INTRODUCTION
Marine natural product discovery increases rapidly with hundreds of new compounds being discovered every year (Faulkner, 2002; Proksch and Míller, 2006). Most of these compounds were isolated from marine invertebrates like ascidians, sponges, bryozoans, and mollusks and few of them are in clinical trials (Blunt et al., 2008; Proksch et al., 2002). The motive for the presence of these active compounds is mainly for their chemical defense and survival of these soft bodied sessile organisms. Among all these invertebrates, ascidians are prolific producer of biologically active compounds with significant activities. Most of these compounds are active in the field of cancer therapy (Rinehart, 2000). Several eudistomins A, D, G, H, I, J, M, N, O, P, and Q, bromo, hydroxy, pyrrolyl and iminoazepino. beta-carbolines have been isolated and their biological function, especially antiviral activity were described in the tunicate Eudistoma olivaceum found in Caribbean (Kobayashi et al., 1984). A series of β-carbolines alkaloids Eudistomin Y1–Y7 which was originally isolated from marine tunicates has been chemically synthesized via acid-catalyzed Pictet-Spengler reaction and proved for their biological activities against breast carcinoma cells (Jin et al., 2013).
A review on synthesis of Eudistomin U, Isoeudistomin U, and related indole compounds and their biological activates like anticancer, antibacterial, antimalarial, and DNA binding were evaluated (Kolodina and Serdyuk, 2018). The studies related to chemistry and antimicrobial activities of ascidians found in Indian coast, especially in the south east coast of India are scanty. So, this research focuses on identification of compounds, especially the biologically important eudistomin group of alkaloids with antimicrobial activities from a green ascidian E. viride which is extensively found in Mandapam, south east coast of India.
MATERIAL AND METHODS
Collection and identification
The ascidian E. viride was collected by snorkeling in the intertidal rocky shallow region of Mandapam, south east coast of India. A small part of the collected ascidian was preserved in 5% formalin after relaxing the sample in menthol, the ascidian was identified using standard keys (Renganathan, 1984). The major part of ascidian samples were air dried and stored for extraction.
Extraction of ascidian
The air-dried samples were stored in methanol (SD Fine Chemicals, Mumbai). The ascidian sample was transported to the laboratory and then extracted with Dichloromethane (SD Fine Chemicals, Mumbai) and then with distilled methanol to yield dark brown gummy extract.
Column chromatography
The column was packed with silica gel and equilibrated with hexane. The crude extract (2 g) was loaded into column to fractionate the crude into different fractions. A step gradient from 100% Hexane to 100% Ethylacetate to 100% Methanol was used to separate out the different compounds from the crude extract. The flow rate was 3.4 ml per minute. Different fractions were collected.
MALDI—TOF analysis of column fractions
The column fractions were spotted in MALDI plate with CCA (alpha-cyano-4-hydroxycinnamic acid) matrix and were analyzed by MALDI-TOF (Ultraflex MALDI TOF/TOF (BrukerDaltonics)) for finding the mass of different molecules present in each fraction.
HPLC
The major fractions with excellent molecules (identified by mass) were subjected to reverse phase High Performance Liquid Chromatography (HPLC) using a semi preparative Agilent ZORBAX 300SB-C18 column with, 5 μm, 9.4 x 250 mm, to purify the individual compounds from a less complex column fractions. The mobile phase used was 95% distilled methanol: 95% water mixed with 0.1%Trifluoroacetic acid. A standardized binary gradient program (from 95% H2O: 95% Methanol) for 60 minutes time was used to separate out the individual molecules. Based on the UV-Visible Spectroscopic scan values, the different dual wavelength detection was made for each of the column fraction.
Antimicrobial activity
The antibacterial activity of the extracts was assessed against 10 human bacterial pathogens (Salmonella paratyphi, Escherichia coli, Vibrio cholerae, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus epidermidis, Klebsiella pneumoniae, Bacillus cereus, Bacillus subtilis, Enterobacter aerogenes). The human test bacterial strains were obtained from Christian Medical College (CMC), Vellore. All the test organisms were cultured in Mueller Hinton broth and the 18–24 hours old cultures were used for the experiment. The antibacterial activity of the extracts was assayed by following the standard disc diffusion technique (Becerro et al., 1994; Ramasamy and Murugan, 2003; Slattery et al., 1995). The sterile Whatman 6-mm discs, impregnated with 100 μg of extracts, were placed in the Petri plates seeded with the bacterial pathogens on Mueller Hinton agar. Discs without the extracts were maintained as control and the assay was carried out in triplicate. After 20–24 hours of incubation at the room temperature, the susceptibility of the test organisms was determined by measuring the diameter of the zone of inhibition to the nearest millimeter. Different concentrations were used for pure molecules tested for antimicrobial activity. The minimum inhibitory concentration (MIC) was determined using different concentrations of the crude extract (25, 50, 75, and 100 μg/disc). Higher concentration (125 μg/disc) was used to determine the enhancement in activity with increase in concentration of the extract.
RESULTS AND DISCUSSION
Eudistoma viride is a sedentary organism and the exact mechanism by which this organism acquires bioactive substances is not known. This ascidian has already been shown to harbor epibacteria on their surface (Ramasamy and Murugan, 2003). So, in this study, the metabolite production is either by ascidian itself or by its associated symbiotic bacteria as previously explained by Martinez-García et al. (2007).
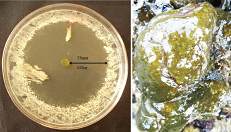 | Figure 1. Antibacterial activity of E.viride crude extract against E. coli. The zone of inhibition measured 33 mm at 125 μ/disc and the ascidian E. viride. [Click here to view] |
Potent wide spectrum of antimicrobial activity was observed in the methanol extract of the ascidian E. viride against all the 10 human pathogenic bacteria tested with zone of inhibition ranging from 7–19 mm. High activity was noticed at 125 μg/disc with a zone of inhibition of 33mm against E. coli bacteria (Fig. 1). The observed inhibition activity of 33 mm against E. coli was higher when compared with the antibacterial activity with an average inhibition zone of 29.1 mm of the crude extracts of the E. viride against 20 marine biofilm bacteria (Ramasamy and Murugan, 2003). The strong antibacterial activity exhibited by the ascidian E. viride in the present study against E. coli coincided with the observation of antimicrobial activity against E. coli, Listeria monocytogenes, and Candida albicans by Clavanins, the peptides found in the blood cells of Styelaclava, a solitary tunicate (Menzel et al., 2002).
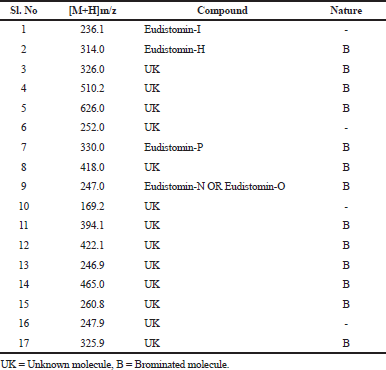 | Table 1. Ultra Flex MALDI-TOF masses of different fractions of E.viride [Click here to view] |
The crude extract was fractionated using the normal phase silica gel column chromatography. A total of 72 individual fractions were collected and each fraction was analyzed in MALDI-TOF to find out the different molecules confined in different fractions. MALDI-TOF spectrum of column chromatographic fractions displayed several compounds eluted in different fractions. Antimicrobial assay for the column fractions directed us with fraction 26 to produce intense zone of inhibition.
The HPLC detection wavelength was fixed based on the UV-VIS scanning pattern of individual column fractions. The individual peaks eluted from the HPLC were separately collected and simultaneously analyzed in mass spectrometry to find the compounds eluted and to ascertain its purity. Most of the HPLC fraction was ≥90% pure. Based on the manner of isotopic pattern obtained from the MALDI-TOF spectrum, it was determined whether the compound possessed bromine moiety or not (Table 1, Fig. 2A–L). It was exciting that most of the individual column fraction co-eluted the brominated and its non-brominated forms of the alkaloid. This may be because the structural similarity of the alkaloid molecules which made it to elute at the same time. In the reverse phase HPLC, most of the fractions revealed the presence of single compounds which separated even the structurally similar brominated and non-brominated compounds. This might be because the reverse phase C18 column might bind the alkaloids strongly, and when percentage of methanol gradient increases slowly, the strong bond between matrix and the molecule detaches slowly based on the molecular structure and their groups. Most of the non-brominated forms of the alkaloids eluted first and then the brominated molecules. This clearly establishes that the bromine moiety strongly bind to the C18 matrix when compared with the non-brominated molecule. Fourier-transform infrared spectroscopy (FTIR) spectrum confirmed the presence of brominated alkaloids in various pure fractions with a sharp peak in the 1,186 cm-1. HPLC of the highly active column fraction 26 displayed two sharp peaks at 18.5 minutes which was less intense compared with that at 20.0 minutes which was highly intense (Fig. 3).
.png) | Figure 2. (A–L) MALDI-TOF spectrum of different fractions of E. viride which shows the isotopic pattern of halogenated and non-halogenated compounds. [Click here to view] |
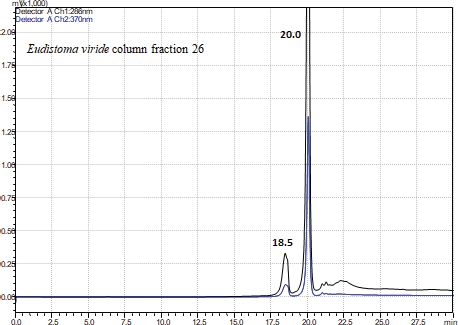 | Figure 3. HPLC spectrum of the column fraction 26 showing 2 peaks at 18.’ and 20.0’. [Click here to view] |
Both fractions were separately analyzed in MALDI-TOF and identified as compound Eudistomin I in fraction 18.5 and Eudistomin H in fraction 20.0. Eudistomin I and Eudistomin H showed wide spectrum of antibacterial activity against all pathogens. The zone of inhibition ranged from 3 to 7 mm for Eudistomin I and traced to 7 mm for Eudistomin H at 100 μg/disc. Anticancer activity of Eudistomin-H isolated from the same ascidian was found to be active at 0.49 μg/ml (Rajesh and Murugan, 2015). Collectively, these results point out that the development of Eudistomin H and its derivative Eudistomin I as antibacterial agents could be promising.
CONCLUSION
In this study, several Eudistomin groups of molecules were identified from organic solvent extract of E. virideusing MALDI-TOF mass spectrometry. Many other unexplored mass traces belonging to different unidentified molecules have been identified which possess bromine moiety. The crude extract and its purified fractions exhibited the presence of anti-microbial activity against several human pathogenic bacteria. The results suggested that Eudistomin-H and its non-brominated form Eudistomin-I were potentially active against a panel of pathogenic bacteria. This study opens new insights on this less explored marine sedentary ascidian E. viride which possess different halogenated molecules with novel bioactivity.
FUNDING
RPR—CSIR-SRA [13[8863-A]/2016] grant.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
PERMISSION FOR SAMPLING AND FIELD STUDIES
Not applicable as this is not a study which involve scheduled organism.
ACKNOWLEDGMENTS
RPR thanks CSIR-SRA [13[8863-A]/2016] grant. RPR thanks the Mass Spectrometry Facility, Molecular Biophysics Unit [MBU], Indian Institute of Science [IISc], Bangalore 560012. We are grateful to Prof. Siddhartha. P. Sarma, MBU, IISc for providing us with laboratory space, equipment, and valuable advice.
REFERENCES
Becerro MA, Lopez NI, Turon X, Uniz MJ. Antimicrobial activity and surface bacterial film in marine sponges. J Exp Mar Biol Ecol, 1994; 179:195–205. CrossRef
Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep, 2008; 25(1):35–94. CrossRef
Faulkner DJ. Marine natural products. Nat Prod Rep, 2002; 19:1–48. CrossRef
Jin H, Zhang P, Bijian K, Ren S, Wan S, Alaoui-Jamali MA, Jiang T. Total synthesis and biological activity of marine alkaloid Eudistomins Y1–Y7 and their analogues. Mar Drugs, 2013; 11, 1427–39. CrossRef
Kobayashi J, Harbour GC, Gilmore J, Rinehart Jr KL. Eudistomins A, D, G, H, I, J, M, N, O, P, and Q, bromo, hydroxy, pyrrolyl and iminoazepino. beta.-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J Am Chem Soc, 1984; 106(5):1526–8; doi: 10.1021/ja00317a080 CrossRef
Kolodina AA, Serdyuk OV. Eudistomin U, Isoeudistomin U, and related indole compounds: synthesis and biological activity. Heterocycles, 2018; 96(7):1171–96. CrossRef
Martinez-García M, Díaz-Valdés M, Ramos-Esplá A, Salvador N, Lopez P, Larriba E Antón J. Cytotoxicity of the ascidian cystodytes dellechiajei against tumor cells and study of the involvement of associated microbiota in the production of cytotoxic compounds. Drugs, 2007; 5:52–70.
Menzel LP, Lee JH, Sjostrand B and Lehrer RI. Immunolocalization of clavanins in Styelaclava hemocytes. Dev Comp Immunol, 2002; 26:505–15. CrossRef
Proksch P, Muller WEG. Frontiers in Marine Biotechnology. Horizon Bioscience, Proksch and Muller, Oxford University Press, UK, 2006.
Proksch P, Edrada RA, Ebel R. Drugs from the seas—current status and microbiological implications. Appl Microbiol Biotechnol, 2002; 59:125–34. CrossRef
Rajesh RP, Murugan A. Anticancer effects of brominated indole alkaloid Eudistomin H from marine ascidian Eudistoma viride against cervical cancer cells (HeLa). Anticancer Res, 2015; 35:283–376.
Ramasamy MS, Murugan A. Chemical defense in ascidians Eudistoma viride and Didemnum psammathodes in Tuticorin, southeast coast of India: bacterial epibiosis and fouling deterrent activity. Indian J Mar Sci, 2003; 32:337–9.
Renganathan TK. New record and redescription of a rare colonial ascidian, Eudistoma viride Tokioka 1955 from the Indian waters. Geobios New Reports, 1984; 3:49–51.
Rinehart KL. Antitumor compounds from tunicates. Med Res Rev, 2000; 20:1–27. CrossRef
Slattery M, McClintock JB, Heine JN. Chemical defenses in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol, 1995; 190:61–77. CrossRef