INTRODUCTION
Passiflora edulis is a member of the Passifloraceae family with two varieties: Passiflora edulis f. edulis (purple fruit) and Passiflora edulis f. Flavicarpa (yellow fruit). This plant is native to Brazil, the biggest producer, but it is cultivated in other tropical, subtropical, and temperate regions in South America, South Africa, India, Malaysia, Australia, New Zealand, Hawaii, and Asia (Lim, 2012). The common name of Passiflora edulis f. Flavicarpa is passion fruit, but it is also known as maracuyá, parchita, and gulupa (Spanish). Its exotic smell and taste have made it a very desirable fruit, and also, it has been reported that it has a high nutritional value and medicinal properties (Zas and John, 2016). The ethnobotanical literature shows that P. edulis has been used in traditional medicine in different parts of the world (Dhawan et al., 2004).
Different phytochemical studies of this plant demonstrate the presence of chemical compounds such as glucose solids, phytochemicals compounds, ascorbic acid, ϒ-lactones, flavor components, volatile constituents, amino acids, carbohydrates, and minerals. In the flavonoids group, the anthocyanins, pelargonin, delphinidin, and cyanidin, stand out. Furthermore, the presence of fatty acids and volatile components such as hexenal, 2-tridecanone, 2-tridecanol, 2-pentadecanone, hexadecanoic and octadecanoic acid (among others), and cyanogenic compounds has been reported (Lim, 2012; Patel, 2009).
The antioxidant activity of extracts from different parts of this plant has been demonstrated (Aguillón et al., 2013; da Silva et al., 2013; Rudnicki et al., 2007). Specifically, the leaves extract has been found to have powerful antioxidant properties in vivo and in vitro (da Silva et al., 2013; Ferreres et al., 2007) due to its high content of phenolic compounds. For that reason, evaluating its anticancer activity has become of great interest. Aqueous extracts of P. edulis prevent the action of matrix-metalloprotease-2 (MMP-2) and MMP-9 enzymes; therefore, they inhibit tumor invasion and metastasis (Puricelli et al., 2003). Furthermore, these extracts have been reported to have cytotoxic potential against SW480 and SW620 colon cancer cell lines (Montoya et al., 2013). In addition, isolated carotenoids and polyphenols of this species, as well as extracts from the fruit and the leaves, have been evaluated in other cancer models, e.g., leukemic cell lines, where a cytotoxic and apoptosis-inducing effect was found (Neira, 2003). In a previous study, our group reported the cytotoxic activity of the ethanolic extract from passion fruit leaves against SW480 and Caco-2 colon cancer cell lines (Ramírez et al., 2017). In the present work, it was evaluated the effect on cell viability, proliferation, cell cycle distribution, and cell death patterns in SW480 and Caco-2 cells treated with an ethanolic extract from P. edulis leaves.
MATERIALS AND METHODS
Preparation of the ethanolic extract from passion fruit leaves
Fresh leaves of P. edulis F. Flavicarpa were collected in the Municipality of La Tebaida, Quindío, Colombia (4,4376°N, 75,8489°W, 1165 Meters above sea level). The specimens were identified at Universidad del Quindío Herbarium (Collection number: 33974). The leaves were desiccated in a circulating air oven at 40°C, pulverized into powder, and it was lixiviated and recirculated for 8 days in 500 ml of 96% ethanol. The chlorophyll was separated by liquid–liquid extraction with ethanol–water (7:1) according to Aguillon et al. (2013). The ethanol was evaporated under reduced pressure (60 mbar) at 40°C and the resulting solid was resuspended in a 20% solution of dimethylsulfoxide (DMSO) and filtered. The extract obtained was stored and protected from the light at 4°C until use.
HPLC-DAD (High-performance liquid chromatography integrated with diode array detection)
The compounds in the extract were identified by a Dionex UltiMate 3000 HPLC-DAD under the following conditions: C18 column (150 × 4.6 mm, 5 μm), mobile phase: solvent A (tetrahydrofuran: isopropanol: acetonitrile 10:2:3 v/v/v) and solvent B (H3PO4 0.5%) (12% A in B) at a wavelength of 340 nm and 1 ml/minute.
Cell lines and maintenance
SW480 and Caco-2 cell lines (American Type Culture Collection Manassas, USA) were maintained and propagated in Dulbecco’s Modified Eagle’s Medium (DMEM) medium (Gibco), supplemented with 25 mM of glucose, 2 mM of L-glutamine, 10% horse serum (Gibco), 100 UI/ml penicillin, 100 μg/ml streptomycin (Gibco), and 1% of nonessential amino acids (Gibco). The cultures were maintained at 37°C and 5% CO2. For the assays, the serum was reduced to 3% and supplemented with 10 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium (ITS medium).
Cell viability assay by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
A total of 5,000 cells/well were cultivated in a final volume of 100 μl of maintenance medium. The cells were established for 48 hours and subsequently treated with different extract concentrations (v/v %), corresponding to 5% (114.53 μg/ml), 5.5% (125.9 μg/ml), and 7% (160.3 μg/ml), according to Ramírez et al. (2017), in order to evaluate their effect at 24, 48, 72, and 96 hours after treatments. At each time point, 20 μl/well of MTT were added [5 mg/ml in Phosphate-buffered saline (PBS)] (Sigma) and cells were incubated at 37°C and 5% CO2 for 4 hours in darkness. Afterward, 100 μl of acidified isopropanol were added (Triton X-100 10%, HCl 0.8%) to each well, which was mixed by orbital shaking for 30 minutes at room temperature in darkness (Stockert et al., 2012). Finally, the absorbance (560 nm) of the samples was measured in the GloMax-Multi Detection SystemTM (Promega) and the viability percentage was estimated using the following equation, in order to determine the mean Lethal Dose (IC50) by linear regression. The percentage of viability = (ODt/ODc) × 100, where ODt is the optical density of treated cultures and ODc is the optical density of the negative control (untreated cells).
Evaluation of the antiproliferative effect
The cloning efficiency assay was used to evaluate the antiproliferative potential of the extracts (Franken et al., 2006). A total of 10,000 cells/well from each cell line were inoculated in a 2 ml volume of ITS medium and maintained for 48 hours before adding the extracts in proportions of 5%, 5.5%, and 7%. After 48 hours of treatment, the cells were detached and resuspended in 1 ml of DMEM medium, and subsequently, an aliquot of 200 cells was taken from each treatment, mixed with 2 ml of ITS medium, and cultivated for 7 days. At the end of the experiments, the cells were fixed with Carnoy’s solution (methanol-acetic acid) and stained with crystal violet (0.5% w/v) for 10 minutes. The number of colonies (50 cells or more) was quantified under an optical microscope and was compared with the number of inoculated cells and the number of colonies found in the negative control. The results are expressed as percentage of absolute cloning efficiency (ACE) and relative cloning efficiency (RCE). The latter was used to establish the antiproliferative effect of each treatment.
ACE = (Number of colonies in treated cells/number of inoculated cells) × 100 and RCE = (ACE treated cells/ACE untreated cells) × 100.
Evaluation of the effect on the cell cycle
The distribution of the cell cycle was analyzed by DNA staining with propidium iodide (PI) (Bertho et al., 2000). The cells were cultivated, treated, and collected as described in previous sections. 1 × 105 cells were fixed with 1ml of Methanol-PBS in a 9:1 (v/v) ratio and stored at −20°C for 2 hours. Afterward, cells were centrifuged at 2,500 rpm for 5 minutes, the supernatant was removed, and cell pellet was washed twice with PBS, to eliminate methanol excess. The cells were resuspended in 500 μl of PBS (250 μg/ml RNAse, 10 μg/ml PI) and incubated at room temperature for 30 minutes in darkness. Later, the distribution of the stages of the cell cycle was evaluated with the Fluorescence-activated Cell Sorting (FACS) Canto II flow cytometer and the data were analyzed with the programs FlowJo (version 6.0) and ModFit LT™ (version 4.0). Finally, the average at each stage of the cell cycle was calculated.
Cell death-inducing effect
The cell-inducing effect was evaluated by PI and Annexin V binding detection (Hingorani et al., 2011). A total of 3 × 105 cells/well were inoculated in ITS medium and incubated for 48 hours. Later, they were treated for 48 hours with 5%, 5.5%, and 7% v/v of the extracts. Cellular suspensions were obtained and mixed with 1 ml of Annexin V binding buffer (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.14 M NaCl, 2.5 mM CaCl2, and pH 7.4), 4 μl Annexin V-Fluorescein-5-isothiocyanate (Annexin-V-FLUOS staining kit, Sigma-Aldrich), 10 μl of PI (1 mg/ml) and then were incubated for 15 minutes at 4°C in darkness. The samples were evaluated with the FACSCanto II flow cytometer and analyzed by the FlowJo platform (version 6.0) to report the average values of positive events in each cell population: Viable cells (Annexin V-negative, PI-negative), early apoptotic cells (Annexin V-positive, PI-negative), late apoptotic cells (Annexin V-positive, PI-positive), and necrotic cells (Annexin V-negative, PI-positive).
Statistical analysis
A completely randomized design was used with “Treatment proportion” as the only factor. The statistical software GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA) was used to perform the one-way analysis of variance (ANOVA), Tukey’s test for multiple comparisons, and Dunnett’s test for comparison between treatments and the negative control. In all cases, the statistically significant differences were considered with p values < 0.05.
RESULTS
Identification of compounds by HPLC-DAD
When the compounds were evaluated by HPLC, two peaks that correspond to highly polar compounds were observed. The first compound showed absorption of 11.0 milli absorption units (mAU) and a retention time of 4.0 minutes (Fig. 1). The second compound showed a mAU of 21.0 and a retention time of 9.0 minutes (Fig. 2). Based on 3-D images, the absorbance spectra of the first and second compound were found to be 284 and 254 nm, respectively.
Possibly, the observed compounds are flavonoids, rutin, and quercetin, as described by (Gutiérrez et al., 2010). They observed the presence of compounds that corresponded to flavonoids in the maximum Ultraviolet radiation absorption spectra of 256 and 356 nm for rutin, and 256 and 369 nm for quercetin. In turn, Zucolotto et al., (2012) evidenced the presence of C-glycosyl flavonoids in leaf extracts from several species of Passiflora, which presented 11 mAU, 9.0 minutes of retention time, and an absorbance spectra of 256 and 271 nm. Likewise, the presence of other flavonoids, such as luteolin-6-C-chinovoside, has been evidenced in ethanolic extracts of Passiflora (Li et al., 2011). Therefore, our results and the chromatographic profiles indicate the presence of flavonoid-type compounds in the extracts.
 | Figure 1. HPLC plot of the first highly polar compound that was found in the ethanolic extract of P. edulis leaves. [Click here to view] |
 | Figure 2. HPLC plot of the second highly polar compound that was found in the ethanolic extract of P. edulis leaves. [Click here to view] |
Effect of the extracts on cell viability
The effect of the extracts on Caco-2 and SW480 cells viability was significant after 24 hours of treatment for concentrations higher than 5%. As shown in Table 1, the effect is stronger on Caco-2 cells at 24 hours of treatment; however, after 96 hours, SW480 cells displayed a greater reduction on cell viability than Caco-2 cells, independent of the extract concentration.
Likewise, the IC50 was estimated for the extract on both cell lines, after 48 hours of treatment. For SW480 cells, it was found an IC50 with a concentration of 16.5% (379 μg/ml) of the extract; on the other hand, for Caco-2 cells, the IC50 was calculated with a concentration of 21.3% of the extract (487 μg/ml) (Data not shown). It is important to note that these concentrations are higher than those evaluated in our experiments.
Evaluation of the antiproliferative effect of the extracts
SW480 and Caco-2 cells were exposed to the extract at 5%, 5.5%, and 7%, in order to evaluate its antiproliferative potential. As shown in Table 2, it was observed a significant reduction of ACE and RCE values in both cell lines, as the concentration of the extract was increased, being stronger the effect over Caco-2 cells; therefore, the antiproliferative effects were attributed to the P. edulis extract.
Effect of the extracts on the distribution of cell cycle phases
In both cell lines, alterations in the distribution patterns of cell cycle phases were observed, being the increase in SubG1 population (which indicates DNA fragmentation) and the decrease in G2/M population, the more remarkable observations. These variations were proportional to the concentration of the extract, since as the concentration was increased, greater was the effect of the treatments on both cell lines.
SW480 cells showed an increase in hypodiploid events of up to 57.7% compared to the negative control, whose average SubG1 population was 13.6% (Fig. 3). Besides, the effect of the extract on SW480 cells resulted in a reduction of the proportion of cells in the other phases (G0/G1, S, and G2/M), significant difference (p < 0.005).
On the other hand, Caco-2 cells treated with the same concentrations showed less variation in the distribution of the cell cycle compared with SW480 cells; however, they also exhibited an accumulation of events in the hypodiploid subpopulation of up to 11.8% compared to the negative control. Furthermore, the extract produced an increase in cell proportion in the S phase and a decrease in G2/M on Caco-2 cells (Fig. 4).
Cell death-inducing effect of the extracts
The results show the potential of the extract to induce necrosis and/or apoptosis in SW480 and Caco-2 cells. The effect on SW480 is shown in Table 3 and Figure 5, where a displacement toward the Q3 quadrant that corresponds to necrotic cells was observed. When the average values of each event were compared, the extract was found to have produced a reduction in the percentage of viable cells and an insignificant increase (when compared to the negative control) in early apoptotic (p = 0.8228) and late apoptotic cell subpopulations (p = 0.6038). The negative control presented an average proportion of necrotic cells of 9.6% and the treatments induced a statistically significant increase in the average values of necrotic cells to 13.6%, 14.6%, and 30.2%, for extract of 5%, 5.5%, and 7% (p = 0.0018).
 | Table 1. Effect of the ethanolic extract of P. edulis leaves on cell viability. [Click here to view] |
 | Table 2. Effect of the treatment with ethanolic extracts of P. edulis leaves on cloning efficiency. [Click here to view] |
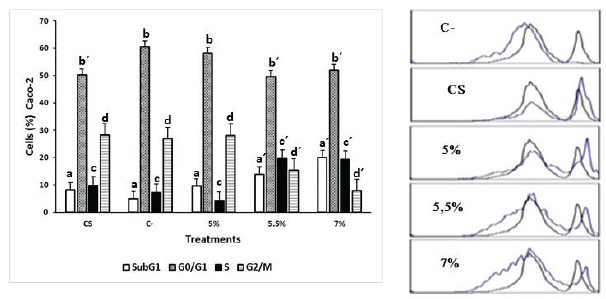 | Figure 3. Effect of the ethanolic extract of P. edulis leaves on SW480 cells treated for 48 hours. C- (untreated cells), CS: (cells exposed to DMSO 0.2% for 48 hours). The results are presented as frequency histograms in comparison to the distribution of untreated cultures and as average percentages of events found for each phase of the cell cycle. Significant difference (p < 0.005) a ≠ a´ b ≠ b´ c ≠ c´ d ≠ d´. [Click here to view] |
 | Figure 4. Effect of the ethanolic extract of P. edulis leaves on Caco-2 cells treated for 48 hours. C- (untreated cells), CS: (cells exposed to DMSO 0.2% for 48 hours). The results are presented as frequency histograms in comparison to the distribution of untreated cultures and as average percentages of events found for each phase of the cell cycle. Significant difference (p < 0.005) a ≠ a´ b ≠ b´ c ≠ c´ d ≠ d´. [Click here to view] |
 | Table 3. Cell death-inducing effect of the extracts. [Click here to view] |
 | Figure 5. Scatter plot of the death-inducing effect of the ethanolic extract of passion fruit leaves on SW480 cells after 48 hours of treatment. C- (untreated cells). [Click here to view] |
 | Figure 6. Scatter plot of the death-inducing effect of the ethanolic extract of passion fruit leaves on Caco-2 cells after 48 hours of treatment. C- (untreated cells). [Click here to view] |
Likewise, the effect of the extracts on Caco-2 cells (Table 3 and Fig. 6) induced a statistically significant increase in the average of necrotic cells to 10.8%, 11.7%, and 19.9%, for extract of 5%, 5.5%, and 7% (p = 0.0003). Furthermore, it was observed a reduction in the percentage of viable cells and a slight increase in the early (p = 0.579) and late apoptotic subpopulations (p = 0.495).
DISCUSSION
The interest in establishing the possible anticancer properties of P. edulis is growing due to a number of studies that describe the different biological activities that have been identified in species of genus Passiflora L. (Correa et al., 2016; Patel et al., 2011). Nevertheless, there is little available information on their anticancer potential, which could be mediated by a cytotoxic, antiproliferative, and/or death-inducing mechanism. Some of these effects have been established in species of the genus Passiflora L. On the one hand, the extract of P. foetida at a dose of 92 mg/kg presented antitumor outcomes and a cell lethality over 50%, when its effects were evaluated in an in vivo model of Walker carcinoma (Pessoa et al., 2006); on the other hand, the ethanolic extract of P. ligularis showed relevant antiproliferative activity over human hepatocellular carcinoma cells (IC50 50 mg/ml), but no cytotoxic effect was observed on primary cultures of hepatocytes (Pessoa et al., 2016). Likewise, the antitumor potential of species in the genus Passiflora L. has been described in an in vitro model of Ehrlich carcinoma, with an IC50 of 800 μg/ml (Sujana et al., 2012).
In the present work, it was evaluated the biological activity of the ethanolic extract of P. edulis f. Flavicarpa leaves on Caco-2 and SW480 colonic adenocarcinoma cells lines. The results revealed that the extract has an effect on cell viability and proliferation, as well as the activation of cell death-related mechanisms. These effects might be related to the presence of some metabolites that have been described in extracts of P. edulis (Dhawan et al., 2004), like terpenes and carbohydrates, which has been found both in aqueous extracts of the fruit and in ethanolic extracts of the leaves; likewise, glycosides such as “Passiflorine” and “Passicapsin,” alkaloids, carotenoids, anthocyanins, ascorbic acid, and lactones, which have been found in different parts of this plant, could be involved in the biological effects observed in the present work (Ingale and Hivrale, 2010). Aguillón et al. (2013) identified the presence of tannins, flavonoids, quinones, glycosides, carotenoids, and carbohydrates in aqueous and ethanolic leaf extract of P. edulis. Other authors have reported other metabolites of interest in this plant, such as vitamin A, vitamin B6, thiamine, riboflavin, flavonoids (Lin et al., 2016), C-glycosyl flavones like isoorientin, and γ-aminobutyric acid (Zeraik et al., 2012), among others.
The biological assays revealed that the employed extract presents activity over Caco-2 and SW480 cells. The observed cytotoxic effect was dose-dependent, which enabled to establish IC50 values after 48 hours of treatment (379 μg/ml for SW480 and 487 μg/ml for Caco-2). Although the IC50 values of the ethanolic extract obtained with the MTT assay exceed the 100 μg/ml proposed by the National Cancer Institute of the United States (WHO, 2000), the viability reduction could be considered as important, taking into account that they are extracts and not pure compounds. In addition, it was established that the effect depended on the dose and the exposure time.
The result of the cloning efficiency assay enabled to determine that the exposure to a concentration of 132 μg/ml (5%) for 48 hours is enough to reduce the clonal proliferation capacity of SW480 and Caco-2 cell lines, even 7 days after treatment. However, it cannot be argued that the effect is maintained after this period of time because some viable cells might recover their replicative potential and establish colonies again.
When the effect on the cell cycle was evaluated, alterations in the distribution of the cell cycle phases were found, like an increase in SubG1 population (indicating DNA fragmentation) and a decrease in G2/M population. SubG1 accumulation may be related to apoptotic events indicating specific and prelytic DNA fragmentation or, less likely, to genetic instability events that produce hypoploid subclones (Bertho et al., 2000). Therefore, further testing is necessary to better understand the accumulation process resulting from the exposure to the ethanolic extract of P. edulis leaves. For this reason, we evaluated the cell-death-inducing effect of the extracts by flow cytometry; in this manner, it was found that treatments induce cell death mainly by necrosis, being more sensitive SW480 cells than Caco-2 cells; however, a dose-dependent response was observed in both cell lines. These observations could be explained by the fact that one of the antitumoral mechanisms that have been proposed for natural compounds is their capacity to induced oxidative stress by accumulation of ROS and the regulation of NRF2 (Schieber and Chandel, 2014; Stepanic et al., 2015). This is critical, because other events such as lipid peroxidation or protein oxidation may thus be triggered and affect key processes involved in cell survival (Aldini et al., 2011). Besides, it is important to note that due to the oxidative state, DNA oxidation may increase and produce random DNA fragmentation. A critical DNA damage and poor repair capacity might lead to an increase in the number of necrosis events (Borges et al., 2008), which might explain our results. As a whole, these results suggest that the ethanolic extract of P. edulis produces a cell death-inducing effect, which agrees with previous reports that demonstrated the same effect of polar, carotenoid and polyphenol extracts from P. edulis, on other carcinogenesis models, including leukemic cell lines MOLT-4, HL-60, CEM, K562 (Neira, 2003), and liver cancer cell lines, like HepG2 (Aguillón et al., 2018).
The differences in the biological effect of P. edulis extract on the two cell lines should be considered to be an intrinsic variation linked to the origin of each these cells. In these regard, it is important to note that Caco-2 cells express high levels of phase II enzymes such as UDP-glucuronosyl-transferases (UGTs) (Galijatovic et al., 1999; Lakshmana and Sankar, 2009; Liu and Vincent, 2015; Nakamura et al., 2008) which has been involved in the glucuronidation of free hydroxyl groups present in the chemical structures of different compounds, like the flavonoids that we observed in our extracts, a process that aims to increase the polarity and water solubility of these compounds for their subsequent excretion (Wu et al., 2011; Zhang et al., 2007). This is very important since the high expression of UGTs in Caco-2 cells could explain its higher resistance to flavonoid-type compounds, when compared with other in vitro colon cancer models, as previously reported (González et al., 2014; Jiang and Hu, 2012; Ramos et al., 2011; Wang et al., 2000). This fact highlights the need to evaluate extracts on the greatest possible number of cell lines because the cellular response is mediated by the heterogeneity of the origin of each cell line, the genomic instability, and the diversity of available molecular phenotypes (Ahmed et al., 2013). In order to reduce this variability, we suggest evaluating the same extract and comparing the responses of other colon cancer cell lines such as HT-29 and HCT-116, among others.
CONCLUSION
The findings of this study enabled to establish the biological potential of the ethanolic extract of P. edulis leaves on SW480 and Caco-2 cell lines. Under the evaluated conditions, the extract presented cytotoxic and antiproliferative effects, as well as the potential to induce cell death on both cell lines. In this manner, the results of this research contribute to the acknowledgment of P. edulis as a potential species in the search for compounds with biological activity for biotechnological use. In addition, they provide a starting point for the fractionation and isolation of different compounds derived from this species that may turn out to be promissory for chemoprevention or the therapeutic treatment of colorectal cancer.
ACKNOWLEDGMENTS
This work was supported by grant P13135 from Instituto Tecnológico Metropolitano and the participation of researchers from Universidad de Antioquia and Universidad del Quindío.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
Aguillón J, Maldonado M, Loango N, Arango S, Landázuri P. Antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits juice Passiflora edulis. Perspect Nutr Humana, 2013; 15(1):13–25.
Aguillón J, Arango S, Uribe D, Loango N. Citotoxyc and apoptotic activity of extracts from leaves and juice of Passiflora edulis. J Liver Res Disord Ther, 2018; 4(2):70–4. CrossRef
Ahmed D, Eide P, Eilertsen I, Danielsen S, Eknæs M, Hektoen M, Lothe R. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis, 2013; 2:e71, 1–8.
Aldini G, Kyung-Jin Y, Etsuo N, Robert M. In: Biomarkers for antioxidant defense and oxidative damage : principles and practical applications. John Wiley Sons Inc., Florida, pp 35–50, 2011.
Bertho L, Santiago M, Coutinho SG. Flow cytometry in the study of cell death. Memórias Do Instituto Oswaldo Cruz, 2000; 95(3):429–33. CrossRef
Borges H, Linden R, Wang J. DNA damage-induced cell death. Cell Res, 2008; 18(1):17–26. CrossRef
Correa R, Peralta R, Haminiuk G, Bracht A, Ferreira I. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci Technol, 2016; 58:79–95. CrossRef
da Silva J, Cazarin C, Colomeu T, Batista Â, Meletti L, Paschoal J, de Lima Z. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: in vitro and in vivo study. Food Res Int, 2013; 53(2):882–90. CrossRef
Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. J Ethnopharmacol, 2004; 94:1–23. CrossRef
Ferreres F, Sousa C, Valentão P, Andrade P, Seabra R, Gil Á. New C-deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agr Food Chem, 2007; 55(25):10187. CrossRef
Franken P, Rodermond H, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc, 2006; 1(5):2315–9. CrossRef
Galijatovic A, Otake Y, Walle U, Walle T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica, 1999; 29(12):1241–56.
González A, Giménez J, Núñez M, Larrosa M, García, M, Tomás F, Espín C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr, 2014; 53(3):853–64.
Gutiérrez Y, Martínez M, Henriques A, del Barrio G. Análisis de flavonoides en una fracción butanólica obtenida de phyllanthus orbicularis HBK flavonoids analysis of a butanol fraction obtained from phyllanthus orbicularis HBK. Rev Cubana Farmacia, 2010; 44(3):367–73.
Hingorani R, Deng J, Elia J, McIntyre C, Mittar D. Detection of apoptosis using the BD annexin V FITC assay on the BD FACSVerseTM system. BD Biosci [Online]. Available via https://www.bdbiosciences.com/documents/BD_FACSVerse_Apoptosis_Detection_AppNote.pdf (Accessed 24 May 2017).
Ingale G, Hivrale U. Pharmacological studies of Passiflora sp. and their bioactive compounds. Plant Sci, 2010; 4(10):417–26.
Jiang W, Hu M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv, 2012; 2(21):7948–63.
Lakshmana R, Sankar G. Caco-2 cells: an overview. JPRHC, 2010; 1(2):260–75.
Li H, Zhou P, Yang Q, Shen Y, Deng J, Li L, Zhao D. Comparative studies on anxiolytic activities and flavonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa‘. J Ethnopharmacol, 2011; 133:1085–90. CrossRef
Lim T. Passiflora edulis. In: Edible med nonmed plants. Ed. Springer Sciences, Nueva York, USA, pp 147–65, 2012. CrossRef
Lin C, Kao C, Huang S, Li C, Chen C, Li H, Chen Y. Chemical constituents of fruit chells Passiflora edulis. Chem Nat Comp, 2016; 52(2):273–4. CrossRef
Liu X, Vincent H. Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact caco-2 TC7 cells using siRNA Xing. Mol Pharm, 2015; 4(6):15–77.
Montoya Y, Orozco P, Arango S, Maldonado M, Aguillón J. Evaluation of cytotoxic activity of the aqueous extract of passion fruit (Passiflora edulis) on groups of cell lines. In Pan American Health care Excahges (PAHCE), Ed. IEEE, Medellin, Colombia, pp 6–10, 2013.
Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Disposition, 2008; 36(8):1461–4.
Neira C. The effects of yellow passion fruit, Passiflora edulis Flavicarpa, phytochemicals on cell cycle arrest and apoptosis of leukemia lymphoma MOLT-4 cell line. University of Florida, Gainesville, FL, 2003.
Patel S. Morphology and pharmacology of Passiflora edulis: a review. J Herb Med Toxicol, 2009; 3(1):1–6.
Patel S, Soni H, Mishra K, Singhai A. Recent updates on the genus passiflora: a review. Int J Res Phytochem Pharmacol, 2011; 1:1–16.
Pessoa C, Veras L, Leyva A, Amaral E, Odorico M. Anticancer potential of Northeast Brazilian plants. In: MTHK Athers (ed.). Lead Molecules from Natural Productus. Nueva York, USA, pp 197–211, 2006. CrossRef
Puricelli L, Dell’Aica I, Sartor L, Garbisa S, Caniato R. Preliminary evaluation of inhibition of matrix-metalloprotease MMP-2 and MMP-9 by Passiflora edulis and P. foetida aqueous extracts. Fitoterapia, 2003; 74(3):302–4. CrossRef
Ramírez V, Arango S, Uribe D, Maldonado M, Aguillón J. Effect of the ethanolic extract of Passiflora edulis F. Flavicarpa leaves on viability, cytotoxicity and apoptosis of colon cancer cell lines. J Chem Pharm Res, 2017; 9(6):135–9.
Ramos S, Rodríguez I, Martín M, Goya L, Bravo L. Dietary flavanols exert different effects on antioxidant defenses and apoptosis/proliferation in Caco-2 and SW480 colon cancer cells. Toxicol Vitro, 2011; 25(8):1771–81.
Rudnicki M, de Oliveira M, Veiga T, Reginatto F, Dal-Pizzol, F, Fonseca J. Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem, 2007; 100(2):719–24. CrossRef
Schieber M, Chandel N. ROS function in redox signaling and oxidative stress. Curr Biol, 2014; 24(10):1–25. CrossRef
Stepanic V, Gasparovic AC, Troselj KG, Amic D, Zarkovic N. Selected attributes of polyphenols in targeting oxidative stress in cancer. Curr Top Med Chem, 2015; 15(5):496–509.
Stockert J, Blázquez A, Cañete M, Horobin R, Villanueva A. MTT assay for cell viability: intracellular localization of the formazan product is in lipid droplets. Acta Histochem, 2012; 114(8):785–96. CrossRef
Sujana N, Ramanathan S, Vimala V, Sundaram M, Pemaiah B. Antitumour potential of passiflora incarnate against ehrlich ascites carcinoma. Int J Pharm Pharm Sci, 2012; 4(2):17–20.
WHO. General guidelines for methodologies on research and evaluation of traditional medicine World Health Organization. 2000 [Online]. Available via http://whqlibdoc.who.int/hq/2000/WHO_EDM_TRM_2000.1.pdf (Accessed 2 June 2017).
Wang W, Heideman L, Chung C, Pelling J, Koehler K, Birt D. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinogen, 2000; 28:102–10.
Wu B, Kulkarni K, Basu S, Zhang S, Hu M. First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. J Pharm Sci, 2011; 100(9):3655–81.
Zas P, John S. Diabetes and medicinal benefits of Passiflora edulis. Int J Food Sci Nutr Diet, 2016; 5(2):265–9.
Zeraik M, Yariwake J, Wauters J, Tits M, Angenot L. Passion fruit rinds (Passiflora edulis): isoorientin quantification by HPLC and evaluation of antioxidant (radical scavenging) capacity. Quim Nova, 2012; 35(3):541–5. CrossRef
Zhang L, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm, 2007; 4(6), 833–45.
Zucolotto S, Fagundes C, Reginatto H, Ramos F, Castellanos, Paulo E. Analysis of C -glycosyl flavonoids from South American Passiflora species by HPLC-DAD. Phytochem Anal, 2012; 23:232–9. CrossRef