INTRODUCTION
Tuberculosis (TB) is a chronic, infectious disease caused by Mycobacterium tuberculosis that has been present for a long time. This disease remains the most large-scale medical and social problem. Approximately 3–4 million people around the world die each year from TB, and every year, approximately 8 million first-ever events of TB are registered. This burden is due to the high susceptibility of human immunodeficiency virus-infected patients (Nunn et al., 2005). The emergence of multidrug-resistant and extensively drug-resistant TB has directed attention to, and scientific interest in, this infectious disease (Bloch et al., 1994; Kochi et al., 1993; Rastogi et al., 1992). For this reason, there is a need to discover new classes of chemical agents that features the diverse mechanisms of action to treat this disease.
Nitrogen-containing heterocyclic compounds have drawn the attention of medicinal chemists due to their various therapeutic properties (Nagesh et al., 2014;Siddesh et al., 2014;Thriveni et al., 2014). Indolizines are bicyclic heteroaromatic compounds containing six- and five-membered condensed rings with bridging nitrogen (Sandeep et al., 2013). Indolizine pharmacophore, with different degrees of substitution and unsaturation, is present in a wide variety of natural alkaloids (Michael, 2001; 2002) and unnatural azacyclic compounds (Halab et al., 2002; Pearson and Hembre, 1996). Synthetic indolizine analogs have been reported for their numerous pharmacological properties, such as their analgesic (Vaught et al., 1990), anti-cancer (Butler, 2008; Sandeep et al., 2016a), anti-diabetic (Mederski et al., 2012; January 31), anti-histaminic (Cingolani et al., 1990), anti-inflammatory (Hagishita et al., 1996; Sandeep et al., 2017; 2018b), anti-leishmanial (Jaisankar et al., 2004), anti-microbial (Hazra et al., 2011), anti-mutagenic (Olejnikova et al., 2009), antioxidant (Nasir et al., 1998), anti-tubercular (Dannhardt et al., 1987; Khedr et al., 2018), antiviral (Mishra and Tiwari, 2011), larvicidal (Sandeep et al., 2016b; 2018a), in vitro, and herbicidal activities (Smith et al., 2005).
.png) | Scheme 1. Reagents and conditions: (i) 4-substituted phenacyl bromide, acetone, 5 hours stir; (ii) K2CO3, dimethylformamide (DMF), 30 minutes stir at room temperature. [Click here to view] |
 | Figure 1. Molecular structure and code of indolizine analogs used for in vitro qualitative anti-TB activity. [Click here to view] |
With these observations in mind, and in continuation of our efforts to develop novel heterocyclic (Khedr et al., 2018; Venugopala et al., 2013; 2016; 2018) and peptide (Narayanaswamy et al., 2011) compounds with anti-TB activity and to screen pharmacologically active heterocyclic compounds based on their polymorphic properties (Munshi et al., 2004; Panini et al., 2014a; 2014b), we evaluated synthesized (Sandeep et al., 2016a) ethyl 7-acetyl-2-substituted-3-(4-substituted benzoyl)indolizine-1-carboxylate analogs 2a–r (Scheme 1; Fig. 1) to determine their qualitative anti-TB activity in vitro using an agar dilution method against a susceptible H37Rv strain (Table 1).
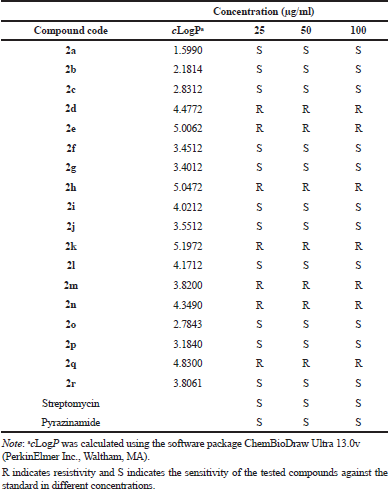 | Table 1. Anti-tubercular activity against H37Rv strain of M. tuberculosis. [Click here to view] |
MATERIALS AND METHODS
The synthetic route for the construction of indolizine scaffolds 2a–r and the characterization of the title compounds are described in our earlier research publication (Sandeep et al., 2016a). The synthetic ethyl 7-acetyl-2-substituted-3-(4-substituted benzoyl)indolizine-1-carboxylates 2a–r have been tested for their qualitative anti-TB activity in vitro against an M. tuberculosis H37Rv strain.
Anti-tubercular activity
The procedure followed for anti-TB screening involves the use of Middlebrook 7H9 broth and the M. tuberculosis strain H37Rv. The basal medium was prepared in accordance with the manufacturer’s instructions (HiMedia Laboratories, Mumbai, India) and sterilized by autoclaving; 4.5 ml of broth was added into each sterile bottle. To this, 0.5 ml of Oleic acid dextrose catalase (OADC) supplement was added, which contained catalase, bovine serum albumin, and dextrose fraction. A volume of 10 mg/ml stock solution of the test compound was then prepared. From this, an appropriate amount of solution was transferred to media bottles to achieve concentrations of 25, 50, and 100 μg/ml. Then, 10 μl of a suspension containing the M. tuberculosis H37Rv strain (100,000 organisms/ml, adjusted by McFarland’s turbidity standard) was transferred to each tube and incubated at 37°C. In addition, one growth control without the compound and drug controls was established. The bottles were examined twice a week to determine growth, for a total period of 3 weeks. Turbidity was considered as growth and was indicative of resistance to the compound. Growth was confirmed by taking a smear from each bottle and conducting a Ziehl–Neelsen (ZN) stain. The antibiotic standards used included streptomycin (7.5 μg/ml) and pyrazinamide (7.5 μg/ml).
RESULTS AND DISCUSSION
The in vitro qualitative anti-TB activity was tested for all of the 2a–r derivatives using the M. tuberculosis strain H37Rv at 25, 50, and 100 μg/ml by an agar dilution method (Sun et al., 2010) (Table 1). Test compounds 2a, 2b 2c, 2f, 2g, 2i, 2j, 2l, 2o, 2p, and 2r were found to be active against M. tuberculosis at all three concentrations. Compounds 2d, 2e, 2h, 2k, 2m, 2n, and 2p were found to be inactive against M. tuberculosis at all three concentrations. The common functionality of inactive compounds 2d, 2e, 2h, 2k, 2m, 2n, and 2p was the presence of a diethyl ester group at position 1 and a methyl or ethyl group at position 2. The common functionality of active compounds 2a, 2b 2c, 2f, 2g, 2i, 2j, 2l, 2o, 2p, and 2r was the presence of a diethyl ester group at position 1, and either hydrogen or diethyl ester at position 2. The acetyl group was found at position 7 and the substituted benzoyl group was noted at position 3 of the indolizine nucleus.
CONCLUSION
In an attempt to select promising indolizine compounds (2a–r) to determine their quantitative anti-TB activity, test compounds 2a, 2b 2c, 2f, 2g, 2i, 2j, 2l, 2o, 2p, and 2r were active against the M. tuberculosis H37Rv strain, while test compounds 2d, 2e, 2h, 2k, 2m, 2n, and 2p were found to be inactive against the M. tuberculosis H37Rv strain. Based on the preliminary results, we proposed to design and synthesize novel indolizine scaffolds having various functional groups for anti-TB activity against multidrug-resistant strains of M. tuberculosis.
ACKNOWLEDGMENTS
The authors would like to thank Sahyadri Science College, Shimoga, for providing laboratory facilities and Maratha Mandal NGS Institute of Dental Science, Belgaum, India for carrying out anti-tubercular activity. KNV would like to thank the National Research Foundation (Grant Nos. 96807 and 98884), South Africa and Durban University of Technology, South Africa, for their support and encouragement.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
Bloch AB, Cauthen GM, Onorato IM, Dansbury KG, Kelly GD, Driver CR, Snider DE Jr. Nationwide survey of drug-resistant tuberculosis in the United States. JAMA, 1994; 271:665–71.
Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep, 2008; 25:475–516.
Cingolani GM, Claudi F, Massi M, Venturi F. Indolizine derivatives with biological activity VI 1-(2-aminoethyl)-3-benzyl-7-methoxy-2-methylindolizine, benanserin structural analogue. Cingolani, 1990; 25:709–12.
Dannhardt G, Meindl W, Gussmann S, Ajili S, Kappe T. Anti-mycobacterial 7-hydroxy-2,3-dihydro-1H-indolizin-5-ones. Eur J Med Chem, 1987; 22:505–10.
Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M. Potent inhibitors of secretory phospholipase A2: synthesis and inhibitory activities of indolizine and indene derivatives. J Med Chem, 1996; 39:3636–58.
Halab L, Becker JA, Darula Z, Tourwe D, Kieffer BL, Simonin F, Lubell WD. Probing opioid receptor interactions with azacycloalkane amino acids. Synthesis of a potent and selective ORL1 antagonist. J Med Chem, 2002; 45:5353–7.
Hazra A, Mondal S, Maity A, Naskar S, Saha P, Paira R, Sahu KB, Paira P, Ghosh S, Sinha C, Samanta A, Banerjee S, Mondal NB. Amberlite-IRA-402 (OH) ion exchange resin mediated synthesis of indolizines, pyrrolo [1,2-a] quinolines and isoquinolines: antibacterial and antifungal evaluation of the products. Eur J Med Chem, 2011; 46:2132–40.
Jaisankar P, Pal B, Manna KN, Pradhan PK, Medda S, Basu MK, Giri VS. Synthesis of antileishmanial (5R)-(-)-5-carbomethoxy-3-formyl-5,6-dihydroindolo-[2,3-a]-indolizine. ARKIVOC, 2004; 2003:150–7.
Khedr MA, Pillay M, Sandeep C, Chopra D, Aldhubiab BE, Attimarad M, Alwassil OI, Mlisana K, Odhav B, Venugopala KN. Molecular modeling studies and anti-TB activity of trisubstituted indolizine analogues; molecular docking and dynamic inputs. J Biomol Struct Dyn, 2018; 36:2163–78.
Kochi A, Vareldzis B, Styblo K. Multidrug-resistant tuberculosis and its control. Res Microbiol, 1993;144:104–10.
Mederski W, Beier N, Burgdorf LT, Gericke R, Klein M, Tsaklakidis C. Indolizine derivatives and the use thereof as antidiabetics. US Patent; January 31, 2012, p. 8.
Michael JP. Simple indolizidine and quinolizidine alkaloids. Alkaloids Chem Biol, 2001;55:91–258.
Michael JP. Indolizidine and quinolizidine alkaloids. Nat Prod Rep, 2002; 19:719–41.
Mishra BB, Tiwari VK. Natural products in drug discovery: clinical evaluations and investigations. Opportunity Challenge Scope Nat Prod Med Chem, 2011; 1–61.
Munshi P, Venugopala KN, Jayashree BS, Row TNG. Concomitant polymorphism in 3-Acetylcoumarin: role of weak C-H•••O and C-H•••π Interactions. Cryst Growth Des, 2004; 4:1105–7.
Nagesh HK, Padmashali B, Sandeep C, Yuvaraj T, Siddesh MB. Synthesis and antimicrobial activity of benzothiophene substituted coumarins, pyrimidines and pyrazole as new scaffold. Int J Pharm Sci Rev Res, 2014; 28(2):6–10.
Narayanaswamy VK, Albericio F, Coovadia YM, Kruger HG, Glenn EM, Pillay M, Govender T. Total synthesis of a depsidomycin analogue by convergent solid phase peptide synthesis and macrolactonization strategy for anti-tubercular activity. J Peptide Sci, 2011; 17:683–9.
Nasir AI, Gundersen LL, Rise F, Antonsen O, Kristensen T, Langhelle B, Bast A, Custers I, Haenen GR, Wikstrom H. Inhibition of lipid peroxidation mediated by indolizines. Bioorg Med Chem Lett, 1998; 8:1829–32.
Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol, 2005; 5:819.
Olejnikova P, Birosova L, Svorc L. Antimicrobial and antimutagenic properties of newly synthesized derivatives of indolizine. Sci Pharm, 2009;77:216.
Panini P, Venugopala KN, Odhav B, Chopra D. Polymorphism in two biologically active dihydropyrimidinium hydrochloride derivatives: quantitative inputs towards the energetics associated with crystal packing. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater, 2014a; 70:681–96.
Panini P, Venugopala KN, Odhav B, Chopra D. Quantitative analysis of intermolecular interactions in 7-Hydroxy-4-methyl-2H-chromen-2-one and Its Hydrate. Proc Natl Acad Sci India Sect A, 2014b; 84:281–95.
Pearson WH, Hembre EJ. Synthesis of novel glycosidase-inhibitory hydroxymethyl-substituted polyhydroxylated indolizidines: ring-expanded analogs of the pyrrolizidine alkaloids alexine and australine. J Organ Chem, 1996; 61:5546–56.
Rastogi N, Ross BC, Dwyer B, Goh KS, Clavel-Seres S, Jeantils V, Cruaud P. Emergence during unsuccessful chemotherapy of multiple drug resistance in a strain of Mycobacterium tuberculosis. Eur J Clin Microbiol Infect Dis, 1992; 11:901–7.
Sandeep C, Padmashali B, Kulkarni RS. Efficient synthesis of indolizines and new imidazo[1,2-a]pyridines via the expected cyclization of aromatic cycloimmonium ylides with electron deficient alkynes and ethyl cyanoformate. Tetrahedron Lett, 2013; 54:6411–4.
Sandeep C, Padmashali B, Venugopala KN, Kulkarni RS, Venugopala R, Odhav B. Synthesis and characterization of ethyl 7-acetyl-2-substituted 3-(substituted benzoyl)indolizine-1-carboxylates for in vitro anticancer activity. Asian J Chem, 2016a; 28:1043–8.
Sandeep C, Venugopala KN, Gleiser RM, Chetram A, Padmashali B, Kulkarni RS, Venugopala R, Odhav B. Greener synthesis of indolizine analogues using water as a base and solvent: study for larvicidal activity against Anopheles arabiensis. Chem Biol Drug Des, 2016b; 88:899–904.
Sandeep C, Venugopala KN, Khedr MA, Padmashali B, Kulkarni RS, Rashmi V, Odhav B. Design and synthesis of novel indolizine analogues as COX-2 inhibitors: computational perspective and in vitro screening. Indian J Pharm Educ Res, 2017; 51:452–60.
Sandeep C, Venugopala KN, Nayak SK, Gleiser RM, García DA, Kumalo HM, Kulkarni RS, Mahomoodally FM, Venugopala R, Mohan MK, Odhav B. One-pot microwave assisted synthesis and structural elucidation of novel ethyl 3-substituted-7-methylindolizine-1-carboxylates with larvicidal activity against Anopheles arabiensis. J Mol Struct, 2018a; 1156:377–84.
Sandeep C, Venugopala KN, Tratrat C, Mahomoodally FM, Aldhubiab BE, Haroun M, Venugopala R, Mohan MK, Kulkarni RS, Attimarad MV, Harsha S, Odhav B. Efficient synthesis and characterization of novel indolizines: exploration of in vitro COX-2 inhibitory activity and molecular modelling studies. New J Chem, 2018b;42:4893–901.
Siddesh M, Basavaraj P, Thriveni K, Sandeep C. Synthesis of thiophene-linked pyrimidopyrimidines as pharmaceutical leads. J Chem Sci, 2014; 126:821–6.
Smith SC, Clarke ED, Ridley SM, Bartlett D, Greenhow DT, Glithro H, Klong AY, Mitchell G, Mullier GW. Herbicidal indolizine-5,8-diones: photosystem I redox mediators. Pest Manag Sci, 2005; 61:16–24.
Sun Z, Zhang J, Song H, Zhang X, Li Y, Tian M, Liu Y, Zhao Y, Li C. Concomitant increases in spectrum and level of drug resistance in Mycobacterium tuberculosis isolates. Int J Tuberculosis Lung Dis, 2010; 14:1436–41.
Thriveni KS, Padmashali B, Siddesh MB, Sandeep C. Synthesis of pyrimidine incorporated piperazine derivatives and their antimicrobial activity. Indian J Pharm Sci, 2014; 76:332–8.
Vaught JL, Carson JR, Carmosin RJ, Blum PS, Persico FJ, Hageman WE, Shank RP, Raffa RB. Antinociceptive action of McN-5195 in rodents: a structurally novel (indolizine) analgesic with a nonopioid mechanism of action. J Pharmacol Exp Ther, 1990; 255:1–10.
Venugopala KN, Chandrashekharappa S, Pillay M, Bhandary S, Kandeel M, Mahomoodally FM, Morsy MA, Chopra D, Aldhubiab BE, Attimarad M, Alwassil OI, Harsha S, Mlisana K. Synthesis and structural elucidation of novel benzothiazole derivatives as anti-tubercular agents: in-silico screening for possible target identification. Med Chem, 2018; doi:10.2174/1573406414666180703121815.
Venugopala KN, Rao GD, Bhandary S, Pillay M, Chopra D, Aldhubiab BE, Attimarad M, Alwassil OI, Harsha S, Mlisana K. Design, synthesis, and characterization of (1-(4-aryl)-1H-1, 2, 3-triazol-4-yl) methyl, substituted phenyl-6-methyl-2-oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylates against Mycobacterium tuberculosis. Drug Des Develop Ther, 2016; 10:2681.
Venugopala KN, Susanta KN, Pillay M, Renuka P, Yacoob MC, Odhav B. Synthesis and antitubercular activity of 2-(substituted phenyl/benzyl-amino)-6-(4-chlorophenyl)-5-(methoxycarbonyl)-4-methyl-3,6-dihydropyrimidin-1-ium chlorides. Chem Biol Drug Des, 2013; 81:219–27.