INTRODUCTION
Agastache foeniculum (Pursh) Kuntze [known as Lophanthus anisatus (Nutt.) Benth.] is a perennial herbaceous plant from Lamiaceae family. This plant can also be found under the common names, such as the fennel giant hyssop, anise hyssop, or “Mexican mint.” Anise hyssop is cultivated in Moldova, Romania, Ukraine, and Russia (Chumakova and Popova, 2013). This honey-bearing plant is used for the production of essential oil. The anise hyssop leaves are also used for infusions, food flavoring, and for different type low-alcohol drinks (Zhekova et al., 2010). In traditional medicine, Lophanthus anisatus finds applications for acute respiratory diseases, functional disorders of the gastrointestinal tract, and inflammatory diseases of the urinary system. Externally, the plant is used for dermatitis of fungal origin, seborrhea, used to strengthen and grow hair (Marcel et al., 2013).
Many studies have confirmed the antimicrobial and fungicidal activities, as well as the antioxidant effect of the anise hyssop (Ownagh et al., 2010). The anise hyssop gives aroma and flavor of a combination of aniseed and mint. Its leaves and flowers are traditionally used either raw or cooked for flavoring many salads, bread, and cooked dishes including pea and lamb (Ravindran, 2017). The major constituent of essential oil from A. foeniculum is methyl chavicol (estragol) (88%–95%) which imparts an anise-like flavor and it is usually used in the manufacture of perfumes, liqueurs, some foods, and beer (Mallavarapu et al., 2004; Ravindran, 2017; Zhekova et al., 2010). Based on the analysis of samples obtained from different geographical origins has been suggested the existence of five chemotypes of anise hyssop, 1—the typical estragol-containing one (aniseed-like aroma type), and four others (mint-like aroma type) with other substances as: 2—menthone (11%–60%), 3—menthone and pulegone (6%–8%), 4—methyleugenol, and 5—methyleugenol and limonene (3%–12%) (Chumakova and Popova, 2013; Shanayda and Shvydkiv, 2008; Zhekova et al., 2010; ZieliÅ„ska and Matkowski, 2014).
The aim of the present investigation was gas chromatography-mass spectrometry (GC-MS) analysis of the essential oil obtained from A. foeniculum (Pursh) Kuntze herb and determination of its antioxidant potential, antimicrobial, and acetylcholinesterase inhibitory abilities. The isolation and chemical identification compounds from waste plant material obtained after steam distillation (terpenoids, flavonoids, and phenolic acids) present the additional interest in this
research.
MATERIALS AND METHODS
Plant material and essential oil extraction
The aerial parts of anise hyssop were purchased from a local drugstore (Dicrassin Ltd., Batch number L02092019, Bulgaria). The plant material was finely ground to powder. The essential oils of dried anise hyssop samples (75 g) were isolated by steam distillation for 3 hours using a glass Clevenger-type apparatus. The extracted yellow-colored essential oils were dried over anhydrous Na2SO4 and were kept at a refrigerator (4°C) in sealed dark glass vials for further analysis. The obtained wastes, as well as the water extract from anise hyssop hydro-distillation, were used for further extraction and analysis.
Extraction procedures
The aqueous layer after steam distillation was extracted twice with petroleum ether. Then, the aqueous layer was extracted in triplicate with ethyl acetate. The combined ethyl acetate extracts were dried over anhydrous Na2SO4 and evaporated under vacuum at 45°C to dryness. The dried ethyl acetate fraction was dissolved in methanol and it was used for further analyses for phenolic acid by high-performance liquid chromatography with diode-array detection (HPLC-DAD) method. Dried anise hyssop wastes (25 g) were put in a round bottom flask of 250 ml and the plant material was extracted with 100 ml methanol under reflux and boiling for 1 hour. The obtained extract was filtered through filter paper and the residue was extracted again with the same volume of used solvent. Both extracts were combined and then evaporated to dryness. The dry extract was used for analyses of triterpenes by HPLC-DAD method.
GC-MS analysis of essential oil
GC-MS analysis was carried out on gas chromatograph Agilent Technology Hewlett Packard 7890 A, coupled with MS detector Agilent Technology 5975 C inert XL EI/CI MSD at 70 eV). Separation of the compounds was performed on a column HP-5ms (30 m × 0.25 mm × 0.25 μm) at temperature regime: from 40°C, held for 3 minutes, then rising at 5°C/minute to 300°C, held for 10 minutes. The injector temperature was set at 250°C and the flow rate of helium was 1.0 ml/minute and split 10:1 was used. The injection volume was 1 μl.The obtained MS spectra were analyzed by 2.64 AMDIS software (NIST, Gaithersburg, MD). Compounds listed in the order of elution from an HP-5ms column with retention indices (RI) determined experimentally by coinjection of C8–C36 alkanes (Ivanov et al., 2018). The identification of the compounds was determined by comparison of mass spectrometry and RI matching on Adams (2001; 2007).
HPLC-DAD analysis of phenolic compounds
Separations and quantitative determination of polyphenolic content were performed on an HPLC instrument Elite Chrome Hitachi, coupled with a diode-array detector (DAD), and ELITE LaChrom software. The separation was performed on a reverse-phase column Supelco, Discovery® HS C18 (5 μm, 25 cm × 4.6 mm) at 30°C, and at wavelength 280 and 320 nm. Elution of polyphenols was achieved with mobile phase A—2% acetic acid and mobile phase B—acetonitrile in gradient mode described before Ivanov et al. (2014) and Marchev et al. (2011) at the flow rate 0.8 ml/minute. The sample injection volume was 20 μl. For the preparation of the standard curves, the standard pentacyclic triterpenes (ursolic acid, oleanolic acid, betulin, and betulinic acid) purchased from SIGMA (Germany) were used. The identification of components in the sample was performed by comparing their DAD spectrum with the range of DAD spectrum obtained from standards. The yields were mathematically calculated and were represented as milligram per gram extract and milligram per 100 g dry weight (dw).
HPLC-DAD analysis of triterpenes
Separations and quantitative determination of pentacyclic triterpenes were performed on an HPLC instrument Elite Chrome Hitachi, coupled with a DAD and ELITE LaChrom software. The elution was performed on a reverse-phase column Supelco, Discovery® HS C18 (5 μm, 25 cm × 4.6 mm) at 26°C, with mobile phase methanol: formic acid (92:8 v/v) isocratic mode at the flow rate of 0.4 ml/minute. Detection was done at wavelength 210 nm. The sample injection volume was 20 μl (Marchev et al., 2012). For the preparation of the standard curves, the standards rosmarinic acid, myricetin, luteolin, and apigenin purchased from SIGMA (Germany) were used. The identification of components in the sample was performed by comparing their DAD spectrum with the range of DAD spectrum obtained from standards. The yields were mathematically calculated and were represented as milligram per gram extract and milligram per 100 g dw.
Antioxidant activity of essential oil (DPPH method)
Essential oil in different concentration (0.15 ml) was added to 2.85 ml solution of freshly prepared 0.1 mol 1,1-diphenyl-2-picrylhydrazyl radical (DPPH, Sigma) in methanol (Merck, Germany). The reaction was performed for 15 minutes at 37°C in darkness. The absorptions were measured at 517 nm against methanol. The IC50 value was calculated. The ability of the essential oil to scavenge DPPH radical was calculated as follows:
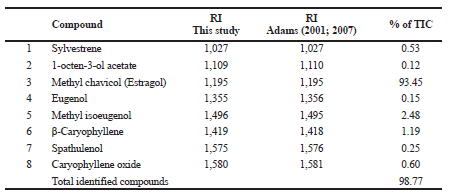 | Table 1. GC-MS analysis of anise hyssop essential oil. [Click here to view] |
where Acontrol was absorbance measure for the mixture of methanol and DPPH solution; and Asample represents absorbance of the different solution of A. foeniculum essential oil and DPPH solution.
Test microorganisms for antimicrobial activity
Eleven microorganisms including five Gram-positive bacteria (Staphylococcus aureus ATCC 25923, Enterococcus faecalis, Curtobacterium flaccumfaciens PM_YT, Listeria monocytogenes, and Bacillus subtilis ATCC 6633); Gram-negative bacteria (Salmonella sp., Escherichia coli ATCC 8739, Proteus vulgaris, Pseudomonas aeruginosa ATCC 9027, and Klebsiella pneumoniae) and yeasts (Candida albicans) from the collection of the Department of Microbiology at the University of Food Technologies, Plovdiv, Bulgaria, were selected for the antimicrobial screening.
Culture media (Luria–Bertani glucose agar)
Luria–Bertani glucose (LBG) medium was used for the cultivation of test microorganisms and determination of minimal inhibitory concentration (MIC). LBG-agar medium was prepared as follows: 10 g tryptone, 5 g yeast extract, 10 g NaCl, 10 g glucose, and 15 g agar in 1 l of distilled water. The pH was adjusted to 7.5 and the medium was sterilized by autoclaving for 20 minutes at 121°C.
Antimicrobial activity
The antimicrobial activity of essential oil from anise hyssop (A. foeniculum) was evaluated on LBG-agar medium by the agar well diffusion method (Tumbarski et al., 2017). The test microorganisms were cultured on LBG-agar medium at 37°C for 24 hours. The final concentration of the viable cells in the suspensions of sterile 0.5% NaCl for inoculation was adjusted to 1.0 × 108 cfu/ml. Then, the suspensions were inoculated in a preliminarily melted and tempered at 45°C–48°C LBG-agar media. The inoculated LBG-agar media were transferred in a volume of 16 ml in sterile Petri dishes (d = 9 cm). After hardening, six wells (d = 6 mm) per dish were cut. A volume of 50 μl samples were put in the agar wells at duplicated. Antibiotics Streptomycin (6 mg/ml), Nystatin (40 μg/ml), and methanol were used as controls. The inoculated Petri dishes were incubated at 37°C. The antimicrobial activity was determined by measuring the diameter of the inhibition zones (ZI) around the wells on the 24th and 48th hour of incubation.
Minimal inhibitory concentration
MIC of anise hyssop essential oil was determined by the conventional method—series of double-diluted samples, ranging from 10.0 to 0.079 μl/ml were prepared. The Petri dishes were incubated with 50 μl at the conditions shown above.
The MIC values were determined as the lowest concentration of the extract inhibiting completely the growth of each test microorganism around the agar well. The determined and recorded MIC values as microliter per milliliter were calculated (Tumbarski et al., 2017).
Acetylcholinesterase inhibitory assay
Acetylcholinesterase (AChE) inhibitory method was performed by using a colorimetric method described by López et al. (2002) with slight modification: 0.86 U AChE (type VI-S; Sigma) was dissolved in a volume of 1.0 ml 50 mmol phosphate buffer (pH 8.0), supplied with 0.15 mol NaCl and 0.05% (v/v) Tween 80 (Duchefa, The Netherlands). Prepared enzyme solution (20 μl) was added into 2.0 ml 50 mmol phosphate buffer (pH 8.0) and mixed with 20 μl of analyzed anise hyssop essential oil. The samples were incubated for 20 minutes at 4°C in darkness, then the reaction was started by adding 20 μl 6.0 mmol (in 50 mmol phosphate buffer with pH 7.0) acetylthiocholine iodide (Sigma) and 20 μl 5.0 mmol (50 mmol phosphate buffer with pH 7.0) 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, Sigma). Samples were vortexed and incubated at 37°C for 20 minutes in darkness. After the reaction time, the samples were cooled down in ice and 20 μl of 1.8 mmol (50 mmol phosphate buffer pH 7.0) Eserine salicylate (Sigma) was added to inactivate the enzyme. A blank sample with pure methanol instead of essential oil was prepared, as well. Positive control samples were developed for both experimental samples and blank sample, following the same procedure, but the enzyme was fully inhibited by adding 20 μl of 1.8 mmol eserine salicylate solution before starting the enzyme reaction. Changes in the absorption of samples against their positive controls were measured at 405 nm wavelength.
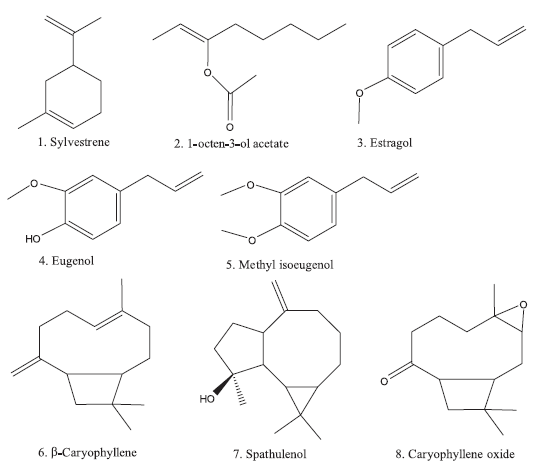 | Figure 1. Chemical structure of identified components in anise hyssop essential oil. [Click here to view] |
RESULT AND DISCUSSION
Chemical composition of anise hyssop essential oil
The GC-MS analysis of the content and composition of essential oil obtained from A. foeniculum (Pursh) Kuntze was presented (Table 1). Three groups of compounds were identified in the essential oil: monoterpenes (sylvestrene and 1-octen-3-ol acetate), phenylpropenes (methyl chavicol, eugenol, and methyl isoeugenol), and sesquiterpenes (β-caryophyllene, spathulenol, and caryophyllene oxide) (Fig. 1). The essential oil from anise hyssop was found to be transparent yellow liquid with low viscosity and was obtained in a yield of 0.20 g/100 g dw. The amount of obtained essential oil from A. foeniculum was in accordance with results obtained by Charles et al. (1991) (who reported the essential oil content from 0.07% to 2.45%). Eight compounds were detected in anise hyssop essential oils, as one of these compounds Sylvestrene (1) was not previously reported (Fig. 1; Table 1). Methyl chavicol (3) was the major oil component 93.45% of TIC. Other dominating oil constituents in A. foeniculum included methyl isoeugenol (5) (2.48%) and β-caryophyllene (6) (1.19%) (Table 1). Moreover, many researchers reported that the essential oil obtained from A. foeniculum contained mainly methyl chavicol (from 6% to 92%) regardless of variety, geographical and climatic conditions, and growth phases. α-Limonene, menthone, β-caryophyllene, and germacrene B were established as the plant major constituents (Charles et al., 1991; Myadelets et al., 2013; Nykanen et al., 1989, Shanayda and Shvydkiv, 2008). The anise hyssop has two main chemotypes, as one of them is with aniseed aroma (the methyl chavicol is the main component of the essential oil), and the other with mint aroma tone (mainly presented iso-mentone and pullegone) (Chumakova and Popova, 2013; Shanayda and Shvydkiv, 2008; Zhekova et al., 2010).
In contrast to other authors, the following components of α-limonene, menthone, and germacrene B were not identified in the investigated sample. In our case, the investigated essential oil from anise hyssop belonged to an “anethol” type, with aniseed aroma, because it contains methyl chavicol (3) as the main compound (93.45% of TIC) (Table 1). Mallavarapu et al. (2004) reported that eugenol (4) in the anise hyssop oil was found in higher concentration at the end of their vegetative stage than in oil obtained from plants at full bloom stage. Also, octenol acetate was presented only in the essential oil distilled at the end of the vegetative stage (Mallavarapu et al., 2004). Moreover, it is obvious that the essential oil of anise hyssop plant was comparable in quality to that reported previously. The results of the chemical composition of the anise hyssop essential oil show that the analyzed sample is at the end of the flowering period. Furthermore, the derivatives of estragol which is the major constituent in this oil are usually used in the natural food products as flavorings and perfumes.
Antioxidant activity of anise hyssop essential oil
The radical scavenging properties of anise hyssop essential oil in a concentration of 10 μl/ml inhibited 77.88% of DPPH radical (Fig. 2). IC50 of this essential oil was calculated to be 6.45 μl/ml. Hashemi et al. (2017) investigated the radicals scavenging activity of essential oil obtained from flowers of A. foeniculum. It was analyzed by DPPH assay the 92.1% of DPPH radical was inhibited in a concentration of 10 mg/ml (Hashemi et al., 2017).
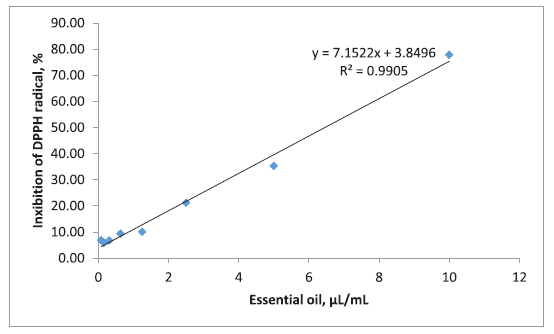 | Figure 2. Antioxidant activity (DPPH radical scavenging ability) of anise hyssop essential oil. [Click here to view] |
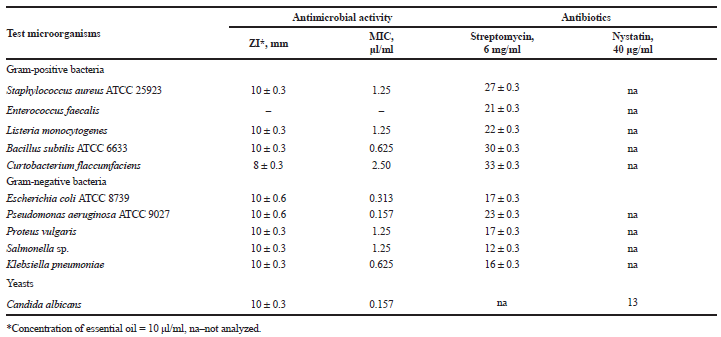 | Table 2. Antimicrobial activity of essential oil obtained from aerial parts of A. foeniculum. [Click here to view] |
Antimicrobial activity of anise hyssop essential oil
Antimicrobial activity is among the most frequently reported properties of essential oils from different Lamiaceae plants. The results from antimicrobial screening demonstrated that the essential oil of A. foeniculum in a concentration of 10 μl/ml possessed moderate inhibitory effect on Gram-positive bacteria S. aureus ATCC 25923, C. flaccumfaciens PM_YT, L. monocytogenes, B. subtilis ATCC 6633, Gram-negative Salmonella sp., E. coli ATCC 8739, P. vulgaris, P. aeruginosa ATCC 9027, K. pneumoniae and yeasts C. albicans, while Gram-positive bacterium E. faecalis remained unaffected. The inhibitory activity of the essential oil of A. foeniculum against the test microorganisms was lower compared to the antibiotics served as controls. Methanol used as solvent showed negative results
(Table 2).
The MIC values, summarized in Table 2, showed that the test microorganisms were sensitive to very low concentrations of the tested oil, ranging from 0.157 μl/ml for P. aeruginosa ATCC 9027 and C. albicans to 2.5 μl/ml for C. flaccumfaciens PM_YT, respectively. At present, there are inadequate data in the scientific literature for the antimicrobial activity of anise hyssop essential oil, although these plants are widespread and famous from the ancient times with their healing properties. Some authors reported that strength of antibacterial and antifungal activity of Agastache essential oil was rather to moderate in all the studied species. Antimicrobial and antifungal activities against S. aureus, L. monocytogenes, Bacillus cereus, B. subtilis, Salmonella typhimurium, Salmonella enteritidis, E. coli, Aspergillus niger, and Aspergillus flavus were investigated of essential oil from anise hyssop (Hashemi et al., 2017). However, essential oil from Agastache rugosa possessed antifungal activity (Shin, 2004; Shin and Kang, 2003). Our results for the antimicrobial potential of essential oil obtained from the aerial part of A. foeniculum enriched the application of this plant in cosmetic and food industries.
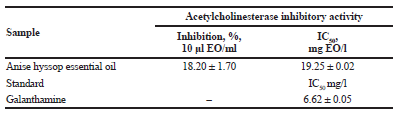 | Table 3. Acetylcholinesterase inhibitory activity of essential oil (EO) from A. foeniculum and positive standard galanthamine. [Click here to view] |
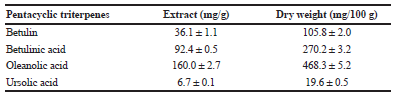 | Table 4. Pentacyclic triterpenes identified in methanol extract obtained from anise hyssop waste by HPLC-DAD. [Click here to view] |
Acetylcholinesterase inhibition of essential oil
The summary of acetylcholinesterase inhibition (AChEI) of essential oil and the standard galanthamine used in this study was given in Table 3. The AChEI of this essential oil was indicated primarily by its IC50. From the IC50 of A. foeniculum, essential oil showed an AChEI capacity similar to that of the reference inhibitor galantamine (Table 3). In the available scientific literature, no data are available for acetylcholinesterase inhibitory activity of A. foeniculum essential oil (IC50 19.25 mg/l). Similar results for acetylcholinesterase inhibitory activity of essential oil from different medicinal plants of Aframomum melegueta (IC50 16.0 mg/l), Crassocephalum crepidioides (IC50 12.1 mg/l), Monodora myristica (IC50 15.6 mg/l), and Ocimum gratissimum (IC50 6.54 mg/l) were found (Owokotomo et al., 2015). The acetylcholinesterase inhibitory activity is due to synergistic and antagonistic interactions between the components of essential oil. Major compound in anise hyssop essential oil was estragol (Table 1). This compound was reported to possess high AChE inhibition activities (IC50 0.337 μmol), followed by eugenol as well (IC50 40.32 μmol) (Farag et al., 2016).
Evaluation of wastes after steam distillation
After steam distillation of anise hyssop, aerial parts for production of essential oil, the waste and aqueous extract were used for analysis. Methanol as solvents and the extraction under reflux for the extraction and isolation of pentacyclic triterpenes was applied. The yield of extract was calculated as −2.9 ± 0.1 g/100 g dw. In the present study, for the first time, these compounds were analyzed and the individual triterpenic acids in anise hyssop waste were identified. Four terpenoids—betulin, betulinic, oleanolic, and ursolic acids—were found in anise hyssop as oleanolic and betulinic acids were in high concentrations: oleanolic acid—160.0 mg/g extract (468.3 mg/100 g dw) and betulinic acid—92.4 mg/g extract (270.2 mg/100 g dw), respectively (Table 4). That was of the great importance because of many valuable biological activities of these terpenoids (Jäger et al., 2009). Indeed, this class of compounds presents several biological activities, including anti-inflammatory (Pádua et al., 2014), antioxidant (Smina et al., 2011), anti-viral (Cichewicz and Kouzi, 2004), anti-diabetic (Alqahtani et al., 2013), anti-tumor (Laszczyk, 2009), hepatoprotective (Prasad et al., 2007), and cardio-protective (Shaik et al., 2012) activities. Due to the revealed pentacyclic triterpenes profile of anise hyssop waste, they showed the potential for application in cosmetic and food formulas.
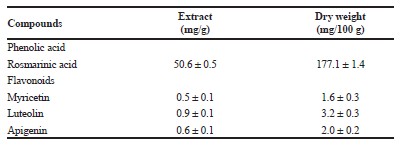 | Table 5. Phenolic compounds identified in the aqueous extract obtained from steam distillation of anise hyssop by HPLC-DAD. [Click here to view] |
From investigated water extracts, the highest yield of rosmarinic acid (50.6 mg/g extract) and the various number of flavonoids with high biological activity (myricetin—0.45 mg/g, luteolin —0.92 mg/g, and apigenin—0.57 mg/g,) was detected (Table 5).Therefore, anise hyssop waste after steam distillation could be used as a promising source of extracts (yield 3.5 ± 0.2 g/100 g dw) with high biological value for the application in pharmaceutical and cosmetic sectors. The similar result has been obtained from anise hyssop aerial parts cultivated in Egypt from Mostafa et al. (2018), they were identified coumarin, ferulic acid, rutin, and luteolin and apigenin.
CONCLUSION
The information for medicinal uses and phytochemical compounds in essential oil and waste plant material from A. foeniculum aerial parts were enriched. This is the first report of its kind to present acetylcholinesterase inhibitory activity of A. foeniculum essential oil (IC50 19.25 mg/l). Moreover, for the first time, the presence of betulin, betulinic acid, and oleanolic acid in anise hyssop waste and sylvestrene (1) in essential oil were detected and reported by us. The obtained essential oil from A. foeniculum possessed strong antioxidant and moderate antimicrobial and acetylcholinesterase inhibitory activity. The phytochemical compounds in different extracts could found possible application in the pharmaceutical industry and also for the natural cosmetic products.
REFERENCES
Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation, Carol Stream, IL, 2001.
Adams RP. Identification of essential oils components by gas chromatography mass spectroscopy. 4th edition, Allured Pubishing Corporation, Carol Stream, IL, 2007.
Alqahtani A, Hamid K, Kam A, Wong K, Abdelhak Z, Razmovski-Naumovski V, Chan K, Li K, Groundwater P, Li G. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem, 2013; 20:908–31.
Charles DJ, Simon JE, Glowacki C, Widrlechner MP. Characterization of essential oil of Agastache species. J Agric Food Chem, 1991; 39(11):1946–9. CrossRef
Chumakova VV, Popova OI. Lofant anisovy (Agastache foeniculum L.). A perspective source of obtaining drugs. Pharm Pharmacol, 2013; 1:39–43 (on Russian). CrossRef
Cichewicz R, Kouzi S. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med Res Rev, 2004; 24:90–114. CrossRef
Farag MA, Ezzat SM, Salama MM, Tadros MG, Serya RAT. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z Naturforsch C, 2016; 71(11–12):393–402. CrossRef
Hashemi M, Ehsani A, Hassani A, Afshari A, Aminzare M, Sahranavard T, Azimzadeh Z. Phytochemical, antibacterial, antifungal and antioxidant properties of Agastache foeniculum essential oil. JCHR, 2017; 7(2):95–104.
Ivanov I, Dincheva I, Badjakov I, Petkova N, Denev P, Pavlov A. GC-MS analysis of unpolar fraction from Ficus carica L. (fig) leaves. IFRJ, 2018; 25(1):282–86.
Ivanov I, Vrancheva R, Marchev A, Petkova N, Aneva I, Denev P, Georgiev V, Pavlov A. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol App Sci, 2014; 3(2):296–306.
Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants—rich sources for a new group of multi-potent plant extracts. Molecules, 2009; 14:2016–31. CrossRef
Laszczyk M. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med, 2009; 75:1549–60. CrossRef
López S, Bastida J, Viladomat F, Codina C. Acetylcholinesterase inhibitory activity of some alkaloids and Narcissus extracts. Life Sci, 2002; 71:2521–9. CrossRef
Mallavarapu GR, Kulkarni RN, Baskaran K, Ramesh S. The essential oil composition of anise hyssop grown in India. Flavour Fragr J, 2004; 19:351–3. CrossRef
Marcel DM, Vârban DI, Muntean S, Moldovan C, Olar M. Use of species Agastache foeniculum (Pursh) Kuntze. Hop Med Plant, 2013; 2:41–2.
Marchev A, Georgiev V, Ivanov I, Badjakov I, Pavlov A. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of Salvia tomentosa Mill. Biotechnol Lett, 2011; 33(9):1873–8.
Marchev A, Ivanov I, Georgiev V, Pavlov A. Determination of di- and triterpenes in Salvia tomentosa Mill. cell suspension culture by high performance liquid chromatography. Sci Works UFT, 2012; 59:229–33.
Mostafa EM, Abdelhady NM, El-Hela AA. Phytochemical and biological activity of Agastache foeniculum (Pursh) Kuntze cultivated in Egypt. JCBPSC, 2018; 8(2):434–43.
Tumbarski Y, Lincheva V, Petkova N, Nikolova R, Vrtancheva R, Ivanov I. Antimicrobial activity of extract from aeral parts of potentilla (Potentilla reptans L.). Ind Technol, 2017; 4(1):37–43.
Myadelets MA, Vorobyeva TA, Domrachev DV. Composition of the essential oils of some species belonging to genus Agastache Clayton ex Gronov (Lamiaceae) cultivated under the conditions of the Middle Ural. Chem Sust Devel, 2013; 21:397–401.
Nykanen I, Holm Y, Hiltunen R. Characterization of essential oil of Agastache foeniculum. Planta Med, 1989; 55:314–5. CrossRef
Ownagh AO, Hasani A, Mardani K, Ebrahimzadeh S. Antifungal effects of thyme, agastache and satureja essential oils on Aspergillus fumigatus, Aspergillus flavus and Fusarium solani. Vet Res Forum, 2010; 1(2):99–105.
Owokotomo IA, Ekundayo O, Abayomi TG, Chukwuka AV. In vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxic Rep, 2015; 2:850–7. CrossRef
Pádua T, de Abreu B, Costa T, Nakamura M, Valente L, Henriques M, Siani A, Rosas E. Anti-inflammatory effects of methyl ursolate obtained from a chemically derivedcrude extract of apple peels: potential use in rheumatoid arthritis. Arch Pharm Res, 2014; 37:1487–95. CrossRef
Prasad S, Kalra N, Shukla Y. Hepatoprotective effects of lupeol and mango pulp extract of carcinogen induced alteration in Swiss albino mice. Mol Nutr Food Res, 2007; 51:352–9. CrossRef
Ravindran PN. The encyclopedia of herbs and spices, 11. Anise Hysspo Agastache foeniculum. CAB International, UK, 2017.
Shaik A, Rasool S, Abdul Kareem M, Krushna G, Akhtar P, Devi K. Maslinic acid protects againstisoproterenol-induce cardiotoxicity in albino Wistar rats. J Med Food, 2012; 15:741–6. CrossRef
Shanayda MI, Shvydkiv OS. Comparative analysis of essential oils of two forms of Lophanthus anisatus Adans. Phytochem Res, 2008; 2:56–60 (On Ukraine).
Shin S. Essential oil compounds from Agastache rugosa as antifungal agent against Trichophyton species. Arch Pharm Res, 2004; 27:295–9. CrossRef
Shin S, Kang CA. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. J Appl Microbiol, 2003; 36:111–5. CrossRef
Smina T, Mathew J, Janardhanan K, Devasagavam T. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ Toxicol Pharmacol, 2011; 32:438–46. CrossRef
Zhekova G, Dzhurmanski A, Dobreva A. Gas-chromatography and organoleptic analysis of the essential oil of Agastache foeniculum (Pursh.) Kuntze. Agric Sci Technol, 2010; 2(2):102–4.
ZieliÅ„ska S, Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem Rev, 2014; 13:391–416.