INTRODUCTION
The waste of physical, chemical, and biological origin, which create or could create a significant risk to the environment and human health, require special methods and means of recycling. They also include pharmaceutical waste, especially unusable medicines that mainly consist of biologically active synthetic compounds, and their analogs are absent in nature and cause their difficult natural safe disposal (Patil et al., 2015). As a result, the unsatisfactory situation and the unresolved issues of waste management in the pharmaceutical industry are constantly growing during recent decades. Thus, the equivalent amount of pharmaceuticals that is thrown away is approximately 364 tons a year (Lubick, 2010). Despite the existing of various social programs and events which have the main goal to gather expired drugs from people, pharmacies, and hospitals, it still hasn’t been developed the efficient utilization method of this type of production.
According to the results of interviews in Ukraine, 86% of respondents consider that the easiest way to get rid of waste is moving all of the useless medicines in the bin and it is unexpectedly that 16% of total amount of drugs is paracetamol of various brands and forms (Goldman et al., 2015; Hromovyk et al., 2012; Wu et al., 2012). Interestingly, that paracetamol-containing drugs production takes place in 26 countries under more than 247 various brands and dosage forms. As a consequence, the importance of recycling of paracetamol-containing drugs is also notified that these medicines are widely used in the treatment of various diseases and included in top 10 analgesics by recipes and the total net ingredient cost according to the National Statistics Prescriptions. On the other hand, the importance of utilization of paracetamol using simple chemical transformations leads to obtain attractive reagents such as 4-aminophenols and its derivatives like vital structural motifs for the synthesis of variety of biologically active compounds such as antitumor (Lozynskyi et al., 2014), antimicrobial (Mkpenie et al., 2016), antioxidant (Bhat and Belagali, 2016), cardiovascular agents (Mota et al., 2015), and steroid sulfatase (El-Gamal et al., 2016) and ribosomal protein kinase inhibitors (Zhou et al., 2016) (Fig. 1). Therefore, the purpose of this work is to analyze the possibility of utilization based on hydrolysis and alkylation reactions of expired and substandard paracetamol of various brands and forms as a source of 4-aminophenol and its derivatives as promising reagents for an organic synthesis.
MATERIALS AND METHODS
The expired and substandard drugs were obtained from pharmacies for further evaluation of their possible utilization. Thus, in current work, paracetamol of various brands and forms (Tylenol extra strength (I) (McNeill Consumer), paracetamol (II) (Stirolbiopharm, Ukraine), Rapidol (III) (“Balkanpharma-Dupnitza AD”, Bulgaria), Efferalgan (IV) (Bristol-Myers Squibb, France), and Daleron С (V) (КРКÐ, Slovenia) were chosen. All chemical reagents were purchased from commercial sources and used without purification. Mass spectra were obtained using electrospray ionization techniques on an Agilent 1100 Series liquid chromatography mass spectrometry (LCMS). The purity of all obtained compounds was checked by thin-layer chromatography (TLC).
General procedure for the acid hydrolysis of some paracetamol-containing drugs
A target paracetamol-containing drug (10 g) without prior grinding was refluxed for 15–35 minutes in 10–15 ml concentrated hydrochloric acid (36.5%–38.0%, Sigma-Aldrich), and then left overnight at room temperature. The precipitated crystals were filtered off, washed with methanol (5–10 ml) and dried at room temperature until constant weight.
General procedure for synthesis of N-[4-(benzyloxy)phenyl] acetamide derived from paracetamol-containing drugs
A mixture of appropriate not a shredded paracetamol-containing drug (10 mmol), benzylchloride (10 mmol) and potassium hydroxide (11.1 mmol) in 15 ml of ethanol was heated under reflux for 2 hours and then left overnight at room temperature. The precipitated crystals were filtered off, washed with methanol (5–10 ml). Recrystallization from ethanol rendered desired products in pure form.
RESULTS AND DISCUSSION
The total active substance content in the expired drugs determined by mass-spectrometry and expressed as N-(4-hydroxyphenyl)-acetamide (Fig. 2). The Figure 2 shows that the content of paracetamol in of the studied drug samples did not change significantly over a period of time according to the LCMS spectra, despite the fact that the use of these expired medicines is prohibited.
In our study, we adopted the method of chemical decomposition and transformation paracetamol of various brands and dosage forms providing to 4-aminophenol and its alkylated analog and established optimum reaction conditions (Scheme 1). The target compounds after the completion of the reactions were obtained as a crystalline state and the reaction medium was removed which contain the auxiliary substances of dosage forms and possible impurities. The obtaining of 4-aminophenol was carried out by acid hydrolysis from the corresponding expired and substandard paracetamol-containing drugs under reflux conditions. The end of hydrolysis of paracetamol was monitored by TLC and after completion of the reaction was obtained target product with various yields. The structure and purity of obtained 4-aminophenol were supported by LCMS spectra and the observed peaks with mass values (M+H)+ confirmed the formation of the product. It should be noted that according to the LCMS data in the crystalline form of 4-aminophenol was observed a small amount of impurity at m/z 157.1 (corresponding to N-(4-hydroxyphenyl)-acetamide) (Fig. 3). As a result, the qualitative content analysis of paracetamol-containing drugs of various brands and forms after acid hydrolysis reveals yields of 4-aminophenol hydrochloride in the range of 16%–87% (Table 1).
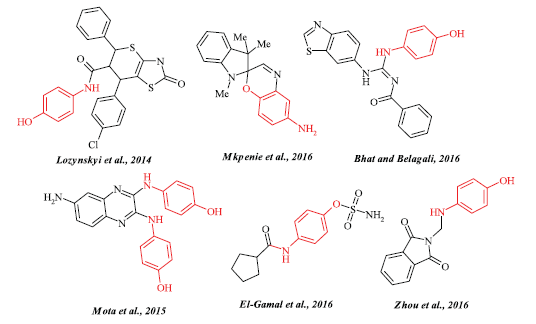 | Figure 1. Some biologically active aminophenol-containing derivatives described in the literature. [Click here to view] |
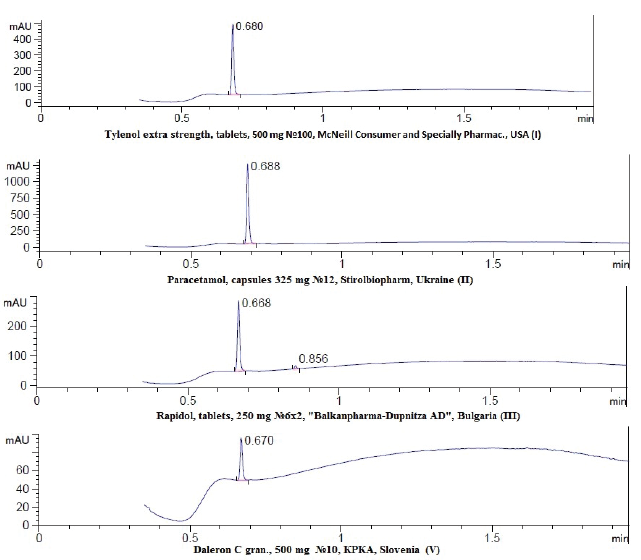 | Figure 2. Chromatogram corresponding to studied drug samples (I–III, V). [Click here to view] |
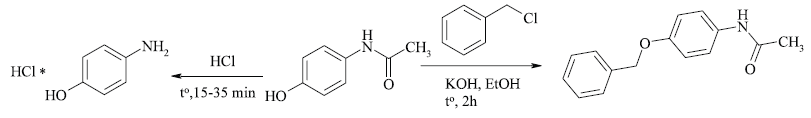 | Scheme 1. The synthesis of 4-aminophenol and N-[4-(benzyloxy)phenyl]acetamide. [Click here to view] |
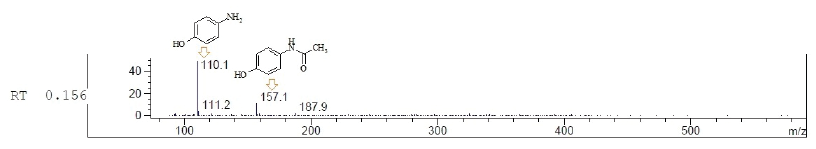 | Figure 3. LCMS spectra of paracetamol hydrolysate and structure of fragments. [Click here to view] |
To expand our investigation, we then studied the reaction of between paracetamol-containing drugs with alkylated reagent benzyl chloride in an ethanolic solution of potassium hydroxide under heating conditions, providing N-[4-(benzyloxy)phenyl] acetamide. The formation of a higher percent of target product was observed by using paracetamol in a capsular form unlike tablets, 58% and 50% yield, respectively (Table 2). Their identity was confirmed in the LCMS analysis which showed, for their peaks molecular ions at m/z 157.2, 159.2, 242.2, and 243.3 corresponding to N-(4-hydroxyphenyl)-acetamide as slight admixture and target N-[4-(benzyloxy)phenyl]acetamide (Fig. 4).
The method of chemical decomposition of drugs and structure-related compounds is a platform for synthesis of a variety of useful acids, aldehydes, alcohols, and amines, as well as fuels (Adsul et al., 2011). In addition, the chemicals which are obtained using this principle could be repeatedly utilized for library synthesis of various molecules according to target-oriented synthesis and diversity-oriented synthesis (DOS) approaches (Burke and Schreiber, 2004). Interestingly, the methods of utilization of paracetamol providing 4-aminophenols on the basis of both synthetic approaches lead to obtain some biologically relevant heterocyclic systems such as thiazole (Subtel’na et al., 2010), thiopyranothiazole (Lozynskyi et al., 2014), pyridine (Kamal et al., 2016), benzothiazole (Bhat and Belagali, 2016), indole (Mkpenie et al., 2016), and isoindole (Zhou et al., 2016). Thus, after the completion of hydrolysis based on above-mentioned synthetic methodologies were observed the variation of 4-aminophenol content in the acid hydrolysate and could be attributed to the effect of different auxiliary substances in dosage forms which prevent to complete hydrolysis of 4-acetamidophenol as confirmed by LCMS data. As a result, the obtained results show that the paracetamol in suppository form isn’t favorable for chemical decomposition providing to 4-aminophenol due to a high percent of impurities, especially in combining utilization with tablets and capsules.On the other hand, the usage alkylated analogs of 4-aminophenol, especially 4-amino(acetamido) phenol building blocks provides a unique opportunity to access of organic dyes and useful reagents for synthesis of drugs, photo and film materials etc. (Ravelli and Fagnoni, 2012). As a result, the elaboration of a method of alkylation reaction of paracetamol allowed to obtain useful reagent for organic an synthesis such N-[4-(benzyloxy)phenyl]acetamide with high efficiency in terms of minimization of synthetic steps together with maximization of complexity. Therefore, alkylation reaction of
paracetamol-containing drugs would suggest that the properties of adjuvants in capsule and tablet dosage forms slightly effect on the yield of a target product N-[4-(benzyloxy)phenyl]acetamide and allow their combining utilization under given reaction conditions.
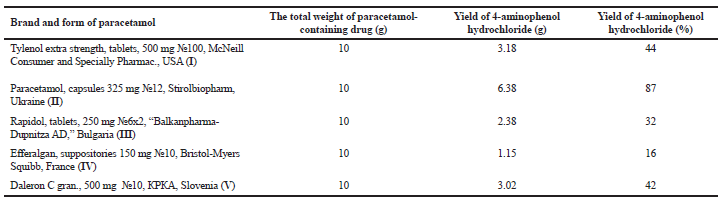 | Table 1. The comparative analysis of the content of 4-aminophenol hydrochloride in paracetamol-containing drugs after acid hydrolysis. [Click here to view] |
 | Table 2. The comparative analysis of the content of N-[4-(benzyloxy)phenyl]acetamide after alkylation paracetamol-containing drugs benzylchloride. [Click here to view] |
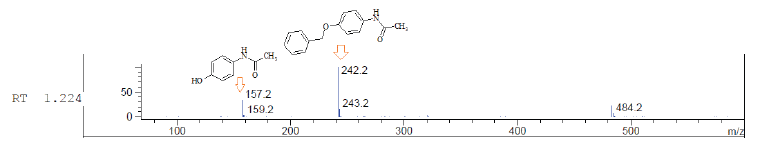 | Figure 4. LCMS spectra of paracetamol-containing drug alkylation reaction products and structure of fragments. [Click here to view] |
CONCLUSIONS
The outcome of the study indicated that the using simple chemical transformation such hydrolysis and alkylation is one of the effective methods of utilization of paracetamol-containing drugs. Thus, it has been reported that the convenient and efficient method of acid hydrolysis of paracetamol of various brands and forms providing 4-aminophenol with good yields. In addition, it was developed an efficient protocol for the synthesis of N-[4-(benzyloxy)phenyl]acetamide as commercially attractive reagent from capsule and tablet forms of paracetamol and benzyl chloride in an alkaline medium. As a result, the obtained data suggest the prospect of development of new eco-friendly methods of chemical decomposition of various drugs for obtaining different useful reagents for organic synthesis and industrial production.
REFERENCES
Adsul MG, Singhvi MS, Gaikaiwari SA, Gokhale DV. Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol, 2011; 102:4304–12. CrossRef
Bhat M, Belagali SL. Guanidinyl benzothiazole derivatives: synthesis and structure activity relationship studies of a novel series of potential antimicrobial and antioxidants. Res Chem Intermed, 2016; 42:6195–208. CrossRef
Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew Chem Int Ed, 2004; 43:46–58. CrossRef
El-Gamal MI, Semreen MH, Foster PA, Potter BV. Design, synthesis, and biological evaluation of new arylamide derivatives possessing sulfonate or sulfamate moieties as steroid sulfatase enzyme inhibitors. Bioorg Med Chem, 2016; 24:2762–7. CrossRef
Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA, 2007; 298:61–9. CrossRef
Hromovyk BP, Pankevych OB, Puzanova IP. Marketing research of home first aid kits. Pharm Rev (in Ukrainian), 2012; 2:86–9.
Kamal A, Ashraf M, Basha ST, Hussaini SA, Singh S, Vishnuvardhan MVPS, Kiran B, Sridhar B. Design, synthesis and antiproliferative activity of the new conjugates of E7010 and resveratrol as tubulin polymerization inhibitors. Org Biomol Chem, 2016; 14:1382–94. CrossRef
Lozynskyi A, Zimenkovsky B, Lesyk R. Synthesis and anticancer activity of new thiopyrano[2,3-d]thiazoles based on cinnamic acid amides. Sci Pharm, 2014; 82:723–34. CrossRef
Lubick N. Drugs in the environment: do pharmaceutical take-back programs make a difference? Environ Health Perspect, 2010; 118:A211–4. CrossRef
Mkpenie VN, Essien EE, Ita BN. Synthesis and biological activities of indolinospirobenzoxazine. J Chem Pharm Res, 2015; 7:787–9.
Mota F, Gane P, Hampden-Smith K, Allerston CK, Garthwaite J, Selwood DL. A new small molecule inhibitor of soluble guanylate cyclase. Bioorg Med Chem, 2015; 23:5303–10. CrossRef
Patil AD, Patil N, Patil R. Accentuating the role of pharmacovigilance and ecopharmacovigilance in context to man and ecology—a review. IJSR, 2015; 4:2048–56.
Ravelli D, Fagnoni M. Dyes as visible light photoredox organocatalysts. ChemCatChem, 2012; 4:169–71. CrossRef
Subtel’na I, Atamanyuk D, SzymaÅ„ska E, Kieć-Kononowicz K, Zimenkovsky, B, Vasylenko O, Gzella A, Lesyk R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg Med Chem, 2010; 18:5090–102. CrossRef
Wu S, Zhang L, Chen J. Paracetamol in the environment and its degradation by microorganisms. Appl Microbiol Biotechnol, 2012; 96:875–84. CrossRef
Zhou W, Li S, Lu W, Yuan J, Xu Y, Li H, Huang J, Zhao Z. Isoindole-1,3-dione derivatives as RSK2 inhibitors: synthesis, molecular docking simulation and SAR analysis. Med Chem Commun, 2016; 7:292–6. CrossRef