INTRODUCTION
Gabapentin (GBP), chemically known as 1-(aminomethyl) cyclohexaneacetic acid (Fig. 1), has a molecular formula of C9H17NO2 and a molecular weight of 171.24 (USP 32, 2009). GBP is a white to almost white crystalline powder that is sparingly soluble in water, slightly soluble in ethanol (96%), and practically insoluble in methylene chloride. It dissolves in dilute acids and dilute solutions of alkali hydroxides. A 2% solution of GBP in water has a pH of 6.5 to 7.5 (BP, 2015; Martindale 36, 2009).
Although GBP was originally developed as anticon-vulsant in the treatment of epilepsy as a structural analogue of gamma-aminobutyric acid, the precise mechanism by which GBP produces its analgesic and antiepileptic actions is yet unknown. However, it is believed to involve voltage-gated N-type calcium ion channels in the central nervous system, reducing calcium influx into the nerve terminals. In this way, the nerves become less excitable, reducing the release of other neurotransmitters (Davies et al., 2007; Fornasari, 2017).
GBP is used for the treatment of complex partial seizures, with or without secondary generalization. It is currently used as a medication to relieve pain, especially neuropathic pain and as an adjunct for the treatment of acute postsurgical pain (Behnam et al., 2012; McNamara, 2011).
.png) | Figure 1. Chemical structure of gabapentin. [Click here to view] |
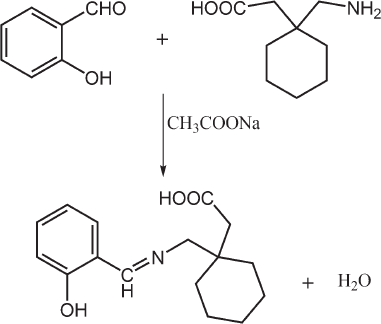 | Figure 2. Schematic representation of proposed reaction of gabapentin with SA. [Click here to view] |
Reviewing the literature revealed that several direct and indirect analytical methods which based on gas chromatography (GC), high performance liquid chromatography (HPLC), capillary electrophoresis (CE), UV-Vis spectrophotometry, and spectrofluorometry have been used for the determination of GBP in pure form, pharmaceutical formulations, and/or biological samples either alone or in combination with other drugs.
Various methods that have been reported for the determination of GBP mostly rely on chromatographic techniques, especially HPLC with various detection methods (Gupta et al., 2008; Ojha et al., 2007; Rao et al., 2009; Yagi et al., 2012). They are more labor-intensive, time-consuming, not easy to work with them, and their columns and solvents are either expensive, hazardous, or not available. In addition, GC (Borrey et al., 2005) and CE methods (Cao et al., 2012) reported for the GBP determination require long and tedious pretreatment of the samples and laborious clean up procedures prior to analysis. They are likely to be limited by routine availability of these techniques.
Several spectroscopic methods were developed for GBP analysis in pure form and in pharmaceutical formulations either with spectrophotometric or spectrofluorimetric determination. Due to the absence of strong absorbing chromophore in GBP, its direct spectrophotometric analysis is problematic and mostly depends on the derivatization with chemical reagents (Adegbolagun et al., 2018; Adegoke et al., 2018; Ambala and Patel, 2011; Fonseca et al., 2017; Gouda and Malah, 2013; Gujral et al., 2009; Saleh et al., 2014; Siddiqui et al., 2010). The official methods for the identification and quantitation analysis of GBP are prescribed in BP and USP (BP, 2015; USP 32, 2009).
UV-Vis spectrophotometry is widely used for the quality control of pharmaceuticals because of its distinctive advantages such as lower cost, robustness, simplicity, good accuracy, and precision. Therefore, a new, simple, sensitive, and accurate derivatization spectrophotometric method for the determination of GBP in pure form as well as in pharmaceutical formulations was developed in which, the use of salicylaldehyde (SA) as a derivatizing reagent for gabapentin was examined, for the first time, to produce a chromophore detectable by UV-Vis detector. The proposed method based on the formation of Schiff’s bases (imine compound) as a result of condensation reaction of primary amino group of GBP with active carbonyl group of SA in the presence of acetate solution in one-step reaction (Fig. 2), which did not require vigorous conditions, extraction, or any clean-up step prior to detection in comparing with other reported procedures (Ambala and Patel, 2011).
MATERIALS AND METHODS
Instruments
Double beam scanning UV-Vis Spectrophotometer (Shimadzu 1601, Japan) with 1 cm matched quartz cell connected to a computer with UV-Probe software. Thermostatically controlled water bath (SB-24, Tokyo Aikakikai, Japan) and pH meter (Shimadzu, Japan) were used.
Reagents and chemicals
GBP standard was kindly donated by Bet Jalla Company (Ramallah, Palestine). Commercially available pharmaceutical dosage form, gabapentin capsules (300 mg/capsule), was purchased from the local market. Analytical grade Methanol was purchased from Merk (Germany). SA, glacial acetic acid, and sodium acetate supplied by (Sigma-Aldrich, Germany). Pharmaceutical grade excipients: starch, lactose monohydrate, magnesium stearate, and talc were kindly supplied by domestic pharmaceutical companies.
Preparation of reagents
SA solution 0.3% (v/v)
SA reagent was prepared by accurately transferring 0.30 ml of SA into 100-ml volumetric flask, mixed thoroughly with warm methanol, and completed the volume to 100 ml with methanol. The solution was freshly prepared daily and protected from light.
Sodium acetate solution 0.6% (w/v)
The solution was prepared by dissolving 0.60 gm of sodium acetate in 100 ml volumetric flask using distilled water.
Acetate buffer solutions
Acetate buffer solutions were prepared by weighing and transferring different amounts of sodium acetate into 1,000 ml volumetric flask, dissolved with distilled water, then different volumes of acetic acid solution 2 M were added and then the volume completed with distilled water to 1,000 ml (USP 32, 2009).
Gabapentin standard solution
Standard GBP stock solution (2 mg/ml) was freshly prepared by dissolving 100 mg of GBP standard in 50-ml volumetric flask using distilled water.
Selection of analytical wavelength for chromogen
Into two 10 ml volumetric flasks, 0.1 and 0.4 ml of GBP stock solution (2 mg/ml) were transferred and then 2 ml of SA 0.3% (v/v) solution followed by 1 ml of sodium acetate 0.6% (w/v) solution were added. The content of each flask was shaken thoroughly and heated on a water bath at 45°C for 20 minutes. The flasks were left to cool for 10 minutes, then volume was completed with methanol to have 20 and 80 μg/ml GBP, respectively. The absorption spectra and absorption maxima of the resulting solutions (chromogen) were determined against a blank prepared in the same manner without the examined drug.
Optimization of reaction conditions
Effect of temperature
The effect of temperature on the reaction was studied by carrying out the reaction at different temperatures: RT, 35°C, 40°C, 45°C, 50°C, 55°C, 60°C, and 70°C.
Effect of solvent
The effect of solvent on the absorbance of chromogen was studied using distilled water and methanol for the dilution of final samples.
Effect of volume of SA 0.3% (v/v) solution
The effect of concentration of SA solution was studied by measuring the absorbance of GBP derivative with different added reagent volumes: 1.0, 1.5, 2.0, and 2.5 ml of SA 0.3% (v/v) solution.
Effect of volume of sodium acetate 0.6% (w/v) solution
The effect of concentration of sodium acetate solution was studied by measuring the absorbance of GBP derivative with different added volumes: 0.5, 1.0, 1.5, and 2.0 ml of sodium acetate solution 0.6% (w/v).
Effect of pH
The effect of pH was studied by measuring the absorbance of GBP derivative at different pH of acetate buffer solutions: 4, 4.5, 5, 5.5, and 7.5.
Effect of reaction time
The effect of reaction time was studied by carrying out the reaction for different time periods: 5, 10, 15, 20, 25, and 30 minutes.
Effect of order of addition
The effect of reagents addition order was studied by carrying out the reaction in different orders.
General procedure for gabapentin determination
Into 10 ml volumetric flask, 0.4 ml of GBP stock solution (2 mg/ml) was transferred followed by 2 ml of SA reagent and 1 ml of sodium acetate solution. The content of the flask was shaken thoroughly and heated on a water bath at 45°C for 20 minutes. The flask left to cool for 10 minutes and the volume was completed with the methanol. The absorbance of the resulting solution was measured at 403 nm against a blank prepared in the same manner without the examined drug.
Construction of calibration curve for gabapentin
Accurately measured aliquots of GBP stock solution were transferred into separate 10-ml volumetric flasks and then the general procedure for gabapentin determination [Section “General procedure for gabapentin determination”] was conducted to produce standard solutions having concentrations of 6–100 μg/ml. The absorbances of these standard solutions were measured at 403 nm and calibration curve was plotted. The correlation coefficient (r), slope and intercept, and molar absorptivity of the drug were determined using the calibration curve.
Method validation
Analytical method validation was performed according to the ICH guidelines (2005) with respect to accuracy, precision, specificity, linearity, limit of detection (LOD), and limit of quantitation (LOQ).
Linearity and range
The linearity of the proposed method was determined by measuring the absorbance of six concentrations (6, 10, 20, 40, 80, and 100 μg/ml of GBP) covering the range (6–100 μg/ml). Five measurements were done for each concentration. The absorbance was plotted against concentration. The regression line and correlation coefficient were evaluated as well as validity of regression line was verified by statistical analysis.
Specificity
The specificity of the method was investigated by adding the common used excipients in the preparation of GBP formulations (starch, magnesium stearate, talc, and lactose) to the drug, then procedure was followed as described and the percent of the drug recovery was calculated.
Accuracy
Accuracy of the method was ascertained by performing recovery studies using standard addition method, in which standard drug aliquots were added at three different levels (20, 40, and 60 μg/ml) to a pre-analyzed sample of GBP capsule powder equivalent to 20 μg/ml. These three concentrations were measured and each of them was repeated three times to calculate the accuracy.
Precision
Intraday precision was assessed by analysis of three different concentrations (20, 80, and 100 μg/ml) of the drug at three different time periods of the same day. The relative standard deviation (RSD) was calculated for each concentration. On the other hand, inter-day precision was studied by repeating the procedure for three different concentrations (20, 80, and 100 μg/ml) of the drug on three different days and RSD was calculated for each concentration.
LOD and LOQ
The LOD is expressed using the following formula (ICH, 2005); LOD= 3.3 σ/S and the LOQ is expressed using the following formula (ICH, 2005); LOQ = 10 σ/S; where, σ is the standard deviation of residuals of the regression line and S is the slope of the regression line.
 | Figure 3. Absorption spectrum of blank solution of SA and sodium acetate against methanol. [Click here to view] |
 | Figure 4. Absorption spectrums of GBP derivative product (20 and 80 μg/ml) [Click here to view] |
Stability of chromogen
The effect of time on the stability of chromogen was studied by following the absorption of the resulting GBP derivative at different time intervals up to 1 hour at 403 nm after the completion of the flask's volume with methanol against the blank.
Application to gabapentin pharmaceutical dosage form (capsules)
Twenty capsules were emptied, weighed, and mixed thoroughly. An accurately weighed portion of the resulting powder equivalent to 100 mg GBP was taken, dissolved in 25 ml distilled water with good shaking for 5–10 minutes, then filtered with filter paper to remove insoluble matter. The filtrate solution was diluted to 50 ml with distilled water to prepare 2-mg/ml solution. 0.2 and 0.4 ml of the resulting solution were transferred into two 10 ml volumetric flasks then the procedure was followed as described above (Section “General procedure for gabapentin determination”). Triplicate measurements were done for each concentration and the percentage of recovery was calculated from the calibration curve.
 | Table 1. Summary of optimum conditions for reaction of GBP with SA. [Click here to view] |
RESULTS AND DISCUSSION
The reaction of aldehydes or ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff base). Water is eliminated in the reaction, which is acid-catalyzed and reversible in the same sense as acetal formation. The pH for imine-producing reactions must be carefully controlled. The rate, at which these compounds are formed, is generally greatest near a pH of 5, and drops at higher and lower pH’s (Sorrell, 2006).
Selection of analytical wavelength for chromogen
The derivatization reaction between GBP and SA was performed and the absorption spectrum of the formed yellow-colored product in methanolic medium was scanned against the blank. The blank was also scanned against methanol in the range of 200–800 nm (Fig. 3). It was found that the absorbance of the derivative product increased directly with GBP concentration and exhibiting λmax at 403 nm (Fig. 4) at which selectivity enhanced through avoiding interferences from excipients in contrast to shorter λmax of other reviewed methods (Siddiqui et al., 2010).
Optimization of derivatization reaction conditions
To optimize the reaction variables, the effects of temperature, solvent, reaction time, pH, and concentrations of SA and sodium acetate on the absorbance of the formed product, with respect to maximum sensitivity and adherence to Beer’s law, were studied through controlled experiments. The results of the optimization studies are summarized in Table 1.
Method validation
Linearity
The calibration curve was constructed by plotting the absorbance of the drug versus the drug concentration within the specified range. A linear correlation was found between concentration and absorbance in the range 6–100 μg/ml (Fig. 5) with a correlation coefficient equal to 0.9961. Molar absorptivity of GBP chromogen was calculated (ε = 684.95 l/mol.cm). The LOD and LOQ for the proposed method were found to be 1.16 and 3.48 μg/ml, respectively. These obtained parameters were comparable to the parameters obtained in published spectrophotometric methods of analysis of GBP (Ambala and Patel, 2011).
 | Figure 5. Calibration curve of GBP analysis (mean of five replicates). [Click here to view] |
 | Table 2. Results of specificity study for GBP analysis. [Click here to view] |
Specificity
The specificity of the method was investigated by observing any interference encountered from common excipients, usually used in the pharmaceutical formulations of GBP. The degree of interference was evaluated by measuring the percentage recovery of GBP at two concentration levels (60 and 90 μg/ml) with various amounts of diverse excipients added to the drug. No interference was found in the analysis of GBP as shown in Table 2.
Accuracy
Accuracy of the method was ascertained by performing recovery studies using a standard addition method. The obtained results in Table 3 showed excellent mean recovery percent values (102.22%–99.28%) with low SD values (≤1.5) which indicate high accuracy of the proposed analytical method.
Precision
Intraday precision was assessed and the results are summarized in Table 4. RSD values were found to be very small (<1.0%) indicating high repeatability of the proposed method. Moreover, the inter-day precision was also assessed and the results are summarized in Table 4. The obtained RSD values were less than 1.34% indicating good precision of the proposed method.
 | Table 3. Results of recovery study for gabapentin analysis. [Click here to view] |
 | Table 4. Evaluation of the precision of the analytical procedure of gabapentin. [Click here to view] |
Stability of chromogen
It was found that the use of methanol as a solvent yielded good results in terms of the stability of GBP derivative which remained at least for 1 hour after dilution and this is sufficient time for the performance of the analysis for many samples.
Application to gabapentin pharmaceutical dosage form
The developed spectrophotometric method has been successfully applied to the determination of GBP in capsules. The percentage of recovery mean for GBP capsules was 101.01 ± 1.23 which is satisfactory and very close to the label claim and lie within the USP accepted range (90%–110%; USP 32, 2009). The percentage of recovery with low values of standard deviation indicates the accuracy and precision of the proposed method (Table 5). The estimated drug content obtained by the proposed method was statistically comparable to the results obtained by a published method (Gujral et al., 2009). No significance difference was found at 95% confidence level (p value < 0.05) as shown in Table 5.
 | Table 5. Determination of gabapentin in capsules by developed method and comparison with validated published method. [Click here to view] |
CONCLUSION
The present study described the development and validation of a simple spectrophotometric assay for the determination of GBP. The obtained results revealed that the proposed method is time saving, precise, accurate and do not require prior extraction procedure or heating and have the advantages of simplicity and sensitivity. Hence, it could be concluded that the developed method could be successfully applied in routine analysis for GBP determination in bulk and in pharmaceutical dosage forms.
REFERENCES
Adegbolagun OM, Thomas OE, Aiyenale EO, Adegoke OA. A new spectrophotometric method for the determination of gabapentin using chromotropic acid. Acta Pharm Sci, 2018; 56(3):93–110. CrossRef
Adegoke OA, Adegbolagun OM, Aiyenale EO, Thomas OE. New spectrophotometric method for the determination of gabapentin in bulk and dosage forms using p-dimethylaminobenzaldehyde. J Taibah Univ Sci, 2018; 12(6):754–64. CrossRef
Ambala PS, Patel NJ. Visible spectrophotometric methods for determination of gabapentin in pharmaceutical tablet and capsule dosage forms. Asian J Pharmacy Life Sci, 2011; 1(3):223–33.
Behnam B, Semnani V, Saghafi N, Ghorbani R, Shori MD, Choobmasjedi SG. Gabapentin effect on pain associated with heroin withdrawal in Iranian crack: a randomized double-blind clinical trial. Iran J Pharm Res, 2012; 11:979–83.
Borrey DCR, Godderis KO, Engelrelst VIL, Bernard DR, Langlois MR. Quantitative determination of vigabatrin and gabapentin in human serum by gas chromatography-mass spectrometry. J Clin Chim Acta, 2005; 354(1–2):147–51. CrossRef
British Pharmacopoeia. Monograph of gabapentin. The Department of Health, The Stationary Office London, UK, 2015.
Cao L, Liang S, Tan X, Meng J. Determination of gabapentin in human plasma by capillary electrophoresis-laser induced fluorescence detection with and without solid-phase extraction. J Microchim Acta, 2012; 178(3–4):285–92. CrossRef
Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2á³ subunits of voltage-gated calcium channels. Trends Pharmacol Sci, 2007; 28:220–8. CrossRef
Fonseca F, Brito de Barros R, Ilharco LM, Garcia AR. Spectroscopic methods for quantifying gabapentin: framing the methods without derivatization and application to different pharmaceutical formulations. Appl Spectrosc, 2017; 71(11):2519–31. CrossRef
Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther, 2017; 6(Suppl 1):25–33. CrossRef
Gouda AA, Malah ZA. Development and validation of sensitive spectrophotometric method for determination of two antiepileptics in pharmaceutical formulations. Spectrochim Acta A Mol Biomol Spectrosc, 2013; 105:488–96. CrossRef
Gujral RS, Haque SM, Shanker P. A sensitive UV spectrophotometric method for the determination of gabapentin. E-J Chem, 2009; 6(S1):163–70.
Gupta A, Ciavarella AB, Sayeed VA, Khan MA, Faustino PJ. Development and application of a validated HPLC method for the analysis of dissolution samples of gabapentin drug products. J Pharm Biomed Anal, 2008; 46(1):181–6. CrossRef
International Conference on Harmonization (ICH). Validation of analytical procedures: text and methodology Q2(R1). International Conference on Harmonization (ICH), Q2(R1), Geneva, Switzerland, pp 1–13.
Sweetman SC. Martindale: the complete drug reference. In Antiepileptics. 36th edition, Pharmaceutical Press, London, UK, pp 482–3, 2009.
McNamara JO. Pharmacotherapy of the epilepsies. In Brunton LL, Chabner BA, Knollman BC (eds.). Goodman & Gilman's the pharmacological basis of therapeutics. 12th edition, The McGraw-Hill Companies, New York, NY, pp 583–608, 2011.
Ojha A, Rathod R, Patel C, Padh H. LC-MS determination of gabapentin from human plasma. Chromatographia, 2007; 66:853–7. CrossRef
Rao BU, Maqdoom F, Nikalje AP. Determination of gabapentin in bulk drug and in pharmaceutical dosage form by HPLC method. J Chil Chem Soc, 2009; 54(4):424–7.
Saleh MS, Youssef AK, Hashem EY, Abdel-Kader DA. A novel spectrophotometric method for determination of gabapentin in pharmaceutical formulations using 2,5-dihydroxybenzaldehyde. Comput Chem, 2014; 2:22–30. CrossRef
Siddiqui FA, Arayne MS, Sultana N, Rehman N. Spectrophotometric determination of gabapentin in pharmaceutical formulations using ninhydrin and π-acceptors. Eur J Med Chem, 2010; 45(7):2761–7. CrossRef
Sorrell TN. Addition-elimination reactions of aldehydes and ketones. In Organic chemistry. 2nd edition, University Science Books, California, pp 669–700, 2006.
United States Pharmacopeia, USP. NF 27. Monograph of gabapentin. 32nd edition, Asian Edition, United States Pharmacopeial Convention, Inc., Rockville, MD, 2009.
Yagi T, Naito T, Mino Y, Takashina Y, Umemura K, Kawakami J. Rapid and validated fluorometric HPLC method for determination of gabapentin in human plasma and urine for clinical application. J Clin Pharm Ther, 2012; 37(1):89–94. CrossRef