INTRODUCTION
Polysaccharides are polymers which are present in several organisms, including tissues of seeds, stems, and leaves of herbal plants, body fluids of animals, shells of crustaceans and insects, cell walls, and extra cellular fluids of bacteria, yeast, and fungi (Singh et al., 2012). Scientists give a huge attention to polysaccharides from the medicinal plants due to their signiï¬cant bioactivities, such as anti-tumor, antioxidant, anticoagulant, antidiabetic, radioprotection, anti-viral, hypolipidemic, and immunomodulatory activities. No side effects reported yet for plant polysaccharides (Xie et al., 2016). There is a need to perform further investigations on these polymers.
Argemone mexicana, an annual herb belonging to family Papaveraceae, is commonly found in tropical and subtropical regions of the world (Husna and Reddy, 2017). Native of tropical America, this species is known as Mexican poppy or Mexican prickly poppy (Sharanappa and Vidyasagar, 2014). It is a prickly and annual herb with 1.2 m in high. In Mali, the plant is locally known in Bamanan as “Bozobo” or “Nienidjeni”. In Northern Nigeria, A. mexicana is locally known in Hausa as “Kaki ruwan Allah,” “Karanko” or “Kwarkwaro”(Ibrahim et al., 2016). In different parts of the world, A. mexicana is used to treat many diseases, including tumors, warts, skin diseases, inflammations, rheumatism, jaundice, leprosy, microbial infections, and malaria (Brahmachari et al., 2013). Earlier authors reported the juice from leaves and the latex of A. mexicana mixed with lemon juice are used in the treatment of malaria (Bapna et al., 2015). Argemone mexicana is one of the most effective plants used traditionally to treat uncomplicated malaria in Mali (Diallo et al., 2006). In addition, the in vitro antiplasmodial activity of A. mexicana was carried out at the Swiss Tropical Institute in Basel in collaboration with the Department of Traditional Medicine of Mali. These authors revealed that the 50% inhibitory concentration values of the decoction, maceration, dichloromethane (DCM), and methanol extracts against the chloroquine-resistant K1 strain of P. falciparum were 5.89, 6.22, 1.22, and 1.00 μg/ml for, respectively (Willcox et al., 2007). A randomized controlled trial comparing A. mexicana aerial parts decoction with artesunate-amodiaquine (standard first-line treatment) in the management of uncomplicated malaria revealed that 89% of patients recovered clinically with the plant decoction versus 95% for artesunate-amodiaquine (Graz et al., 2010). These last authors found also that there are no signiï¬cant differences between the groups in most of the outcome measures, and both treatments were well tolerated. In addition, there was no deterioration of severe malaria in patients >5 years but 1.9% deterioration in children ≤5 years were observed in clinical trials (Willcox et al., 2011). On other hand, analgesic, antispasmodic, possibly hallucinogenic, and sedative properties make this weed plant a medicine traditionally used to treat malaria, warts, cold sore, skin disease, and itches (Motilal et al., 2017). Wound healing property, vasoconstrictor and vasorelaxant effects, antimicrobial, fungitoxic, anti-stress and anti-allergic, analgesic, anti-inflammatory, antifertility, nematicidal, larvicidal, molluscicide, hepatoprotective, cytotoxic, anticancer, anti-HIV, antioxidant, anti-diabetic, and antimalarial activities of the plant extracts were reported (Brahmachari et al., 2013; Husna and Reddy, 2017; Ibrahim et al., 2016; Sharanappa and Vidyasagar, 2014). Besides pharmaceutical efficacies, toxicity effects (acute toxicity, epidemic dropsy, hepatotoxicity, etc.) of certain parts of the plant related to some alkaloids (e.g., sanguinarine) are also narrated (Brahmachari et al., 2013).
Beyond alkaloids, this plant species contains other chemical classes, such as terpenoids, steroids, carbohydrates, long-chain aliphatic alcohols and carboxylic acids, amino acids, flavonoids, and other phenolics (Brahmachari et al., 2013).
In Mali, the results from sub-chronic toxicity studies of the decoction of A. mexicana in rats revealed the safety of the aerial parts of the plant (Sanogo et al., 2008). The Department of traditional medicine in Mali produces A. mexicana as a standardized phytomedicine for the management of malaria. The three protoberberine alkaloids (berberine, protopine, and allocryptopine) present in A. mexicana showed similar high antiplasmodial activities (Willcox et al., 2011). But, the absorption of berberine is poor in some animal models, while the pharmacokinetic of protopine and allocryptopine has not been studied yet in humans. In addition, earlier antiplasmodial tests using the freeze dried A. mexicana decoction were unsuccessful both in mouse and in rat models using Plasmodium berghei and Plasmodium chabaudi, respectively (Willcox et al., 2011). Water soluble compounds having immunomodulatory properties may be involved in such antimalarial activity. Very few previous studies have been reported on polysaccharides from A. mexicana decoction. Among many insects Drosophila melanogaster and Artemia salina are widely used as model organism to perform the pharmacological and toxicological activities of chemical compounds and natural products (Siddique et al., 2005; Zemolin et al., 2014). Drosophila melanogaster has been extensively studied as a decisive model in biology about a century ago. The fly shares several basic biological, biochemical, neurological, and physiological similarities with mammals. About 75% of human disease-causing genes have functional homolog in D. melanogaster (Abolaji et al., 2013). In addition, the fly can well be kept at low cost in the laboratory, and it has been recommended as an alternative model to vertebrate usage. Therefore, its genetically importance coupled with its low cost and its high sensitivity to toxic substances make it a suitable model (Bezerra et al., 2017). The aim of this study is to evaluate the safety of the polysaccharides from A. mexicana using D. melanogaster.
MATERIALS AND METHODS
Plant material
The aerial parts of A. mexicana were collected at Blendio in Mali, September 2014, and identified by a taxonomist of the Department of Traditional Medicine (DMT came from its french name which is Département de Médecine Traditionnelle), Bamako, Mali. Then, a voucher specimen (2948/DMT) is deposited at the herbarium of the DMT. The aerial parts were dried, pulverized to a fine powder by a mechanical grinder, and kept for further investigations.
Extraction and fractionation of polysaccharide fractions
Sample was extracted using an Accelerated Solvent Extractor (Dionex ASE350, Sunnyvale, CA, USA). Powdered aerial parts, (500 g) were weighed and mixed with diatomaceous earth (125 g). Due to the capacity of the stainless steel cells, 316.5 g of the mixture only were packed in eight stainless steel cells of 100 ml two times. Each set was performed using 1,500 psi, with 5 minutes heating, 5 minutes static time, and a 250 seconds purge for a total of three cycles.
To remove low molecular (LM) weight compounds, the plant material was pre-extracted three times with dichloromethane at 40°C, followed by 96% ethanol (EtOH) at 60°C. Then, the residue was extracted three times with 50% ethanol-water (EtOH-H2O) at 50°C, followed by distilled water at 100°C. The water extracts from the two extraction sets were gathered and submitted to the ultrafiltration (cut off 5,000 Da) to separate the LM weight part to the high molecular (HM) weight. The LM weight was concentrated then lyophilized, while the HM weight was dialyzed at cut-off 3,500 Da. The dialyzed HM was then fractionated by an ion exchange chromatography as described by Zou et al. (2014) with slight modifications. Five acidic fractions called High molecular weight (polysaccharide fraction) of the water extract from Argemone mexicana 1 (HMAm1), HMAm2, HMAm3, HMAmA1, and HMAmA2 were isolated and kept to determine their effect on the flies.
Drosophila melanogaster stock and culture
Drosophila melanogaster (Harwich strain) fruit flies were obtained from the fly laboratory of Africa Centre of Excellence in Phytomedicine Research and Development (ACEPRD), University of Jos, Nigeria. The flies were maintained at 25°C ± 1°C and 60%–70% relative humidity and were kept with a 12 hours dark/light cycle. The basal culture medium or diet contained 100.0 g of corn flour, 20.0 g of yeast, 16.0 g of agar, and 1700.0 ml of distilled water and methyl paraben (1 g in 5 ml of ethanol) were added to prevent bacteria contamination as described by Chen et al. (2018) with some modifications. All experiments were performed with the same strain.
Polysaccharides exposure and survival rate
Drosophila melanogaster (both genders), 1–3 days old, were divided into 15 groups of 30 flies each. Five polysaccharide fractions were investigated for their effect on the flies. Among the 15 groups, two doses of each fraction were compared to the control consisting of 1% dimethyl sulfoxide (DMSO). Polysaccharide fractions were dissolved in 1% DMSO: Water (1:99) to get 12.5 and 25 μg/ml as plant treatments. Test substances (supplemented diets) were prepared by adding 1% DMSO, 12.5 and 25 μg/ml for each polysaccharide fraction in the basal diet to obtain ï¬nal concentrations of 10% of these treatments. Hence, for the control the diet (9 g) was mixed with 1% DMSO (1 g), while plant polysaccharides were obtained by adding 1 g of each concentration to 9 g of the basal diet in a beaker. Ten grammes of each supplemented diet were poured into the bottom of a vial and dried for the test.
Exposure of flies to polysaccharide fractions was performed as described by Coutinho et al. (2017) with some modifications. For each fraction, three vials received the following supplemented diets: control (culture medium+ 1% DMSO) and 12.5 and 25 μg/ml, respectively. Then, 30 adult flies (males and females of 1–3 days old) anesthetized on ice were placed in each of the 15 vials containing the supplemented diet. Survival readings of the flies have done at 3, 6, 12, 24, 48, and 72 hours. At the end of each experiment, all tested doses were compared to the mean of the control. The results are presented as percentage (%) of live flies (mean ± standard error of mean) obtained from three independent experiments.
Locomotor assay
The locomotor capacity was evaluated by using the negative geotaxis behavior as described by Abolaji et al. (2014) with some modifications.
Briefly, 150 adult flies (4–6 days old: both genders) were used per experiment. After 72 hours exposure to the control (1% DMSO) and the different doses (12.5 and 25 μg/ml) of each polysaccharide fractions, 10 flies from each supplemented diet vial were transferred randomly in an empty vial prior to place them in a vertical glass column (length: 15 cm; diameter: 2.5 cm) for the locomotor capacity. Then, flies were gently tapped to the bottom of the glass column, and the number of flies that climbed up to the 6 cm mark of the glass column in 6 seconds as well as those that remained below this mark after this time, were recorded. For each batch of 10 flies, this procedure was repeated three times at 1-minute interval. The scores represent the mean of the number of flies at the top (ntop) expressed as a percentage of the total number of flies (ntot) recorded in three independent experiments.
RESULTS AND DISCUSSION
Polysaccharides exposure and survival rate
After 72 hours of the exposure, there was no significant change in the survival rate of flies (Fig. 1). The finding showed that the survival percent was higher than 94%. The exposure period was too short to kill 50% of flies with each polysaccharide fraction; however, this period is enough to evaluate the toxicity of antimalarial agent in D. melanogaster. In our laboratory conditions, the highest dose (25 μg/ml in 10 g of diet) of most of the fractions kept the flies alive at least for 48 hours. HMAm2 showed the highest safety with 100% survival observed for both doses 25 and 12.5 μg/ml in 10 g of diet. There is a slight difference at 12.5 μg/ml between HMAm1 and HMAm2. On other hand, the two concentrations of HMAm3 affected more flies. The control group and those treated with 12.5 μg/ml showed more cases of mortality than 25 μg/ml, this could explain the protection in dose dependent manner of the tested polysaccharide fractions. The mixture of polysaccharides from A. mexicana increased the survival time in mice with tumoral cells of lymphocytic leukemia P-388 and sarcoma 37 (Gil et al., 2005). To our knowledge, few studies have been performed on the polysaccharides from A. mexicana. Previous investigations of the Argemone decoction administrated per oral revealed that the leaves of A. mexicana are nontoxic in rats (Sanogo et al., 2008) and the phytomedicine made with this plant species is safe and tolerable in human (Willcox et al., 2011). However, the plant extract administrated intraperitoneally in mice (18–25 g and averagely aged between 4 and 6 weeks) exhibited an acute toxicity in mice, where the LD50 value was 400 mg/kg body weight (Ibrahim and Ibrahim, 2009). This toxicity could be due to the mode of administration.
Locomotor assay
Locomotor performance of exposed flies to the control (1% DMSO) and polysaccharide fractions at 25 and 12.5 μg/ml for 72 hours are presented in Figure 2. The capacity of flies to climb after polysaccharide exposure is a good factor to support the safety of these natural substances. In overall, climbing behavior of treated flies with polysaccharides were not really affected when compared with the control group. At 12.5 μg/ml, the following samples HMAm1 and HMAm2 exhibited the highest capacity to cross the 6 cm mark in 6 seconds when compared to the control (Fig. 2). The climbing percent at 12.5 μg/ml were 93.3, 90.0, and 86.6 for HMAm1, HMAm2, and HMAmA2, respectively. For 25μg/ml, the highest climbing percent were 92.2%, 87.8%, and 84.5% for HMAmA1, HMAm2, and HMAm1, respectively. The lowest climbing percent at 12.5 μg/ml were 83.3% and 84.4% for HMAmA1 and HMAm3, respectively, while at 25 μg/ml HMAm3 and HMAmA2 presented the lowest climbing percent with 77.8% each. Overall, 12.5 μg/ml showed a higher protection of the flies than 25 μg/ml. Through these two doses (12.5 and 25 μg/ml), HMAm3 was the least protective polysaccharide fraction for flies. Contrary to heat shock protein 70 (hsp70), one of the most expressed stress protein families was increased by Argemone seed oil at 1.0 μl/ml in D. melanogaster third-instar larvae (Mukhopadhyay et al., 2002).
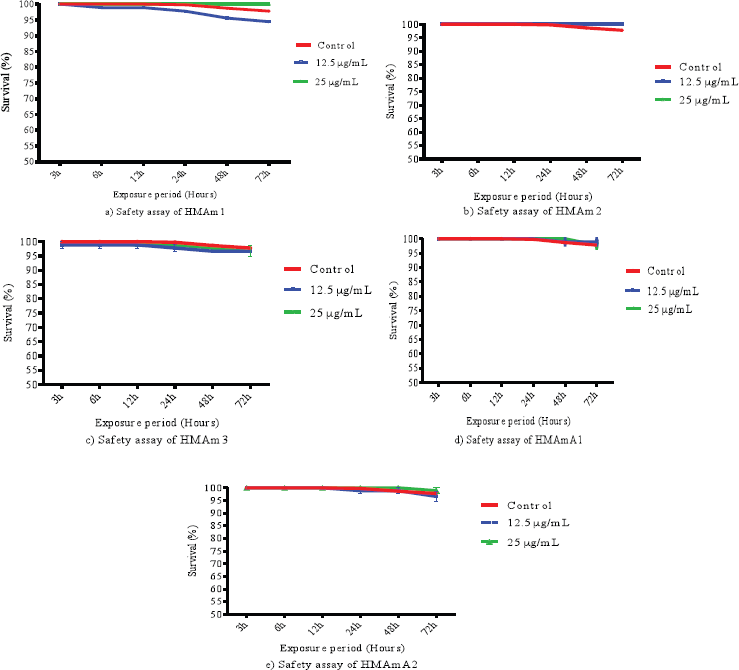 | Figure 1. Survival rate of the five polysaccharide fractions (HMAm1, HMAm2, HMAm3, HMAmA1, and HMAmA2) from A. mexicana. [Click here to view] |
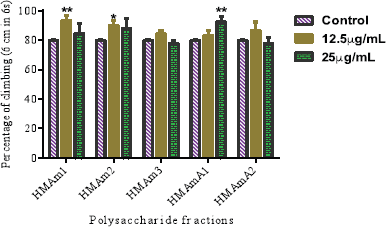 | Figure 2. Locomotor capacity of the five polysaccharide fractions (HMAm1, HMAm2, HMAm3, HMAmA1, and HMAmA2) from A. mexicana. Data are presented as mean ± SEM of three independent experiments. [Click here to view] |
CONCLUSION
The results collectively indicated that the polysaccharide fractions from A. mexicana are safe for D. melanogaster when the flies are exposed to different concentrations of these polysaccharides. These results support the innocuousness of the water extract from A. mexicana aerial parts used in the traditional medicine against various indications. Further biological investigations will be undertaken on these polysaccharide fractions to reveal their possible effect against malaria.
ACKNOWLEDGEMENTS
The authors would like to thank the EU project “Multidisciplinary University Traditional Health Initiative (MUTHI)”, Theme HEALTH, Coordination and support action, Grant Agreement No.: 266005 which supported a part of this work. The authors also acknowledge the World Bank through the Africa Centre of Excellence in Phytomedicine Research and Development (ACEPRD) of University of Jos, Nigeria for its ï¬nancial support. Department of Pharmacy, section Pharmaceutical Chemistry, University of Oslo is also grateful for their technical support. The first author thanks also all the staff of ACEPRD’s fly laboratory for their technical assistance.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Abolaji AO, Kamdem JP, Farombi EO, Rocha JBT. Drosophila melanogaster as a promising model organism in toxicological studies. Arch Bas App Med, 2013; 1:33–38.
Abolaji AO, Kamdem JP, Lugokenski TH, Nascimento TK, Waczuk EP, Farombi EO, da Silva Loreto EL, Rocha JBT. Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic Biol Med, 2014; 71:99–108. CrossRef
Bapna S, Choudhary PK, Ramaiya M, Chowdhary A. Antiplasmodial activity of Argemone mexicana: An in vitro and in vivo study. World J Pharm Research, 2015; 4(11):1653–1663.
Bezerra JWA, Costa AR, da Silva MAP, Rocha MI, Boligon AA, da Rocha JBT, Barros LM, Kamdem JP. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (Lamiaceae) in Drosophila melanogaster and Artemia salina. South Afr J Bot, 2017; 113:437–42. CrossRef
Brahmachari G, Gorai D, Roy R. Argemone mexicana: chemical and pharmacological aspects. Revista Brasileira de Farmacognosia. Braz J Pharmacog, 2013; 23(3):559–75; https://doi.org/10.1590/S0102-695X2013005000021 CrossRef
Chen Y, Liu X, Wu L, Tong A, Zhao L, Liu B, Zhao C. Physicochemical characterization of polysaccharides from Chlorella pyrenoidosa and its anti-ageing effects in Drosophila melanogaster. Carbohy Polym, 2018; 185:120–6. CrossRef
Coutinho HDM, de Morais Oliveira-Tintino CD, Tintino SR, Pereira RLS, de Freitas TS, da Silva MAP, Franco JL, da Cunha FAB, da Costa JGM, de Menezes IRA, Boligon AA, da Rocha JBT, Rocha MI, dos Santos JFS. Toxicity against Drosophila melanogaster and antiedematogenic and antimicrobial activities of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae). Environ Sci Pollut Res, 2017; 25(11):10353–61. CrossRef
Diallo D, Graz B, Falquet J, Traoré AK, Giani S, Mounkoro PP, Berthé A, Sacko M, Diakité C. Malaria treatment in remote areas of Mali: use of modern and traditional medicines, patient outcome. Trans R Soc of Trop Med Hyg, 2006; 100(6):515–20. CrossRef
Gil RM, Cabrera ADA, Labori RE, Loo YC, Cepero WQ, Garciga JLB, Panfet C. Actividad antitumoral de una mezcla de polisacáridos obtenida de la especie Argemone mexicana L. Rev Cubana Plant Med, 2005; 10(3–4):9.
Graz B, Willcox ML, Diakite C, Falquet J, Dackuo F, Sidibe O, Giani S, Diallo D. Argemone mexicana decoction versus artesunate-amodiaquine for the management of malaria in Mali: policy and public-health implications. Trans R Soc Trop Med 2010; 104(1):33–41. CrossRef
Husna SA, Reddy VJ. A Review on Argemone Mexicana. Int J Pharmacol Res, 2017; 7(09):170–4.
Ibrahim HA, Ibrahim H. Phytochemical screening and toxicity evaluation on the leaves of Argemone mexicana Linn. (Papaveraceae). Int Jor App Sci, 2009; 3:39–43.
Ibrahim HA, Umar MA, Bello BA, Aliyu A, Ahmad A. Analgesic and Anti-Inflammatory Studies on The Roots Argemone Mexicana Linn(Family: Papaveraceae). J Pharm Biol Sci, 2016; 11(3):92–5.
Motilal BS, Pathan IB, Nitin N. Evaluation of diuretic and laxative activity of aqueous extract of Argemone mexicana leaves in rats. Ars Pharm, 2017; 58(2):53–8; http://dx.doi.org/10.4321/S2340-98942017000200002 CrossRef
Mukhopadhyay I, Nazir A, Mahmood K, Saxena DK, Das M, Khanna SK, Chowdhuri DK. Toxicity of argemone oil: E¡ect on hsp70 expression and tissue damage in transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Cell Biol Toxicol, 2002; 18:1–11. CrossRef
Sanogo R, Maiga A, Djimdé A, Doumbia L, Guirou C, Diallo D, Doumbo O (2008). Etude de la toxicité sub-chronique du décocté de Argemone mexicana utilisé dans le traitement du paludisme. Pharmacopée et Médecine et Traditionnelles Africaines, 2008; 15:26–31.
Sharanappa R, Vidyasagar GM. Plant profile, phytochemistry and pharmacology of Argemone mexicana Linn. Areview. Int J Pharm Pharm Sci, 2014; 6(7):45–53.
Siddique HR, Gupta SC, Dhawan A, Murthy RC, Saxena DK, Chowdhuri DK. Genotoxicity of industrial solid waste leachates in Drosophila melanogaster. Environ Mol Mutagen, 2005; 46(3):189–97. CrossRef
Singh V, Kumar P, Sanghi R. Use of microwave irradiation in the grafting modification of the polysaccharides––a review. Prog Polym Sci, 2012; 37(2):340–64; https://doi.org/10.1016/j.progpolymsci.2011.07.005 CrossRef
Willcox ML, Graz B, Falquet J, Sidibé O, Forster M, Diallo D. Argemone mexicana decoction for the treatment of uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg, 2007; 101(12):1190–8. CrossRef
Willcox ML, Graz B, Falquet J, Diakite C, Giani S, Diallo D. A “reverse pharmacology” approach for developing an anti-malarial phytomedicine. Malar J, 2011; 10(1):S8. CrossRef
Xie JH, Jin ML, Morris GA, Zha XQ, Chen HQ, Yi Y, Li J-E, Wang Z-J, Gao J, Nie S-P, Shang P, Xie M-Y. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr, 2016; 56(supp 1): S60–84. CrossRef
Zemolin APP, Cruz LC, Paula MT, Pereira BK, Albuquerque MP, Victoria FC, Pereira AB, Posser T, Franco JL. Toxicity induced by Prasiola crispa to fruit fly Drosophila melanogaster and cockroach Nauphoeta cinerea: evidence for Bioinsecticide action. J Toxicol Environ Health A, 2014; 77(1–3):115–24. CrossRef
Zou Y-F, Zhang B-Z, Barsett H, Inngjerdingen KT, Diallo D, Michaelsen TE, Paulsen BS. Complement fixing polysaccharides from Terminalia macroptera root bark, stem bark and leaves. Molecules, 2014; 19(6):7440–58; doi:10.3390/molecules19067440 CrossRef