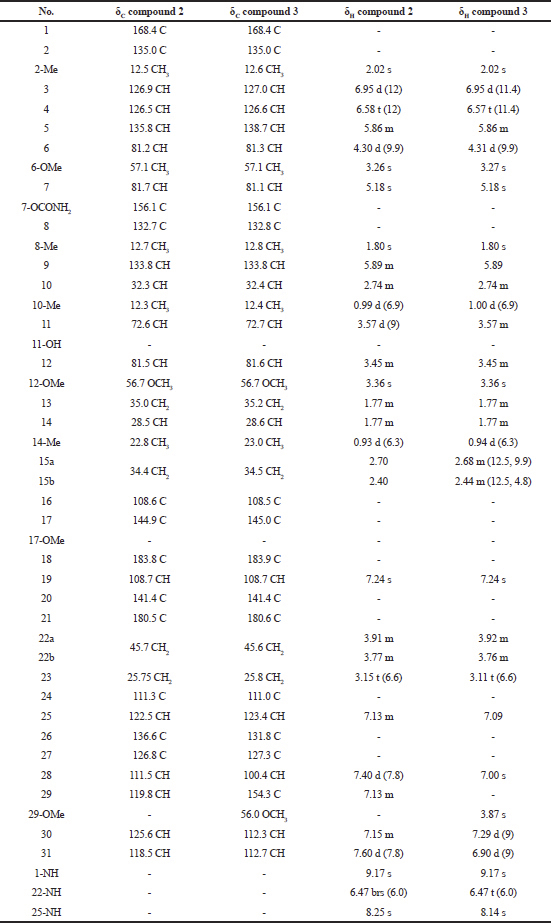 | Table 2. 1H-NMR and 13C-NMR spectral data of compound 2 and compound 3. [Click here to view] |
Compound (2): The mass spectrum showed a [M+Na]+ ion at m/z 711.3384 (molecular formula : C38H48N4O8). The structure was fully elucidated by 1H-NMR, 13C-NMR spectroscopy, DEPT-135, and 2D-NMR spectral data (Table 2).
Compound (3): The mass spectrum showed a [M+Na]+ ion at m/z 741.3482 (molecular formula : C39H50N4O9). The structure was fully elucidated by 1H-NMR, 13C-NMR spectroscopy, DEPT-135, and 2D-NMR spectral data (Table 2), and the spectral data of this compound were compared with the spectral data of compound 1 (Table 3).
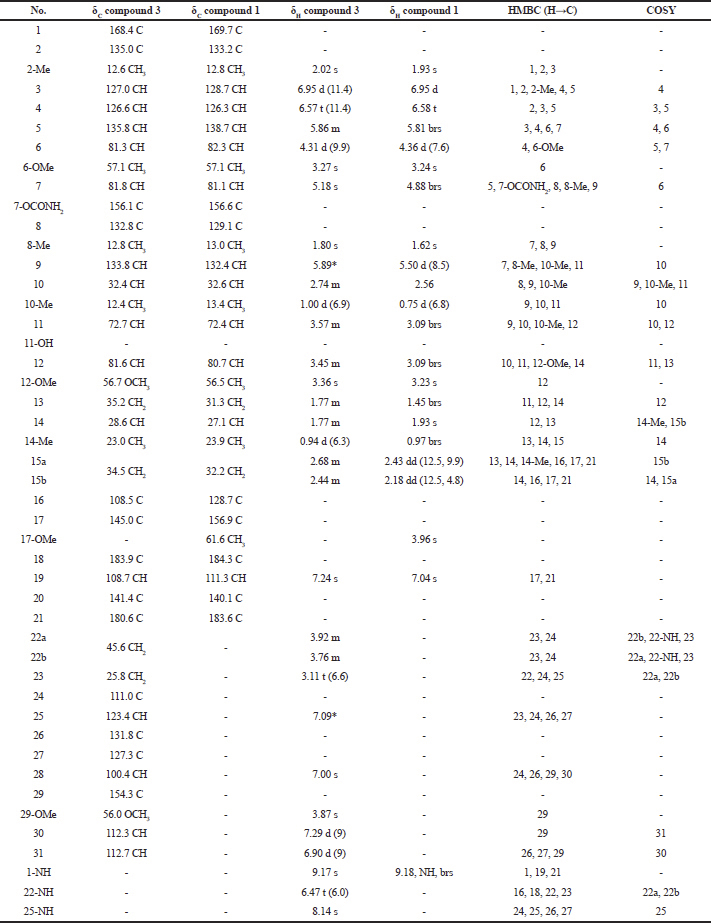 | Table 3. 1H-NMR and 13C-NMR spectral data of compound 3 and compound 1. [Click here to view] |
The water solubility of geldanamycin (1) was found to be 151.60 μM (Table 4). In contrast, the solubility of its derivatives (2 and 3) in water was 290 and 306 μM, respectively, about 1.91 and 2.01 times higher than that of geldanamycin, respectively. These data suggest that the conjugation of a tryptamine moiety to geldanamycin at the C17 position greatly enhanced their water solubility.
The effect of geldanamycin and its derivatives on influenza virus propagation was evaluated at various concentrations in embryonated chicken eggs. The virus yields were determined by HA test. The virus propagation was obtained only in control, whereas no virus was detected in the compound treatments. In addition, the effect of geldanamycin and its derivatives on viral adsorption to chicken erythrocytes was carried out. Interestingly, as expected, the compounds 2 and 3 inhibited viral binding to the cells with HAI titer of 1:50, whereas geldanamycin could not inhibit viral binding to the cells (Table 4). These data suggested that geldanamycin and its derivatives inhibited influenza virus propagation, but tryptamine-geldanamycin hybrids could inhibit the viral adsorption (early step) of influenza virus infection. Heparin at the concentration of 20 μg/ml could completely inhibit both viral propagation and viral absorption (data not shown).
Geldanamycin and its derivatives were evaluated for cytotoxicity activity against LLC-MK2 and Vero cell lines using the MTT assay. The compounds 2 and 3 exhibited weak cytotoxicity activity toward LLC-MK2 and Vero cells with IC50 values of >200.00 μg/ml (Table 5). The results show that two novel geldanamycin possesses low toxicity to normal cells and can display potential application in antiviral chemoprevention and chemotherapy.
DISCUSSION
Influenza virus causes seasonal outbreaks in temperate regions, with an increase in disease and mortality rate which is a serious problem. With the expectation of exploiting the potency of Hsp90 inhibitor against influenza virus, we investigated an in vitro inhibitory activity of geldanamycin and its derivatives against influenza virus as they were promising candidates in vitro.
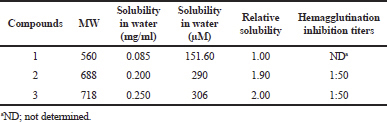 | Table 4. Water solubility and HAI titers of geldanamycins. [Click here to view] |
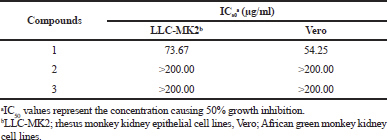 | Table 5. Cytotoxicity activity (IC50) of geldanamycins. [Click here to view] |
The toxicity and water solubility of geldanamycin have been a marked hindrance for its development for chemotherapy use. These have incentive scientists to pay attention to develop less toxic geldanamycin derivatives. In this study, compounds 2 and 3 also exhibited less cytotoxicity than geldanamycin in the normal cell lines. We also found that these compounds showed a greater increase in water solubility. It should be mentioned that the antiviral activity of geldanamycin and its derivatives appeared in influenza virus propagation, which suggests that geldanamycin and its derivatives are an option choice in terms of antiviral agents in the viral propagation step. The introduction of a tryptamine or 5′-methoxytryptamine group at the C17-position of geldanamycin could not interfere with the binding of geldanamycin derivatives to Hsp90, but it had a largely decreased toxicity and increased water solubility. As stated by the crystal structure of the geldanamycin-Hsp90 complex (Stebbins et al., 1997), the substitution in the C17 methoxyl of geldanamycin is revealed to the external cavity of the Hsp90 protein, while the difference of substituents of geldanamycin is crucial for the interaction with the Hsp90 protein. According to the report by Li et al. (2010), 17-amino-17-demethoxygeldanamycin derivatives have a great potential for antiviral activity, whereas the 19-substituted geldanamycin modification was not a possibility in terms of antiviral agents. Due to the introduction of a group, the C19-position of geldanamycin could interfere with the binding of geldanamycin derivatives to Hsp90 by the steric effects (Li et al., 2010). These results led us to contemplate that Hsp90 could be a target for antiviral infection and that geldanamycin and its derivatives have a great potential for antiviral propagation by interfering with Hsp90 in the protein folding and stabilizing of virus-infected cells.
The invention of tryptamine-geldanamycin hybrids has been designed at the C17-position of geldanamycin by nucleophilic substitution reactions. These compounds inhibited not only on viral propagation but also on viral absorption. It suggested that tryptamine-geldanamycin hybrids could protect viral infection in both the steps. According to this effect, Sun et al. (2019) reported that tryptophan dendrimers could block receptor binding of enterovirus A71, and these compounds could prevent binding and internalization of the virus. Furthermore, the Chinese Academy of Science, Shanghai, has shown that tryptamine derivatives had an antiviral activity against hepatitis B virus (Qu et al., 2017). This study discovered the effects of the tryptamine-geldanamycins on inhibition of influenza virus propagation and adsorption. This study will help the researcher to uncover the structural modifications of the compounds to improve biological activities.
CONCLUSION
In summary, the antiviral activity, low toxicity, and enhanced water solubility of two new tryptamine-geldanamycins, compounds 2 and 3, were presented in this work, in comparison with geldanamycin. In particular, all of these compounds showed antiviral activity in viral propagation, whereas the tryptamine-geldanamycins not only inhibit viral propagation but also inhibit viral absorption which has low toxicity and good water solubility better than geldanamycin. These results show that the functions of Hsp90 can be inhibited by these compounds and the virus cannot be propagated. This suggests a new antiviral approach. Therefore, Hsp90 could be an excellent antiviral target, and the tryptamine-geldanamycins could be considered as a new choice for antiviral agents.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Chanjira Jaramornburapong and Ms. Chanakan Winyakul in the Department of Chemistry, Faculty of Science, Silpakorn University, Thailand, for measuring NMR and Mass Spectrometry (MS) data, respectively. This work was supported by the Faculty of Science, Silpakorn University, Nakhon Pathom, Thailand.
REFERENCES
Amraiz D, Zaidi NSS, Fatima M. Antiviral evaluation of an Hsp90 inhibitor, gedunin, against dengue virus. Trop J Pharm Res, 2017; 16:997–1004. CrossRef
Basha W, Kitagawa R, Uhara M, Imazu H, Uechi K, Tanaka J. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibit gene expression and replication of human cytomegalovirus. Antivir Chem Chemother, 2005; 16:135–46. CrossRef
Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. Heat shock protein 90: inhibitors in clinical trials. J Med Chem, 2010; 53:3–17. CrossRef
Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets for geldanamycin and its analogs. Leukemia, 2002; 16:455–62. CrossRef
Brauer R, Chen P. Influenza virus propagation in embryonated chicken eggs. J Visualized Exp, 2015; 97:e52421; doi:10.3791/52421 CrossRef
Burch AD, Weller SK. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J Virol, 2005; 79:10740–49. CrossRef
Chase G, Deng T, Fodor E. HSP90 inhibitors reduce influenza virus replication in cell culture. Virology, 2008; 377:431–9. CrossRef
Connor JH, McKenzie MO, Parks GD, Lyles DS. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology, 2007; 362:109–19. CrossRef
Fukuyo Y, Hunt CR, Horikoshi N. Geldanamycin and its anti-cancer activities. Cancer Lett, 2009; 290:24–35. CrossRef
Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Gen Dev, 2007; 21:195–205. CrossRef
Hu J, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci USA, 1996; 93:1060–4. CrossRef
Hu J, Toft DO, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J, 1997; 16:59–68. CrossRef
Hung JJ, Chung CS, Chang W. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J Virol, 2002; 76:1379–90. CrossRef
Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta, 2012; 1823:742–55. CrossRef
Kitson RR, Chang CH, Xiong R, Williams HE, Davis AL, Lewis W, Dehn DL, Siegel D, Roe SM, Prodromou C, Ross D. Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein hsp90. Nat Chem, 2013; 5:307–14. CrossRef
Kousara S, Anjuma SN, Jaleela F, Khana J, Naseema S. Biomedical significance of tryptamine: a review. J Pharmacovigil, 2017; 5:239. CrossRef
Li YH, Lu QN, Wang HQ, Tao PZ, Jiang JD. Geldanamycin, a ligand of heat shock protein 90, inhibits herpes simplex virus type 2 replication both in vitro and in vivo. J Antibiot, 2012; 65:509–12. CrossRef
Li YH, Tao PZ, Liu YZ, Jiang JD. Geldanamycin, a ligand of heat shock protein 90, inhibits the replication herpes simplex virus type 1 in vitro. Antimicrob Agents Chemother, 2004; 48:867–72. CrossRef
Li YP, Shan GZ, Peng ZG, Zhu JH, Meng S, Zhang T, Gao LY, Tao PZ, Gao RM, Li YH, Jiang JD, Li ZR. Synthesis and biological evaluation of heat-shock protein 90 inhibitors: geldanamycin derivatives with broad antiviral activities. Antivir Chem Chemother, 2010; 20:259–68. CrossRef
Lin Z, Peng R, Li Z, Wang Y, Lu C, Shen Y, Wang J, Shi G. 17-ABAG, a Novel geldanamycin derivative, inhibits LNCaP-cell proliferation through heat shock protein 90 inhibition. Int J Mol Med, 2015; 36:424–32. CrossRef
Luo H, Zhang J, Dastvan F, Yanagawa B, Reidy MA, Zhang HM, Yang D, Wilson JE, McManus BM. Ubiquitin-dependent proteolysis of cyclin D1 is associated with coxsackievirus-induced cell growth arrest. J Virol, 2003; 77:1–9. CrossRef
Miyata Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr Pharm Des, 2005; 11:1131–8. CrossRef
Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res, 2011; 17:5132–9. CrossRef
Ochel HJ, Eichhorn K, Gademann G. Geldanamycin: the prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones, 2001; 6:105–12. CrossRef
ÅŒmura S, Nakagawa A, Sadakane N. Structure of herbimycin a new ansamycin antibiotic. Tetrahedron Lett, 1979; 20:4323–6. CrossRef
Pacey S, Banerji U, Judson I, Workman P. Hsp90 inhibitors in the clinic. Handb Exp Pharmacol, 2006; 172:331–58. CrossRef
Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell, 1997; 90:65–75. CrossRef
Qin HL, Panek JS. Total synthesis of the Hsp90 inhibitor geldanamycin. Organic Lett, 2008; 10:2477–9. CrossRef
Qu SJ, Wang GF, Duan WH, Yao SY, Zuo JP, Tan CH, Zhu DY. Tryptamine derivatives as novel non-nucleosidic inhibitors against hepatitis B virus. Bioorg Med Chem, 2011; 19:3120–7. CrossRef
Richter K, Buckner J. Hsp90: chaperoning signal transduction. J Cell Physiol, 2001; 188:281–90. CrossRef
Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem, 1999; 42:260–6. CrossRef
Schang LM, Bantly A, Knockaert M, Shaheen F, Meijer L, Malim MH, Gray NS, Schaffer PA. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and HIV-1 by targeting cellular, not viral, proteins. J Virol, 2002; 76:7874–82. CrossRef
Shan GZ, Peng ZG, Li YH, Li D, Li YP, Meng S, Gao LY, Jiang JD, Li ZR. A novel class of geldanamycin derivatives as HCV replication inhibitors targeting on Hsp90: synthesis, structure-activity relationships and anti-HCV activity in GS4.3 replicon cells. J Antibiot, 2011; 64:177–82. CrossRef
Smith DR, McCarthy S, Chrovian A, Olinger G, Stossel A, Geisbert TW, Hensley LE, Connor JH. Inhibition of heatshock protein 90 reduces Ebola virus replication. Antiviral Res, 2010; 87:187–94. CrossRef
Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FL, Pavletich NP. Crystal structure of an HSP90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell, 1997; 89:239–50. CrossRef
Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri ES, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem, 1997; 272:8007–12. CrossRef
Sun L, Lee H, Thibaut HJ, Lanko K, Rivero-Buceta E, Bator C, Martinez-Gualda B, Dallmeier K, Delang L, Leyssen P, Gago F. Viral engagement with host receptors blocked by a novel class of tryptophan dendrimers that targets the 5-fold-axis of the enterovirus-A71 capsid. PLoS Pathog, 2019; 15:e1007760. CrossRef
Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol, 1995; 36:305–15. CrossRef
Taechowisan T, Chaisaeng S, Phutdhawong WS. Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07; an endophyte in Boesenbergia rotunda (L.) Mansf A. Food Agri Immunol, 2017; 28:1330–46. CrossRef
Taechowisan T, Dumpin K, Phutdhawong WS. Isolation of avian influenza a (H5N2) from free-grazing ducks in Thailand and antiviral effects of tea extracts on viral propagation. Asian J Poult Sci, 2018; 12:7–13. CrossRef
Taechowisan T, Puckdee W, Phutdhawong WS. Streptomyces zerumbet, a Novel species from Zingiber zerumbet (L.) Smith and isolation of its bioactive compounds. Adv Microbiol, 2019; 9:194–219. CrossRef
Taechowisan T, Lumyong S. Activity of endophytic actinomycetes from roots of Zingiber officinale and Alpinia galanga against phytopathogenic fungi. Ann Microbiol, 2003; 53: 291-8.
Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem, 2004; 12:5317–29. CrossRef
Wang C, Liu P, Luo J, Ding H, Gao Y, Sun L, Luo F, Liu X, He H. Geldanamycin reduces acute respiratory distress syndrome and promotes the survival of mice infected with the highly virulent H5N1 influenza virus. Front Cell Infect Microbiol, 2017; 15:267. CrossRef
Waxman L, Whitney M, Pollok BA, Kuo LC, Darke PL. Host cell factor requirement for hepatitis C virus enzyme maturation. Proc Natl Acad Sci USA, 2001; 98:13931–5. CrossRef
Wrona IE, Gozman A, Taldone T, Chiosis G, Panek JS. Synthesis of reblastatin, autolytimycin, non-benzoquinone analogues: potent inhibitors of heat shock protein 90. J Org Chem, 2010; 75:2820–35. CrossRef