INTRODUCTION
An antimicrobial is an agent that kills microorganisms or stops their growth. There are many types of antimicrobial drugs in the markets, e.g., penicillins, cycloserine, aminoglycosides, chloramphenicol, quinolones, tetracyclines, and glycopeptides, but the resistance of microorganisms to antimicrobial drugs by internal resistance or acquired resistance decreased the activities of these drugs. Therefore, the development of newer antimicrobial compounds for the treatment of the resistance of microorganisms has become a major objective of medicinal chemists
Literature survey revealed that substituted pyrazoline could act as anticancer, antiviral, antioxidant, anti-inflammatory, antimicrobial, antidepressant, antiprotozoal, and antidiabetic agents (Havrylyuk et al., 2016; Marella et al., 2013; Silva et al., 2018). Derivative A showed potent antibacterial proï¬le against the tested Gram-positive [Minimal Inhibition Concentration (MIC) = 8 μg/ml] and Gram-negative (MIC= 32 μg/ml) bacterial strains (Sharma et al., 2010). Compound B exhibit good activities against Staphylococcus aureus [inhibition zone (IZ)= 21 mm] and Candida albicans (IZ = 24 mm) (Sharshira et al., 2012). Compound C exhibited the most potent antimicrobial activities against S. aureus, Pseudomonas Aeruginosa, and C. albicans with MIC= 3.12 μg/ml (Ahmad et al., 2016). Also, some drugs bearing a pyrazoline moiety in their structures, e.g., phenazone and propyphenazone have analgesic and antipyretic effects. Metamizole is a spasm reliever, fever reliever, and it has anti-inflammatory effects (Fig. 1).
Further literature survey revealed that benzofuran or pyrazole moieties have been implemented as anticancer, antiviral, antioxidant, antimicrobial, anti-inflammatory, and antimalarial agents (Chand et al., 2017; Karrouchi et al., 2018; Shamsuzzaman et al., 2015). Moreover, the compound D bearing benzofuran and pyrazole moieties showed excellent antimicrobial activities for Ralstonia solanacearum, Klebsiella pneumoniae, Fusarium oxysporum and Aspergillus flavus (Lingaraju et al., 2017). Compound E bearing benzofuran and pyrazoline moieties exhibited excellent antimicrobial activities in comparison with the standard drug used (Rangaswamy et al., 2012) (Fig. 1).
Based on above information and in continuation of our research program to find new potent antimicrobial and anticancer agents (Abd El-All et al., 2016; Abo-Ghalia et al., 2017; Al-Salem et al., 2017; Amr et al., 2018; El-Naggar et al., 2018; Elgemeie et al., 2008; Hafez et al., 2013; Hassan and Hafez, 2018; Hassan et al., 2019; 2015a; 2017a; 2018a; 2017b; 2015b; 2017c; 2015c; Kassem et al., 2019; Khatab et al., 2019; Moustafa et al., 2018; 2019; Naglah et al., 2017; 2013; Osman et al., 2014; 2009), a series of pyrazolines 6–14 incorporating benzofuran and pyrazole moieties have been synthesized to evaluate their antimicrobial activity against some of pathogenic microorganisms. Also, the calculation of the pharmacokinetic properties and drug-likeness of all compounds were studied.(Fig. 2)
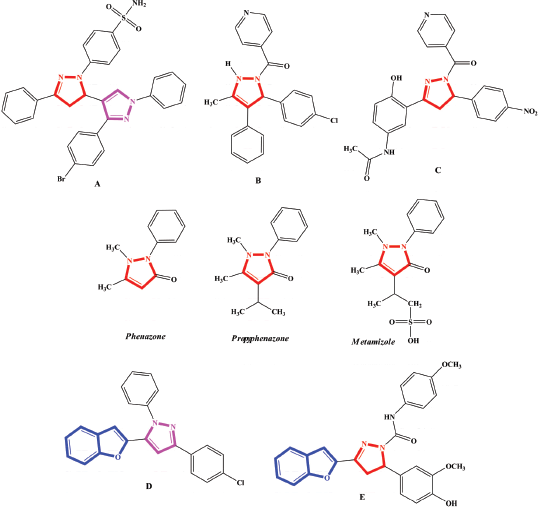 | Figure 1. Examples of pyrazolines, benzofurans, and pyrazoles as antimicrobial activities and the structures of some drugs bearing pyrazoline moiety. [Click here to view] |
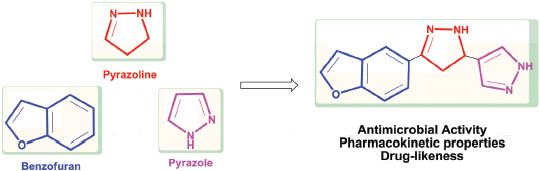 | Figure 2. Design of pyrazoline derivatives incorporating benzofuran and pyrazole moieties. [Click here to view] |
MATERIALS AND METHODS
Antimicrobial activities
The synthesized compounds (Chalcones 3–5, 1H-pyrazolines 6–8, N-phenylpyrazolines 9–11, and N-acetylpyrazolines 12–14) were evaluated their in vitro antimicrobial properties against Escherichia coli (ATCC 25922), Bacillus subtilis (NRRL-B-4219), Aspergillus niger (ATCC 16888), and C. albicans (ATCC 10231) and comparison with antibiotic drugs (Negram, Vancomycin, and Nystatin) as standards by use of an agar well-diffusion method (MacLowry et al., 1970; Othman et al., 2011; Rocha et al., 1995; Valgas et al., 2007).
RESULTS AND DISCUSSION
Chemistry
The starting materials [khellinone 1 (Osman et al., 2012) and 3-substituted-1-phenyl-1H-pyrazole-4-carbaldehydes 2a–c (Jadhav et al., 2013)] were prepared according to the synthetic methods in Scheme 1.
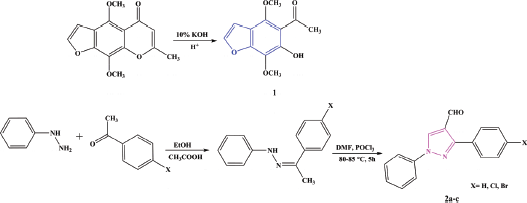 | Scheme 1. Synthesis of khellinone 1 and 3-substituted-1-phenyl-1H-pyrazole-4-carbaldehydes 2a–c. [Click here to view] |
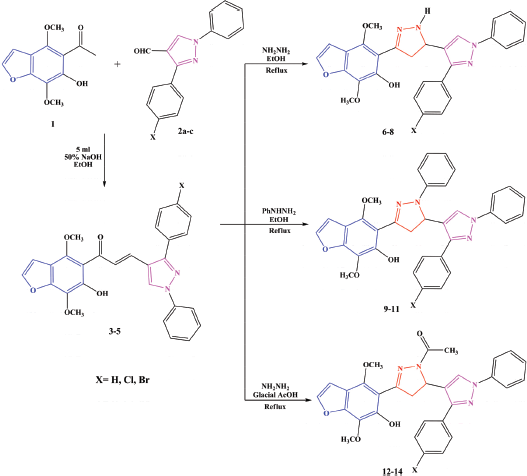 | Scheme 2. Synthesis of 1H-pyrazolines (6–8), N-phenylpyrazolines (9–11), and N-acetylpyrazolines (12–14). [Click here to view] |
The synthetic route used to synthesize the target series of pyrazolines incorporating benzofuran and pyrazole moieties is outlined in Scheme 2. Chalcones 3–5 have synthesized via the condensation of khellinone 1 with pyrazole aldehydes 2a–c. Then, described a synthesis of 1H-pyrazolines 6–8, N-phenylpyrazolines 9–11, and N-acetylpyrazolines 12–14 via the cyclocondensation of 3–5 with hydrazine hydrate or phenyl hydrazine in refluxing ethanol or glacial acetic acid (Hassan et al., 2016) (Scheme 2).
Biological evaluations
In vitro antimicrobial activity
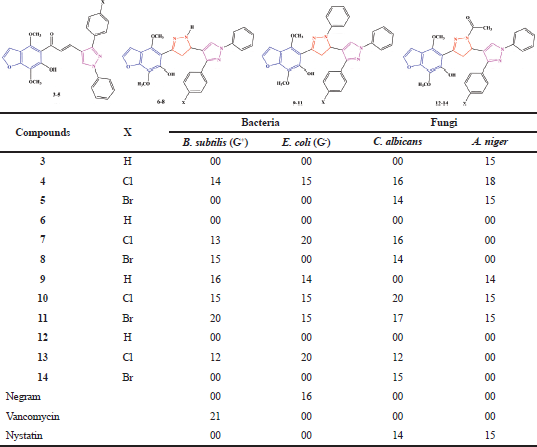
The antibacterial and antifungal activities of the synthesized compounds 3–14 against a panel of pathogenic tested organisms are represented in Table 1 and Figure 3. The results revealed that some synthesized derivatives exhibited excellent to moderate inhibitory effect.
In case of B. subtilis (G+), compound 11 [inhibition zone (IZ) = 20 mm] recorded excellent inhibitory effect and equipotent to the antibacterial reference drug (Vancomycin, IZ = 21 mm). Compounds (4, 7, 8, 9, 10, and 13) showed a moderate inhibitory effect and recorded IZ diameter ranged from 12 to 16 mm. On the other hand, the rest of compounds (3, 5, 6, 12, and 14) did not show any inhibitory effect.
In case of E. coli (G-), the two compounds 7 and 13 (IZ = 20 mm) showed more potent inhibitory effect in comparison to the antibacterial reference drug (Negram, IZ = 16 mm). Compounds (4, 9, 10, and 11) showed activity (IZ rang = 14–15 mm) nearly equal to the activity of the antibacterial drug used (Negram, IZ = 16 mm), while the other tested compounds have not any inhibition effect.
 | Table 1. In vitro antimicrobial (inhibition zone of growth IZ, mm) of chalcones 3–5 and pyrazolines 6–14 against panel of pathogenic tested organisms. [Click here to view] |
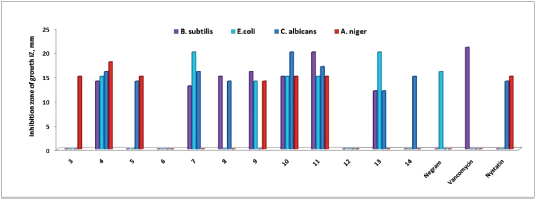 | Figure 3. Antimicrobial activity of chalcones 3–5 and pyrazolines 6–14 against panel of pathogenic tested organisms. [Click here to view] |
By testing the compounds against C. albicans, compounds (4, 7, 10, 11, and 14) were more potent (IZ rang = 15–17 mm) than antifungal drug used (Nystatin, IZ = 14 mm). The two compounds (5 and 8) showed activity equal to antifungal drug used in this study (Nystatin, IZ = 14 mm). Also, the derivative 13 (IZ = 12 mm) showed a moderate inhibition effect.
In case of the pathogenic fungi, A. niger, compound 4 showed excellent inhibitory effect (IZ = 18 mm) more than (Nystatin, IZ = 15 mm). The four compounds (3, 5, 10, and 11) have activity equal to antifungal drug used (Nystatin, IZ = 15 mm). Also, compound 9 (IZ = 14 mm) showed moderate activity. The compounds (6–8 and 12–14) have not any activity.
 | Table 2. Lipinski’s rule of five for the compounds, chalcones 3–5 and pyrazolines 6–14. [Click here to view] |
 | Table 3. Drug likeness calculations of the compounds, chalcones 3–5 and pyrazolines 6–14. [Click here to view] |
Finally, we recommend for using compounds 7 and 13 in the treatment of Gram-negative pathogenic microorganisms, compound 11 in the treatment of Gram-positive, compound 10 in the treatment of C. albicans and compound 4 in the treatment of A. niger.
Pharmacokinetic properties and drug-likeness
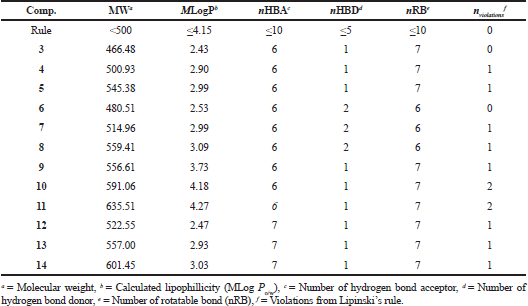
Lipinski’s rule of five for the compounds, chalcones 3–5 and pyrazolines 6–14
To qualify Chalcones 3–5 and pyrazolines 6–14 as a drug candidate, the molecular weight (MW), lipophilicity (MLogP), the number of hydrogen bond acceptors (nHBA), donors (nHBD), and the number of rotatable bond (nRB) of Lipinski’s rule of five (Lipinski et al., 2001) were calculated using SwissADME web (http://swissadme.ch/index.php#undefined). The computed molecular properties are shown in Table 2.
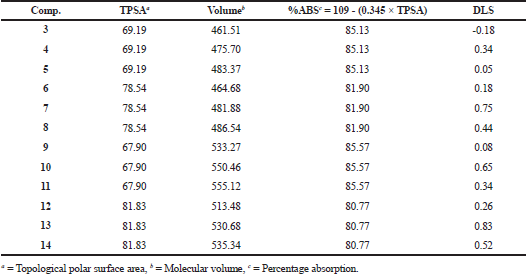
Drug likeness calculations of the compounds, chalcones 3–5 and pyrazolines 6–14
Molecular polar surface area (TPSA) is an affected parameter in the prediction of drug transport properties. Molecular volume was calculated by using the MolSoft website (http://molsoft.com/mprop/.). The percentage of absorption (%ABS) was calculated by using %ABS = 109 − (0.345 × TPSA) and referred to the degree of absorption (Desai et al., 2014).
Computed drug-likeness scores of the compounds, Chalcones 3–5 and pyrazolines 6–14 are presented in Table 3. Compound 3 has a negative value (DLS = −0.18) should not be considered as drug-like candidate. Compounds 7 and 13 possessed maximum drug-likeness model score (DLS) of 0.75 and 0.83, respectively.
CONCLUSION
In conclusion, we have synthesized a series of chalcones 3–5, 1H-pyrazolines 6–8, N-acetylpyrazolines 12–14 9–11, and N-acetylpyrazolines incorporating benzofuran and pyrazole moieties. All the synthesized compounds were screened for their in vitro antimicrobial activity. The evaluations showed that compounds 4, 7, 10, 11, and 13 were the most active compounds against a panel of pathogenic tested organisms. Also, the pharmacokinetic properties and calculation of drug likeness exhibited the two compounds 7 and 13 were found to be maximum DLS of 0.75 and 0.83, respectively.
ACKNOWLEDGMENTS
The authors wish to express their thanks to the National Research Centre for the facilities provided.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
FINANCIAL SUPPORT
None.
REFERENCES
Abd El-All AS, Hassan AS, Osman SA, Yosef HAA, Abdel-Hady WH, El-Hashash MA, Atta-Allah SR, Ali MM, El Rashedy AA. Synthesis, characterization and biological evaluation of new fused triazine derivatives based on 6-methyl-3-thioxo-1,2,4-triazin-5-one. Acta Pol Pharm, 2016; 73:79.
Abo-Ghalia MH, Moustafa GO, Alwasidi AS, Naglah AM. Cytotoxic investigation of isophthaloyl cyclopentapeptides. Lat Am J Pharm, 2017; 36:1957.
Ahmad A, Husain A, Khan SA, Mujeeb M, Bhandari A. Synthesis, antimicrobial and antitubercular activities of some novel pyrazoline derivatives. J Saudi Chem Soc, 2016; 20:577. CrossRef
Al-Salem HSA, Naglah AM, Moustafa GO, Mahmoud AZ, Al-Omar MA. Synthesis of novel tripeptides based on dibenzofuran-2-sulfonyl-[aromatic and hydroxy aromatic residues]: towards antimicrobial and antifungal agents. J Comput Theor Nanosci, 2017; 14:3958. CrossRef
Amr AE, Abo-Ghalia MH, Moustafa GO, Al-Omar MA, Nossier ES, Elsayed EA. Design, synthesis and docking studies of novel macrocyclic pentapeptides as anticancer multi-targeted kinase inhibitors. Molecules, 2018; 23:2416. CrossRef
Chand K, Rajeshwari, Hiremathad A, Singh M, Amelia Santos M, Keri RS. A review on antioxidant potential of bioactive heterocycle benzofuran: natural and synthetic derivatives. Pharmacol Rep, 2017; 69:281. CrossRef
Desai NC, Kotadiya GM, Trivedi R. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg Med Chem Lett, 2014; 24:3126. CrossRef
Elgemeie GH, Elsayed SH, Hassan AS. Design and synthesis of the first thiophene thioglycosides. Synth Commun, 2009; 39:1781. CrossRef
Elgemeie GH, Elsayed SH, Hassan AS. Direct route to a new class of acrylamide thioglycosides and their conversions to pyrazole derivatives. Synth Commun, 2008; 38:2700. CrossRef
El-Naggar M, Hassan AS, Awad HM, Mady MF. Design, Synthesis and Antitumor Evaluation of Novel Pyrazolopyrimidines and Pyrazoloquinazolines. Molecules, 2018; 23:1249. CrossRef
Hafez TS, Osman SA, Yosef HAA, Abd El-All AS, Hassan AS, El-Sawy AA, Abdallah MM, Youns M. Synthesis, structural elucidation and in vitro antitumor activities of some pyrazolopyrimidines and Schiff bases derived from 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides. Sci Pharm, 2013; 81:339. CrossRef
Hassan AS, Askar AA, Nossier ES, Naglah AM, Moustafa GO, Al-Omar MA. Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules, 2019; 24:3130. CrossRef
Hassan AS, Awad HM, Magd-El-Din AA, Hafez TS. Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med Chem Res, 2018a; 27:915. CrossRef
Hassan AS, Hafez TS, Ali MM, Khatab TK. Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res J Pharm Biol Chem Sci, 2016; 7:417.
Hassan AS, Hafez TS, Osman SA, Ali MM. Synthesis and in vitro cytotoxic activity of novel pyrazolo[1,5-a]pyrimidines and related Schiff bases. Turk J Chem, 2015c; 39:1102. CrossRef
Hassan AS, Hafez TS, Osman SA. Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci Pharm, 2015b; 83:27. CrossRef
Hassan AS, Hafez TS. Antimicrobial activities of ferrocenyl complexes: a review. J App Pharm Sci, 2018; 8:156. CrossRef
Hassan AS, Mady MF, Awad HM, Hafez TS. Synthesis and antitumor activity of some new pyrazolo[1,5-a]pyrimidines. Chin Chem Lett, 2017c; 28:388. CrossRef
Hassan AS, Masoud DM, Sroor FM, Askar AA. Synthesis and biological evaluation of pyrazolo[1,5-a]pyrimidine-3-carboxamide as antimicrobial agents. Med Chem Res, 2017a; 262909. CrossRef
Hassan AS, Moustafa GO, Awad HM, Synthesis and in vitro anticancer activity of pyrazolo[1,5-a]pyrimidines and pyrazolo[3,4-d][1,2,3]triazines. Synth Commun, 2017b; 47:1963. CrossRef
Hassan AS, Osman SA, Hafez TS. Phenyl-2-furaldehyde: synthesis, reactions and biological activities. Egypt J Chem, 2015a; 58:113. CrossRef
Havrylyuk D, Roman O, Lesyk R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline-thiazolidine-based hybrids. Eur J Med Chem, 2016; 113:145. CrossRef
Jadhav SY, Shirame SP, Kulkarni SD, Patil SB, Pasale SK, Bhosale RB. PEG mediated synthesis and pharmacological evaluation of some fluoro substituted pyrazoline derivatives as antiinflammatory and analgesic agents. Bioorg Med Chem Lett, 2013; 23:2575. CrossRef
Karrouchi K, Radi S, Ramli Y, Taoufik J, Mabkhot YN, Al-aizari FA, Ansar M. Synthesis and pharmacological activities of pyrazole derivatives: a review. Molecules, 2018; 23:134. CrossRef
Kassem AF, Moustafa GO, Nossier ES, Khalaf HS, Mounier MM, Al-Yousef SA, Mahmoud SY. Design, synthesis, in vitro anticancer potentiality and molecular modeling study of novel amino acid derivatives based on n1, n3-bis (1-hydrazinyl-1- oxopropan-2-yl) isophthalamide. J Enzyme Inhib Med Chem, 2019; 34(1):1247–58. CrossRef
Khatab TK, Hassan AS, Hafez TS. V2O5/SiO2 as an efficient catalyst in the synthesis of 5-aminopyrazole derivatives under solvent free condition. Bull Chem Soc Ethiop, 2019; 33:135. CrossRef
Lingaraju GS, Rakesh S, Kumar KSV, Raob KP, Sreenivasa MY, Sadashiva MP. Synthesis of new benzofuran-pyrazole hybrids as potential antibiofilm agents. Lett Drug Des Discov, 2017; 14:1. CrossRef
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility in drug discovery and development settings. Adv Drug Deliv Rev, 2001; 46:3. CrossRef
MacLowry DJ, Jaqua MJ, Selepak ST. Detailed methodology and implementation of a semiautomated serial dilution microtechnique for antimicrobial susceptibility testing. J Appl Microbiol, 1970; 20:46. CrossRef
Marella A, Ali MR, Alam MT, Saha R, Tanwar O, Akhter M, Shaquiquzzaman M, Alam M.M. Pyrazolines: A Biological Review. Mini Rev Med Chem, 2013; 13:921. CrossRef
Moustafa GO, El-Sawy AA, Abo-Ghalia MH. Synthesis of novel cyclopeptide candidates: I-cyclo-[Nα-isophthaloyl-bis-(Glycine-amino acid)-L-lysine] derivatives with expected anticancer activity. Egypt J Chem, 2013; 56:473. CrossRef
Moustafa GO, Khalaf H, Naglah A, Al-Wasidi A, Al-Jafshar N, Awad H. The synthesis of molecular docking studies, in vitro antimicrobial and antifungal activities of novel dipeptide derivatives based on N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide. Molecules, 2018; 23:761. CrossRef
Moustafa GO, Younis A, Al-Yousef SA, Mahmoud SY. Design, synthesis of novel cyclic pentapeptide derivatives based on 1,2-benzenedicarbonyl chloride with expected anticancer activity. J Comput Theor Nanosci, 2019; 16:1733. CrossRef
Naglah AM, Moustafa GO, Al-Omar MA, Al-Salem HAS, Hozzein WN. Synthesis, characterization and in vitro antimicrobial investigation of novel amino acids and dipeptides based on dibenzofuran-2-sulfonyl-chloride. J Comput Theor Nanosci, 2017; 14:3183. CrossRef
Osman SA, Mousa HA, Yosef HAA, Hafez TS, El-Sawy AA, Abdallah MM, Hassan AS. Synthesis, characterization and cytotoxicity of mixed ligand Mn(II), Co(II) and Ni(II) complexes. J Serb Chem Soc, 2014; 79:953. CrossRef
Osman SA, Yosef HAA, Hafez TS, El-Sawy AA, Mousa HA, Hassan AS. Synthesis and antibacterial activity of some novel chalcones, pyrazoline and 3-cyanopyridine derivatives based on khellinone as well as Ni(II), Co(II) and Zn(II) complexes. Aust J Basic Appl Sci, 2012; 6:852.
Othman M, Lohb HS, Wiartc C, Khooa TJ, Lima KH, Ting KN. Optimal methods for evaluating antimicrobial activities from plant extracts. J Microbiol Meth, 2011; 84:161. CrossRef
Rangaswamy J, Kumar HV, Harini ST, Naik N. Synthesis of benzofuran based 1,3,5-substituted pyrazole derivatives: as a new class of potent antioxidants and antimicrobials--a novel accost to amend biocompatibility. Bioorg Med Chem Lett, 2012; 22:4773. CrossRef
Rocha L, Marston A, Potterat O, Kaplan MAC, Stoeckli-Evans H, Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericumbrasiliense. Phytochemistry, 1995; 40:1447. CrossRef
Shamsuzzaman HK. Bioactive benzofuran derivatives: a review. Eur J Med Chem, 2015; 97:483. CrossRef
Sharma PK, Kumar S, Kumar P, Kaushik P, Kaushik D, Dhingra Y, Aneja KR. Synthesis and biological evaluation of some pyrazolylpyrazolines as antiinflammatory–antimicrobial agents. Eur J Med Chem, 2010; 45:2650. CrossRef
Sharshira EM, Hamada NMM. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules, 2012; 17:4962. CrossRef
Silva VLM, Elguero J, Silva AMS. Current progress on antioxidants incorporating the pyrazole core. Eur J Med Chem, 2018; 156:394. CrossRef
Valgas C, De Souza SM, Smânia EFA, Smânia Jr A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol, 2007; 38:369. CrossRef