INTRODUCTION
The medical use of 2-thiopyrimide derivatives began many decades ago. Thiobarbiturates exhibit psychotropic activity and are used as medicines for anesthesia (Russo and Bressolle, 1998). Propylthiouracil is used as an antithyroid drug for the treatment of hyperthyroidism (Azizi et al., 2017; Cooper, 2005). Recently, the range of pharmacological activity of 2-thiopyrimidines was significantly expanded. Thus, among them were found the reverse transcriptase inhibitors of human immunodeficiency virus type 1 (HIV-1) of non-nucleoside structure, and the effective concentration of which in vitro was in the nanomolar range (Mai et al., 2001; Nawrozkij et al., 2008). The inhibitors of herpes simplex virus (HSV) of type 1 and 2 (Shigeta et al., 2003), antagonists of P2Y12 receptors that possess an antiplatelet activity (Crepaldi et al., 2009), anticancer (Gorneva et al., 2005) and antibacterial agents (Basavaraja et al., 2010) which are 2-thiopyrimidine derivatives were also created.
High psychotropic activity of pyrimidines has attracted the interest of scientists, whose focus is on developing new pyrimidine containing drugs that affect the central nervous system. A successful strategy for the synthesis of new antiepileptic drugs was the introduction of the exocyclic sulfur atoms into molecules of compounds (Levin et al., 1986; Matias et al., 2017).
The positive experience of our own research concerning the synthesis of new anticonvulsants in the series of pyrimidin-4(3H)-one and 4-thione derivatives (Severina et al., 2013; 2015) and determination of the thioacetamide fragment (Saidov et al., 2014) effect on increase of antiepileptic activity prompted us to search for the new highly potent anticonvulsants among thioalkyl pyrimidine derivatives.
Therefore, this research aimed at synthesizing new potential anticonvulsants—S-alkylated derivatives of 6-methyl-2-thioxo-2,3 ethyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one.
MATERIALS AND METHODS
Chemistry
All of the solvents and reagents were obtained from the commercial sources. The progress of the reactions was monitored by thin layer chromatography (TLC) using aluminum silica gel plates. The melting points (°C) were determined in a capillary using an electrothermal IA9100X1 (Bibby Scientific Limited, Staffordshire, UK) digital melting point apparatus. 1H Nuclear magnetic resonance (NMR) spectra were recorded on a Varian Mercury-400 (Varian Inc., Palo Alto, CA) spectrometer (300 MHz) in hexadeuterodimethyl sulfoxide (DMSO-d6) using tetramethylsilane (TMS) as an internal standard (chemical shifts are in ppm). 13C NMR spectra were recorded at Bruker Avance 400 (100.6 MHz). Chemical shifts were reported in ppm downfield from TMS as internal standards. The elemental analysis was performed on a Euro Vector EA-3000 (Eurovector SPA, Redavalle, Italy) microanalyzer. Elemental analyses were within ±0.4% of the theoretical values. LCMS was recorded with PE SCIEX API 150EX chromatograph.
6-Methyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one 3 was obtained according to the method reported in the literature (Novikov et al., 2005).
General procedure of the synthesis of S-alkylated derivatives of 6-Methyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one, 5.1-15
A mixture of 7, 34 mmol of 2-thiouracil (3) and 10, 85 mmol of potassium carbonate in 10 ml of Dimethylformamide (DMF) was stirred at 70°C–80°C for 1 hour, and the reaction mixture was cooled to room temperature; a solution of 7, 34 mmol of appropriate chloroacetanilide (4) in 10 ml of DMF was added, stirred for 5 hours, and left for 12 hours afterward. The reaction mixture was filtered and the filtrate evaporated in a vacuum; the residue was treated with 100 ml of cold water. The formed precipitate was filtered, air dried, and recrystallized from an acetone-DMF mixture.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-phenylacetamide, 5.1
Yield: 82%, melting point (mp) 241°C –3°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.52 (1H, br. s, NH-3), 10.16 (1H, s, NHCO), 7.59–7.27 (4H, m, H-2′,3′,5′,6′), 7.05 (1H, t, J = 7.5, H-4′), 5.99 (1H, s, CH-5), 4.07 (2H, s, SCH2), 2.13 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 169.82 (C=O, amide), 166.39 (6-C=O), 165.22, 164.12 (C-S), 139.3, 129.14 (2C), 123.72, 119.54 (2C), 107.66 (5-CH, br), 35.61 (CH2), 23.84 (CH3). Found, m/z: 276.32 [M+H]+. The Anal. Calcd. was for C13H13N3O2S: C, 56.71; N, 15.26; S, 11.65%; we found: C, 56.79; N, 15.31; and S, 11.68%.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-methylphenyl)acetamide, 5.2
Yield: 79%, mp 253°C –5°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.52 (1H, br. s, NH-3), 10.16 (1H, s, NHCO), 7.45 (2H, d, J = 8.4, H-2′,6′), 7.10 (2H, d, J = 8.4, H-3′,5′), 5.99 (1H, s, CH-5), 4.05 (2H, s, SCH2), 2.24 (3H, s, CH3), 2.13 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 169.78 (C=O, amide), 167.38 (6-C=O), 166.46 (C-S), 165.33, 143.36, 136.82, 132.61, 129.48, 119.67 (2C, Ar), 107.13 (5-CH, br), 35.45 (CH2), 23.75 (CH3), 20.77 (CH3). Found, m/z: 290.09 [M+H]+. The Anal. Calcd. was for C14H15N3O2S: C, 58.11; N,14.52; S, 11,08; we found: C, 58.01; N, 14.57; and S, 11.05.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(3-methoxyphenyl)acetamide, 5.3
Yield: 76%, mp 192°C–4°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.52 (1H, br. s, NH-3), 9.99 (1H, s, NHCO), 7.28 (1H, s, H-2′), 7.12 (1H, t, J = 8.2, H-5′), 7.02 (1H, d, J = 7.5, H-6′), 6.52 (1H, d, J = 7.5, H-4′), 5.99 (1H, s, CH-5), 4.00 (2H, s. SCH2), 3.75(3H, s, OCH3), 2.19 (3H, s, OCH3). 13C NMR (126 MHz, DMSO-d6): δ 166.46 (C=O, amide), 166.21 (6-C=O), 165.46, 164.12 (C-S), 159.92, 140.39, 129.89, 111.87, 109.21, 105.44, 107.13 (5-CH, br), 55.33 (OCH3,) 35.62 (CH2), 23.74 (CH3). Found, m/z: 306.08 [M+H]+. The Anal. Calcd. was for C14H15N3O3S: C, 55.07; N, 13.76; S, 10.04; we found: C, 55.25; N, 13.71; and S, 9.99.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-chlorophenyl)acetamide, 5.4
Yield: 76%, mp >282°C–4°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.48 (1H, br. s, NH-3), 10.22 (1H, s, NHCO), 7.56 (2H, d J = 8, H-3′,5′), 7.32 (2H, d, J = 8, H-2′,6′), 5.99 (1H, s, CH-5), 4.09 (2H, s. SCH2), 2.45 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 166.61 (C=O, amide), 166.43 (6-C=O), 165.54, 164.09 (C-S), 138.28, 129.09 (2C), 127.38, 121.25 (2C), 107.48 (5-CH, br), 35.59 (CH2), 23.32 (CH3). Found, m/z: 309.03 [M+H]+. The Anal. Calcd. was for C13H12ClN3O2S: C, 50.40; N, 13.56; S, 10.35; we found: C, 50.32; N, 13.61; and S, 10.31.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-bromophenyl)acetamide, 5.5
Yield: 79%, mp >259°C –61°C; 1H NMR (300 MHz, DMSO-d 6, δ (ppm)): 12.48 (1H, br. s, NH-3), 10.22 (1H, s, NHCO), 7.61 (2H, d, J = 7.9, H-3′,5′), 7.42 (2H, d, J = 7.9, H-2′,6′), 5.98 (1H, s, CH-5), 4.05 (2H, s. SCH2), 2.15 (3H, s, CH3). Found, m/z: 353.99 [M+H]+. The Anal. Calcd. was for C13H12BrN3O2S: C, 44.08; N, 11.86; S, 9.05; we found: C, 43.95; N, 11.90; and S, 9.02.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2,3-dichlorophenyl)acetamide, 5.6
Yield: 80%, mp 230°C–2°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.50 (1H, br. s, NH-3), 10.10 (1H, s, NHCO), 7.82 (1H, d, J = 8,2, H-4′), 7.41-7.28 (2H, m, H-5′, 6′), 6.01 (1H, s, CH-5), 4.12 (2H, s. SCH2), 2.19 (3H, s, CH3). Found, m/z: 344.21 [M+H]+. The Anal. Calcd. was for C13H11Cl2N3O2S: C, 45.36; N, 12.21; S, 9.32; we found: C, 45.29; N, 12.23; and S, 9.30.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2,5-dimethylphenyl)acetamide, 5.7
Yield: 72%, mp >248°C –50°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.42 (1H, br. s, NH-3), 9.52 (1H, s, NHCO), 7.22 (1H, s, H-6'), 7.12 (1H, d, J = 8, H-4′), 6.89 (1H, d, J = 8, H-3′), 6.01 (1H, s, CH-5), 4.05 (2H, s, SCH2), 2.29 (3H, s, CH3), 2.15 (3H, s, CH3); 2.09 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 166.37 (C=O, amide), 166.21 (6-C=O), 165.34, 164.12 (C-S), 136.35, 135.2, 130.46, 128.87, 126.39, 125.62, 107.48 (5-CH, br), 34.75 (CH2), 23.52 (CH3-4), 20.95 (CH3), 17.75 (CH3). Found, m/z: 304.11 [M+H]+. The Anal. Calcd. was for C15H17N3O2S: C, 59.38; N, 13.85; S, 10.57; we found: C, 59.19; N, 13.89; and S, 10.55.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2,5-dimethoxyphenyl)acetamide, 5.8
Yield: 68%, mp 188°C–90°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.43 (1H, br. s, NH-3), 9.39 (1H, s, NHCO), 7.86 (1H, s, H-6′), 6.90 (1H, d, J = 7.9, H-4′), 6.56 (1H, d, J = 7.9, H-3′), 6.05 (1H, s, CH-5), 4.01 (2H, s, SCH2), 3.75 (6H, s, 2CH3); 2.23 (3H, s, CH3). Found, m/z: 336.11 [M+H]+. The Anal. Calcd. was for C15H17N3O4S: C, 53.72; N, 12.53; S, 9.56; we found: C, 53.60; N, 12.58; and S, 9.53.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2,4,6-trimethylphenyl)acetamide, 5.9
Yield: 69%, mp >271°C–3°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.33 (1H, br. s, NH-3), 9.39 (1H, s, NHCO), 6.85 (2H, s, H-3′,5′), 6.00 (1H, s, CH-5), 4.02 (2H, s, SCH2), 2.22 (6H, s, 2CH3); 2.11 (6H, s, 2CH3). 13C NMR (126 MHz, DMSO-d6): δ 166.43 (C=O, amide), 166.09 (6-C=O), 165.43, 164.12 (C-S), 135.82, 135.26 (2C), 132.67, 128.66 (2C), 107.84 (5-CH, br), 34.32 (CH2), 23.7 (CH3 -4), 20.92 (CH3), 18.27 (2CH3). Found, m/z: 318.12 [M+H]+. The Anal. Calcd. was for C16H19N3O2S: C, 60.54; N, 13.24; S, 10.10; we found: C, 60.42; N, 13.19; and S, 10.05.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2,4,6-trichlorophenyl)acetamide, 5.10
Yield: 76%, m.p. >258°C–60°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.50 (1H, br. s, NH-3), 10.01 (1H, s, NHCO), 7.69–7.51 (2H, m, Ar-H), 6.03 (1H, s, CH-5), 4.10 (2H, s, SCH2), 2.22 (3H, s, CH3). Found, m/z: 379.66 [M+H]+. The Anal. Calcd. was for C13H10Cl3N3O2S: C, 41.23; N, 11.10; S, 8.47; we found: C, 41.19; N, 11.06; and S, 8.44.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(2-methyl-3-chlorophenyl) acetamide, 5.11
Yield: 75%, mp >262°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.50 (1H, br. s, NH-3), 10.01 (1H, s, NHCO), 7.69–7.51 (3H, m, Ar-H), 6.01 (1H, s, CH-5), 4.09 (2H, s, SCH2), 2.17 (3H, s, CH3). Found, m/z: 324.05 [M+H]+. The Anal. Calcd. was for C14H14ClN3O2S: C, 51.93; N, 12.98; S, 9.90; we found: C, 51.79; N, 12.93; and S, 9.87.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-benzyl-acetamide, 5.12
Yield: 66%, mp 196°C –8°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.50 (1H, br. s, NH-3), 10.01 (1H, t, J = 5.1, CH2NHCO), 7.60–7.27 (4H, m, H-2′,3′,5′,6′), 7.05 (1H, t, J = 7.5, H-4'), 6.05 (1H, s, CH-5), 4.11 (2H, s. SCH2), 4.01 (2H, d, J = 5.3, NHCH2Ph), 2.18 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 167.58 (C=O, amide), 166.43 (6-C=O), 166.09, 165.43 (C-S), 139.61, 128.7 (2C), 127.59 (2C), 127.27, 107.84 (5-CH, br), 42.98 (CH2), 34.27 (CH2, amide), 23.83 (CH3). Found, m/z: 290.09 [M+H]+. The Anal. Calcd. was for C14H15N3O2S: C, 58.11; N, 14.52; S, 11.08; we found: C, 58.01; N, 14.48; and S, 11.04.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-cyclohexlyl-acetamide, 5.13
Yield: 65%, mp 245°C–7°C; 1H NMR (300 MHz, DMSO-d6, δ (ppm)): 12.51 (1H, br. s, NH-3), 7.88 (1H, s, NHCO), 5.98 (1H, s, CH-5), 4.00 (2H, s. SCH2), 2.21 (3H, s, CH3) 1.90–1.18 (M, 11H, C6H11). 13C NMR (126 MHz, DMSO-d6): δ 167.58 (C=O, amide), 166.4 (6-C=O), 166.09, 165.43 (C-S), 107.65 (5-CH, br), 48.42, 36.66, 34.6, 32.74, 31.99, 25.55, 24.66, 23.53 (CH3). Found, m/z: 282.19 [M+H]+. The Anal. Calcd. was for C13H19N3O2S: C, 55.49; N, 10.68; S, 11.40; we found: C, 55.36; N, 10.65; and S, 11.36.
Ethyl 2-[2-(4-methyl-6-oxo-1,6-dihydropyrimidin-2-ylthio)-acetylamino]-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate, 5.14
Yield: 55%, mp 230°C –2°C; 1H NMR (300 MHz, DMSO-d6,, δ (ppm)): 12.61 (1H, br. s, NH-3), 11.29 (1H, s, NHCO), 6.03 (1H, s, CH-5), 4.35 (2H, q, OCH2CH3), 4.10 (2H, s. SCH2), 2.90-2.70 (2H, m, CH2), 2.52–2.31 (4H, m, 2CH2), 2.12 (3H, s, CH3) 1.20 (3H, t, J = 5.2, OCH2CH3). 13C NMR (126 MHz, DMSO-d6): δ 167.66 (C=O amide), 166.10 (6-C=O), 166.05, 166.01, 164.12, 150.52, 141.37, 132.21, 108.54, 107.65 (5-CH, br), 60.81, 33.99 (CH2), 30.37, 28.86, 27.7, 23.69 (CH3) 14.42 (CH3). Found, m/z: 393.48 [M+H]+. The Anal. Calcd. was for C17H19N3O4S2: C, 55.49; N, 10.68; S, 11.40; we found: C, 55.36; N, 10.72; and S, 11.35.
2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-phenoxy-phenyl)-acetamide, 5.15
Yield: 60%, mp 224°C–6°C; 1H NMR (300 MHz, DMSO-d6,, δ (ppm)): 12.45 (1H, br. s, NH-3), 10.08 (1H, s, NHCO), 7.75–7.55 (2H, m, Ar-H), 7.40–7.29 (2H, m, Ar-H), 7.10–6.91 (5H, m, Ar-H), 5.98 (1H, s, CH-5), 4.08 (2H, s. SCH2), 2.21 (3H, s, CH3). 13C NMR (126 MHz, DMSO-d6): δ 166.43 (C=O, amide), 166.09 (6-C=O), 165.43, 164.12 (C-S), 157.78, 152.44 (2C), 135.25, 130.43(2C), 123.49 (2C), 121.37, 119.94 (2C), 118.39, 107.83 (5-CH, br), 35.54 (CH2), 23.91 (CH3). Found, m/z: 368.43 [M+H]+. The Anal. Calcd. was for C19H17N3O3S: C, 62.11; N, 11.44; S, 8.73; we found: C, 62.02; N, 11.40; and S, 8.69.
Anticonvulsant activity
Animals
The experiments were carried out on 95 adult male rats (130–150 g) and adult random-bred albino mice of either sex weighing 22–28 g. The animals were bred in the vivarium of National Pirogov Memorial Medical University, Vinnytsya housed in cages under standard conditions with a temperature of (22°C ± 1°C), relative humidity of (55% ± 15%) with free access to food and water and a 12-hour light/darkness cycle (8.00–20.00), respectively. All of the experiments were conducted in accordance with “Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes,” with the procedures and requirements of the State Expert Center of the Ministry of Health of Ukraine and with the rules of European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986), resolution of the First National Congress on Bioethics (Kyiv, 2001), with the Law of Ukraine National Congress on Bioethics (Kyiv, 2001) and with the Law of Ukraine â„–3447-IV “On Protection of Animals from Cruel Treatment” dated 02.21.2006.
Animals were randomly assigned to the experimental and control groups. The substances tested were dissolved in 1% starch gel and administered by oral gavage cannula at a volume of 0.5 ml/100 g body weight in rats and 0.2 ml/10 g body weight in mice 1 hour before seizure induction. Screening dose for testing compounds was 80 and 50 mg/kg. Referent compounds (phenobarbital, lamotrigine, and carbamazepine) were given the same manner in their median anticonvulsant doses 20, 20, and 15 mg/kg body weight, respectively (Metcalf et al., 2017). The determination of time for the experiment was based on the data concerning the peak of the anticonvulsant activity of the drug, described in the literature (Fisher, 1989; Löscher, 2011). Control rats received equivolume amounts of solvents. All seizures [both chemical (PTZ) and electrical (MES)] were induced between 9:00 and 11:00 to minimize possible inconsistencies arising from circadian rhythms (Löscher, 1996).
Pentylenetetrazole-induced seizures
Seizures in animals were modeled by a single subcutaneous pentylentetrazole injection (Sigma) at a dose of 80 mg/kg. The animals were administered intragastrically with a freshly prepared suspension of experimental compounds and reference drugs phenobarbital and lamotrigine (20 mg/kg). The anticonvulsant activity was assessed by the dynamic of the latent period, the intensity and duration of seizures in minutes, and the lethality rate of mice.
The intensity of seizures was evaluated using a 5-point scale, taking as a basis the following criteria (including the number of animals that died) (Gerald, 1973): 0—no seizure activity; 1—hyperkinesia; 2—trembling, twitching; 3—clonic seizures of upper limbs with the rise on their lower limbs; 4—pronounced tonic-clonic seizures, the animal’s fall to the side, and the available phase of tonic extension; and 5—repeated tonic-clonic seizures, loss of posture, and death.
Anticonvulsant effect was considered an animal protection based on the clonic and tonic seizures and the lethality.
Maximal electroshock seizure testing in mice (MES)
The research was conducted on the non-linear mice of both sexes, weighing 25–28 grams. The animals were divided into four groups with 10 animals in each group. Group one served as the control group. Animals of other groups were receiving intragastrically compounds (5.1-15) (50 mg/kg), lamotrigine (20 mg/kg), or carbamazepine (15 mg/kg). The investigation of anticonvulsant activity was conducted 1 hour later after the administration of the experimental compounds. The MES test consisted of electrical stimulation of the cornea using 60 Hz of alternating current (150 mA), with a stimulus duration of 0.2 second, using a custom-build MES stimulator. The electrodes were soaked with a 0.9% sodium chloride solution. A number of animals with hind limb flexion-extension and mortality rate were estimated.
After estimation of the most active substance, its acute toxicity (LD50), neurotoxicity (TD50), and protective index (PI = LD50/TD50) were determined.
Neurotoxic activity
The possible neurotoxic effects in mice were quantified using a rotarod test. The test compound was administered intraperitoneally to animals at a dose rate of 10–200 mg/kg and the control mice received an equivalent amount of solvent. Mice were placed on a rotating knurled rod (12 rpm). Neurotoxic action was manifested in the form of violations of coordination of motion. Mice were considered impaired if they fell off the rotarod three times during the 1-minute observation period performed immediately prior to stimulation (rotarod failure). The mice of the control group were kept on the rods for several minutes. The value of TD50 was calculated by the probit analysis method.
Acute toxicity
During this experiment, we used the white nonlinear mice (24 ± 3g), which were divided into five groups. Animals of the experimental groups received a substance 5.5, ranging in doses from 100 mg/kg to 400 mg/kg. The drug was administered in appropriate doses intragastrically, dissolving it in the required amount of 1% starch gel. Animal observations were carried out within 14 days; then, the number of dead animals in each group was noted and using the method implemented by Prozorovsky (2007), acute toxicity (LD50 and its confidence interval) was evaluated. We recorded the behavior and body weight of mice, the clinical signs of intoxication, the general condition of animals, the nature of motor activity, the characteristics of breathing, the condition of hair and skin, the presence of a vessel, the consumption of food and water, and also we noted the number of dead animals in each group and the value of LD50 was defined.
Statistical analysis
All values were expressed as the means ± S.E.M. The data were analyzed by ANOVA (analysis of variance) followed by Dunnett’s test (Statistical package for social sciences, SPSS 16.0). An X2 analysis was performed to compare the neurotoxicity differences (number of failures per test). p values ≤ 0.05 was considered as significant.
RESULTS AND DISCUSSION
Chemistry
To establish the prospects of synthesis and optimization of further pharmacological screening, we performed a prediction of the biological activity that was planned for the synthesis of compounds using a PASS computer program (http://www.pharmaexpert.ru/passonline/). 6-Methyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one acetamides were selected for synthesis. High psychotropic activities such as antiepileptic, anxiolytic, antidepressant, antineurotic, and anticonvulsant activities (Pa ≥ 0.50) were predicted for these compounds.
The synthesis of initial 6-methyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one 3 consisted of condensation of the ethyl acetoacetate 1 with a 2.5-fold molar excess of thiourea 2 (Fig. 1). The reaction occurred successfully in refluxing absolute methanol with a 2.6–2.8-fold molar excess of sodium methylate as reported in known methods used (Novikov et al., 2005).
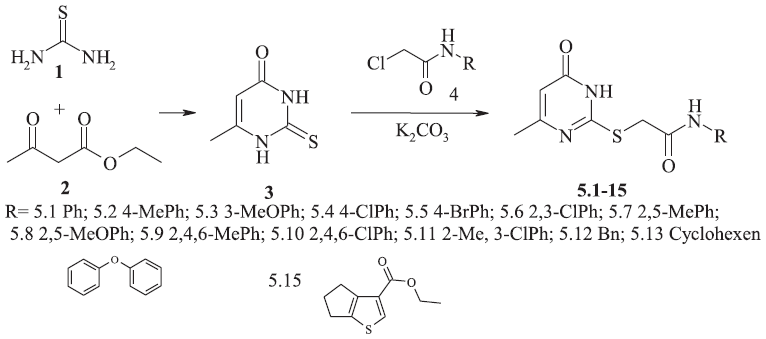 | Figure 1. The synthesis of 2-[(1,6-dihydro-6-oxo-4-methyl-2-pyrimidinyl)thio]-N-acetamides. [Click here to view] |
Alkylation of the resulting 6-methyl-2-thiopymidin-4-one 3 was carried out by an equimolar amount of N-arylsubstituted 2-chloroacetamides, 2-chloro-N-benzylacetamide, and ethyl 2-[(chloroacetyl)amino]-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate according to known procedure of alkylation of thiopyrimidines (Gagnon et al., 2007; Rakhimov and Titova, 2007). The reaction was conducted in dimethylformamide solution in the presence of an excess of potassium carbonate and led to the formation of only 2-[(1,6-dihydro-6-oxo-4-methyl-2-pyrimidinyl)thio]-N-acetamides 5.1-15. The yield of reaction products was 55%–82%.
Because the sulfur atom in the molecule of 4-methyl-2-thiopyrimidin-4-one 3 is the most nucleophilic, the alkylation reaction on this direction was expected and most likely.
In the literature (Danel, 1998), it was noted that the usage of primary alkyl halides as alkylating agents leads to the formation of a complex mixture consisting of S-mono, SN1- and SN3-disubstituted alkylation products. TLC, LCMS, and 1H NMR spectroscopy data confirmed the formation of only S-derivatives which probably can be explained by using sizable alkylating agents that shielded Nitrogen atoms.
The 1H NMR spectra of synthesized compounds 5.1–5.15 contain a wide singlet proton signal of the NH group in the third position of the pyrimidine cycle located at δ 12.52–12.43 ppm, a singlet signal of NHCO proton group of acetamide residue at δ 11.23–9.39 ppm, a signal of a methine proton at 5 position of the pyrimidine cycle at δ 6.05–5.98 ppm, and a singlet of a methylene SCH2-group within a range 4.12–4.00 ppm. Protons of the phenyl radical resonate at δ 7.92–6.52 ppm and their multiplicity and intensity correspond to the nature and location of substituents.
13C NMR spectra of all 2-[(1,6-dihydro-6-oxo-4-methyl-2-pyrimidinyl)thio]-N-acetamides 5.1-15 allow for reliably identifying the signals of only some carbon atoms: C=O amide, 6-C=O, C-S, 5-CH, 6-CH3, CH2, and CH3. It is incorrect to relate all other signals to any specific carbon atom (especially in the aromatic region of the spectrum) without additional two-dimensional experiments. However, they also give useful information—at least, concerning the number of carbon atoms in the molecule. 13C NMR spectra for compounds 5.5, 5.6, 5.8, and 5.10 were not recorded due to insufficient solubility.
Anticonvulsant activity
In the control group, pentylentetrazole administration led to the development of seizures in all animals. The duration of the latent period averaged 4.7 minutes and the duration of seizures —9.7 minutes (Table 1). The seizures which developed in this group of rats were accompanied by severe periodically repeated tonic-clonic convulsions. There was a clear phase of tonic extension (epistotonus). The lethality in this group of rats was 100%. Phenobarbital prevented the development of the seizures in all animals. At the same time, after administration of lamotrigine in rats, pentylentetrazole induced some manifestations of the seizures (convulsive twitching, jumps, and contractions of the upper limbs), but the duration of the latent period was statistically reliably lengthened (in 5.8 times), the degree of the seizure severity, and their total duration were significantly lower than in the control group. Lamotrigine prevented the lethality in 80% of animals.
All investigated compounds reduced the development of seizures in the chemoconvulsive seizure model (Table 1). After their administration, the extension of the latent period was observed in relation to the control group; however, compounds 5.4, 5.10, 5.12, 5.14, and 5.15 slightly increased it (in 0.2–1.5 times).
The extension of the latent period of seizures, reduction of their intensity, and duration of seizures were most observed while administering the compounds 5.2, 5.5, 5.8, 5.13, and 5.11.
Compounds 5.5, 5.7, 5.8, 5.11, and 5.13 prevented the lethality in 100% of rats similarly to phenobarbital. Compounds 5.1, 5.2, 5.3, and 5.10 and lamotrigine reduced lethality, respectively, in 80% and 60% of the experimental rats. 100% lethality of the experimental animals, similar to the control group, was observed while administering the compounds 5.4, 5.12, and 5.15.
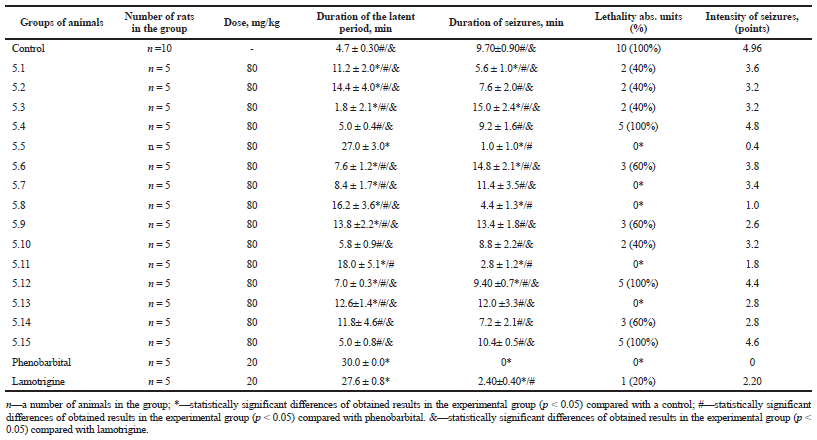 | Table 1. The effect of the synthesized compounds 5.1–5.15, lamotrigine, and phenobarbital on seizures caused by administration of pentylentetrazole in rats. [Click here to view] |
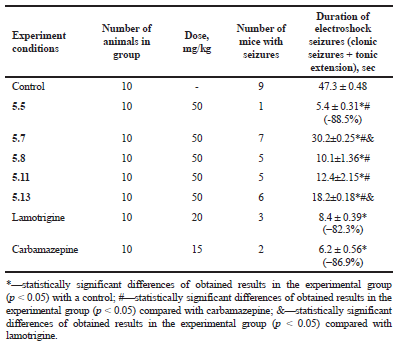 | Table 2. The effect of the synthesized compounds (5.5, 5.7, 5.8, 5.11, and 5.13), carbamazepine, and lamotrigine on seizures caused by maximal electroshock in mice. [Click here to view] |
According to our research, the most pronounced anticonvulsant activity was shown by compound 5.5, which contained 4-bromophenyl radical. This compound reduced the duration of seizures in 2.4 times and the seizure severity in 5.5 times.
Structure-activity relationship (SAR) studies
Analysis of the results of screening studies of anticonvulsant activity of thiopyrimidine derivatives 5.1–5.15 on the model of pentylentetrazole seizures in rats allowed making some general conclusions about the influence of structural fragments on anticonvulsant activity of synthesized compounds.
It can be assumed that the introduction of the N-phenyl radical into the structure of compounds contributes to the increase of anticonvulsant activity, since the replacement of the N-phenyl radical with benzyl, 4-phenoxyphenyl, and cyclopentathiophene (5.12, 5.14, and 5.15) leads to reduction of the latent period, prolongation of the duration of seizures, and an increase the percentage of lethality of the experimental animals (100%, 100%, and 60%, respectively).
Substituents in the phenyl radical also have a significant role in an anticonvulsant activity. 4-, 2,3- and 2,4,6-chloro-substituted derivatives (5.4, 5.6, and 5.10) minimally extended the latent period, did not reduce the duration and severity of the seizures and did not prevent the lethality of animals, whereas, Me-, MeO- and, in particular, 4-Br-substituted derivatives improved this data.
The following pattern was observed: with the increase of the number of chlorine atoms, the lethality of the experimental animals decreased: 5.4, 5.6, and 5.10 = 4-Cl, 2,3-diCl, 2,4,6-triCl = 100%, 60%, 40%. Such results were unexpected as there is a significant amount of literary data describing an increase of anticonvulsant activity when introducing chlorine atoms.
The next step in our research was focused on investigating the effects of the synthesize substances on the models of primary generalized seizures caused by maximal electroshock which is essential for identifying potential anticonvulsants.
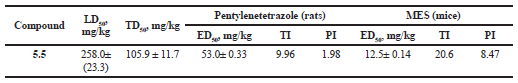 | Table 3. Characteristic of anticonvulsant activity of 2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-bromophenyl)acetamide (5.5) in intragastric administration. [Click here to view] |
The results presented in Table 2 showed that the ability of the compounds to exhibit anticonvulsant effects on the MES model was different. The potent anticonvulsant activity in this study showed compounds 5.8 and 5.11, but the highest anticonvulsant activity among the experimental compounds was shown by compound 5.5. Thus, after its administration, the number of dead animals was 10% versus 90% in control, and the total duration of seizure was 88.5% less (p < 0.05) than in animals without pharmacological correction. Due to the ability to prevent seizures, this compound was not statistically different from carbamazepine and prevailed lamotrigine taken in their median effective doses.
Based on the data obtained in this study, the dose-dependent parameters (ED50) in the pentylenetetrazole-induced seizures model, MES test, acute (LD50) and neurotoxicity (TD50) indexes, as well as the therapeutic (TI) and protective (PI) indexes were established for the lead compound 5.5 (Table 3).
CONCLUSIONS
Based on the PASS prediction, the synthesis of potential anticonvulsants in a series of S-alkylated derivatives of 6-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one was designed. By the interaction of thiourea and acetoacetate in a medium of sodium methylate, 6-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one was obtained.
It was found that as the result of alkylation of 6-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one by N-arylsubstituted 2-chloroacetamides, 2-chloro-N-benzylacetamide, and ethyl 2-[(chloroacetyl)amino]-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate in DMF medium in the presence of potassium carbonate, only monosubstituted S-acetamide derivatives of 6-methyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one were formed.
Using the models of pentylenetrazole-induced seizures and maximal electroshock test, a screening study of the anticonvulsant activity of synthesized compounds was carried out. A lead compound- 2-[(4-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio]-N-(4-bromophenyl)acetamide was revealed. For these compound parameters of ED50, acute (LD50) and neurotoxicity (TD50) as well as TI and PI indexes were determined. Some relationships “structure-anticonvulsant activity” were established.
CONFLICTS OF INTEREST
The authors have declared there is no conflicts of interest.
REFERENCES
Azizi F, Malboosbaf R. Antithyroid drug treatment: a systematic review and meta-analysis. Thyroid, 2017; 27:1223–31. CrossRef
Basavaraja HS, Jayadevaiah KV, Mumtaz MH, Vijay Kumar MM. Synthesis of novel piperazine and morpholine linked substituted pyrimidine derivatives as antimicrobial agents. J Pharm Sci Res, 2010; 2:5–12.
Cooper DS. Antithyroid drugs. N Engl J Med, 2005; 352:905–17. CrossRef
Crepaldi P, Cacciari B, Bonache MC, Spalluto G, Varani K, Borea PA, Kügelgen IV, Hoffmann K, Pugliano M, Razzar C, Cattaneo M. 6-Amino-2-mercapto-3H-pyrimidin-4-one derivatives as new candidates for the antagonism at the P2Y12 receptors. Bioorg Med Chem, 2009; 17:4612–21. CrossRef
Danel К, Pedersen EB, Nielsen C. Synthesis and anti-HIV-1 activity of novel 2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-7-ones. J Med Chem, 1998; 41:191–8. CrossRef
Fisher RS. Animal models of the epilepsies. Brain Res Rev, 1989; 14:245–78. CrossRef
Gagnon A, Amad AH, Pierre R, Bonneau PR, Coulombe R, DeRoy PL, Doyon L, Duan J, Garneau M, Guse I, Jakalian A, Jolicoeur E, Landry S. Thiotetrazole alkynylacetanilides as potent and bioavailable non-nucleoside inhibitors of the HIV-1 wild type and K103N/Y181C double mutant reverse transcriptases. Bioorg Med Chem Lett, 2007; 17:4437–41. CrossRef
Gerald MC, Riffee WH. Acute and chronic effects of d- and 1-amphetamine on seizure susceptibility in mice. Eur J Pharmacol, 1973; 21:323–30. CrossRef
Gorneva G, Mateva R, Gugova R, Golovinsky E. The study of the apoptogenic effect of pyrimidine derivatives on murine leukemia cells. Arch Oncol, 2005; 13:62–4. Available via http://www.pharmaexpert.ru/passonline/ CrossRef
Levine JA, Ferrendelli JA, Covey DF. Alkyl-substituted thiolo-, thiono-, and dithio-γ-buthyrolactones: new classes of convulsant and anticonvulsant agents. J Med Chem, 1986; 29:1996–9. CrossRef
Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure, 2011; 20:359–68. CrossRef
Loscher W, Fiedler M. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. VI. Seasonal influences on maximal electroshock and pentylenetetrazole seizure thresholds. Epilepsy Res, 1996; 25:3–10. CrossRef
Löscher W, Hönack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res, 1991; 8:171–89. CrossRef
Mai A, Sbardella G, Artico M, Ragno R, Massa S, Novellino E, Greco G, Lavecchia A, Musiu C, La Colla M, Murgioni C, La Colla P, Loddo R. Structure-based design, synthesis, and biological evaluation of conformationally restricted novel 2-Alkylthio-6-[1-(2,6-difluorophenyl)alkyl]- 3,4-dihydro-5-alkylpyrimidin-4(3H)-ones as non-nucleoside inhibitors of HIV-1 reverse transcriptase. J Med Chem, 2001; 44:2544–54. CrossRef
Matias M, Campos G, Silvestre S, Falcão A, Alves G. Early preclinical evaluation of dihydropyrimidin(thi)ones as potential anticonvulsant drug candidates. Eur J Pharm Sci, 2017; 102:264–74. CrossRef
Metcalf CS, West PJ, Thomson K, Edwards S, Smith MD, White HS, Wilcox KS. Development and pharmacological characterization of the Rat 6 Hz model of partial seizures. Epilepsia, 2017; 58:1073–84. CrossRef
Nawrozkij MB, Rotili D, Tarantino D, Botta G, Eremiychuk AS, Musmuca I, Ragno R, Samuele A, Zanoli S, Armand-Ugón M, Clotet-Codina I, Novakov IA Orlinson BS, Maga G, Esté JA, Marino Artico M, Antonello Mai A. 5-Alkyl-6-benzyl-2-(2-oxo-2-phenylethylsulfanyl)pyrimidin-4(3H)-ones, a series of anti-HIV-1 agents of the dihydro-alkoxy-benzyl-oxopyrimidine family with peculiar structure–activity relationship profile. J Med Chem, 2008; 51:4641–52. CrossRef
Novikov MS, Ozerov AA, Sim OG. Synthesis of 5-[2-(phenoxy)ethyl] derivatives of 6-methyluracil, 6-methyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one and 2-imino-6-methyl-2,3-dihydro-1H-pyrimidin-4-one. Chem Heterocycl Compd, 2005; 8:1036–40. CrossRef
Prozorovsky VB. Statistic processing of data of pharmacological investigations. Psychopharmacol Biol Narcol, 2007; 7:2090–120.
Rakhimov AI, Titova ES. Synthesis of 2-alkyl(aralkyl)Sulfanyl-6-methylpyrimidin-4(3H)-ones and 4-alkyl(aralkyl)oxy-2-alkyl (aralkyl)sulfanyl-6-methylpyrimidines. Russ J Org Chem, 2007; 43:92–8. CrossRef
Russo H, Bressolle F. Pharmacodynamic and pharmacokinetic of thiopental. Clin Pharmakokinet, 1998; 35:95–134. CrossRef
Saidov NB, Kadamov IM, Georgiyants VA, Taran AV. Planning, synthesis, and pharmacological activity of alkyl derivatives of 3-Mercapto-4-Phenyl-5-Arylaminomethyl-1,2,4-Triazole-(4H). Pharm Chem J, 2014; 47:581–5. CrossRef
Severina AI, Georgiyants VA, Shtrygol SYu, Kavraiskyi DP. Synthesis and alkylation of 1-aryl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-ones as possible anticonvulsant agents. Der Pharma Chem, 2015; 7:43–8.
Severina AI, Skupa OO, Georgiyants VA, Voloshchuk NI. Screening of anticonvulsant activity of new pyrimidin-4(3H)-one derivatives [ONLINE] Med educ in Siberia, 2013. Available via http://www.ngmu.ru/cozo/mos/article/text_full.php?id=1034.
Shigeta S, Mori S, Kira T, Takahashi K, Kodama E, Konno K, Nagata T, Kato H, Wakayama T, Koike N, Saneyoshi M. Anti-herpesvirus activities and cytotoxicities of 2-thiopyrimidine nucleoside analogues in vitro. Antivir Chem Chemother, 2003; 10:195–209. CrossRef