INTRODUCTION
The prevalence of cancer worldwide continues to increase significantly. International Agency for Research Cancer estimated that in 2018 there are 18,100,000 new cancer patients and 9,600,000 cancer deaths. Lung cancer, breast cancer, and colorectal cancer are the types of cancer that have the most incidence (Press Release, 2018). For more than six decades, cancer chemotherapeutic agents have been developed and used as one approach for cancer treatment. Unfortunately, the use of chemotherapeutic agents generally may produce irreversible chronic and delayed toxicities against many vital organs, such as kidneys, heart, and lungs, because of low specificity for cancer cells (Roche, 2012). Moreover, some patients develop resistance to anticancer drugs, such as 5-fluorouracil (5-FU) (Chibaudel et al., 2008). Therefore, there is a significant need to develop a new anticancer agent with better efficacy and selectivity.
Curcumin was well known to possess many biological activities, such as anti-inflammatory inhibition, growth inhibition in various tumor cells, and chemopreventive effects on certain cancers with low toxicity (Anand et al., 2008). The curcumin’s antitumor mechanism is multiple, involving apoptosis induction, proliferation inhibitory, G1/S arrest, and the mitotic block (Kunnumakkara et al., 2017; Srivastava et al., 2007). Although curcumin has evidence as anti-cancer, its therapeutical usage of curcumin is restricted by low of water solubility, chemical and metabolical stability, and relatively poor in vivo bioavailability (Anand et al., 2008). The chemical structure of curcumin has been modified intensively to find the analogs had better physical and chemical properties, as well as better biological activity. New analogs that show an inhibitory activity of cancer cells growth 30 times than curcumin and other drugs often used to treat cancer were identified (Ohori et al., 2006). Monocarbonyl analogs of curcumin (MACs) with cyclohexanone as central can inhibit the growth of colon, ovarian, and breast cancer cells better than cisplatin (Adams et al., 2004; Liang et al., 2009; Yerdelen et al., 2015). Recently, our research group reported that methoxy- and methyl-substituted of asymmetrical mono-carbonyl analogs of curcumin (AMACs) (Fig. 1) showed moderate cytotoxicity against MCF-7 (Prasetyaningrum et al., 2018). Aminomethylation is one of the feasible and cost-efficient procedures for drug development (Biersack et al., 2018). Several aminomethyl derivatives had been synthesized and reported to have better anticancer activity than the parent analogs, such as aminomethyl derivatives of chalcones, acetophenones, benzylidenecyclohexanones, carbazoles, 4,11-dihydroxynaphthol[2,3-f]indole-5,10-dione, gatifloxacin, 8-hydroxyquinoline, benzothiazoles, 2-propoxybenzylidene-isonicotino hydrazide, fluoroquinolones, 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones, and MACs (Bala et al., 2014; Dimmock et al., 1992; Roman, 2015; Subramaniapillai, 2013; Yerdelen et al., 2015). The phenol derivatives having quaternary ammonium group are bioactive compounds. They act as DNA interstrand cross-linking agent to inhibit transcription and furthermore the apoptosis of tumor cells (Song et al., 2006). Diethylaminomethyl derivatives of methyl-substituted of AMACs (Fig. 1) exhibited moderate cytotoxicity against MCF-7 but low selectivity against normal cells (Prasetyaningrum et al., 2018). Thereby, as continuation study of our research group, we synthesized a series of new aminomethyl derivatives of methyl-substituted asymmetrical curcumin mono-carbonyl [2-(4-hydroxy-3-methoxy-benzylidene)-6-(4-methyl-benzylidene)-cyclohexanone] and evaluated their anticancer potential.
MATERIAL AND METHODS
General procedures
The measurement of melting points was performed using analog melting point apparatus (Model SMP11, Stuart Scientific) and the values obtained are uncorrected. The purity of the compounds was checked by thin layer chromatography (TLC) on silica gel Si 60 F254 plates (Merck). Infrared spectral data were attained by an FT-IR spectrophotometer (8400S, Shimadzu). Proton Nuclear Magnetic Resonance (NMR) and Carbon NMR spectra were obtained on NMR spectrometer (Agilent), and Mass spectra were recorded in positive mode on High Resolution Mass Spectrometer (LCT Premier XE-TOF) (Waters Corp.). The known compounds: 2-(4-methyl-benzylidene)-cyclohexanone (1), 2-(4-hydroxy-3-methoxy-benzylidene)-6-(4-methyl-benzylidene)-cyclohexanone (2), 2-(3-diethylaminomethyl-4-hydroxy-5-methoxy-benzylidene)-6-(4-methyl-benzylidene)-cyclohexanone (3e), and 2-(4-hydroxy-3-methoxy-5-morpholin-4-ylmethyl-benzylidene)-6-(4-methyl-benzylidene)-cyclohexanone (3f) were obtained from earlier reasearcher (Prasetyaningrum et al., 2018; Putri et al., 2018).
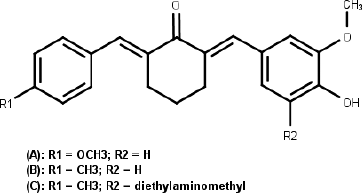 | Figure 1. (A) Methoxy-substituted, (B) Methyl-substituted, and (C) Diethylaminomethyl derivatives of methyl-substituted of AMACs (Prasetyaningrum et al., 2018). [Click here to view] |
Synthesis of compounds 3a–d
The compounds were prepared according to the synthesis method of compound 3e and 3f reported earlier with little modifications (Prasetyaningrum et al., 2018; Putri et al., 2018). To a cold solution of compound 2 and appropriate secondary amine compound (2,6-dimethylmorpholine/diethylamine/pyrrolidine/1-methylpiperazine) in ethanol, formaldehyde solution was added dropwise while stirring in an ice bath. After stirring for 30 minutes at r.t., the reaction mixture was refluxed for 7–11 hours (TLC monitoring). Upon completion, evaporation of the solvent and residue dissolution in methanol was done twice, then the solution warmed and poured gradually into cold distilled water (with constant stirring) to obtain the precipitate product. The product was separated by means of decantation, filtration, washing with cold distilled water, and drying at room temperature. Purification was done by column chromatography to obtain pure 3a–d.
2-[3-(2,6-Dimethylmorpholin-4-ylmethyl)-4-hydroxy-5-methoxy-benzylidene]-6-(4-methyl-benzylidene)-cyclohexanone (3a)
Yellow powder, yield 64.5%, mp 103°C–105°C. FT-IR (KBr) cm−1: 2,933–2,860 (C-H aliphatic), 1,737 (carbonyl), 1,662, 1,600, 1,494 (C=C), 1,271 (C-N), and 1,157 (C-O-C). 1H-NMR (CDCl3, 500 MHz), δ: 1.17 ppm (6H, d, J = 6 Hz, two CH3CH-, 2,6-dimethylmorpholine), 1.89 and 2.85 ppm (4H, t, J = 10 Hz, and d, J = 12 Hz, two CHCH2-N 2,6-dimethylmorpholine), 4.08 and 3.70 ppm (2H, m, two N-CH2CH(CH3)-O 2,6-dimethylmorpholine), 3.90 and 3,91 ppm (3H, s, 3-CH3-O) (Untung et al., 2017), 1.80 ppm (2H, p, J = 7 Hz, CH2CH2CH2 cyclohexanone), 2.37 ppm (3H, s, 4-CH3Ar); 2.90 and 2.94 ppm (4H, t overlap, J = 8 Hz, two CH2CH2C cyclohexanone), 3.72 ppm (2H, s, ArCH2-N), 6.82 ppm (1H, d, J = 2 Hz, H phenyl), 6.99 ppm (1H, d, J = 2 Hz, H phenyl), 7.20 ppm (2H, d, J = 8 Hz, two H phenyl), 7.38 ppm (2H, d, J = 8 Hz, two H phenyl), 7.71 and 7.77 ppm (1H, s, and 1H, s, two H methylidene). 13C-NMR (CDCl3, 125 MHz), δ: 19.1 ppm (2C, two CH3-, 2,6-dimethylmorpholine), 21.5 ppm (1C, 4-CH3Ar), 23.2, 28.6 and 29.8 ppm (3C, three CH2 cyclohexanone), 56.1 ppm (1C, CH2-N-), 58.5 (2C, CH2-N- 2,6-dimethylmorpholine), 61.6 ppm (1C, 4-CH3-O), 71.81 ppm (2C, CH2-O- 2,6-dimethylmorpholine), 113.7, 120.8, 124.0, 127.3, 129.2, 130.6, 137.4, and 138.9 ppm (8C, CAr), 133.4, 133.9, 135.6, and 136.8 ppm (4C, -C=C methylidene), 147.8 and 148.3 ppm (2C, C-O), 190.2 ppm (1C, carbonyl) (Silverstein et al., 2005). Calcd masses for C29H35NO4 : 461.5925, HR-ESI-MS (m/z) found 462.2637 ([M+H]+).
2-(3-Dimethylaminomethyl-4-hydroxy-5-methoxy-benzylidene)-6-(4-methyl- benzylidene)-cyclohexanone (3b)
Red caramel-like solid, yield 63.2%, mp 96.97°C. FT-IR (KBr) cm−1: 2,945–2,829 (C-H aliphatic), 1,662 (carbonyl), 1,597, 1,489 (C=C), 1,255 (C-N), and 1,159 (C-O-C). 1H-NMR (CD3OD, 500 MHz), δ: 1.77 ppm (2H, p, J = 6 Hz, CH2CH2CH2 cyclohexanone), 2.34 ppm (3H, s, 4-CH3Ar); 2.38 ppm (6H, s, two CH3-N), 2.86 and 2.92 ppm (4H, t, J = 6 Hz, two CH2CH2C cyclohexanone), 3.72 ppm (2H, s, Ar-CH2-N), 3.85 ppm (3H, s, 3-CH3-O), 6.92 ppm (1H, s, H phenyl), 7.02 ppm (1H, d, J = 2 Hz, H phenyl), 7.21 ppm (2H, d, J = 8 Hz, two H phenyl), 7.34 ppm (2H, d, J = 6 Hz, two H phenyl), 7.64 and 7.65 ppm (1H, s, and 1H, s, two H methylidene). 13C-NMR (CD3OD, 125 MHz), δ: 21.4 ppm (1C, 4-CH3-Ar), 24.1, 29.4, and 29.6 ppm (3C, three CH2 cyclohexanone), 44.4 ppm (2C, two CH3-N-, dimethylamine), 56.5 ppm (1C, ArCH2-N), 61.5 ppm (1C, 3-CH3-O), 114.9, 123.0, 126.6, 127.3. 130.2, 131.6, 139.3, and 140.2 ppm (8C, CAr), 134.3, 134.5, 137.0, and 137.7 ppm (4C, -C=C- methylidene), 149.3 and 151.2 ppm (2C, C-O), 191.8 ppm (1C, carbonyl) (Silverstein et al., 2005). Calcd masses for C25H29NO3 : 391.507, HR-ESI-MS (m/z) found 392.2222 ([M+H]+).
2-[4-Hydroxy-3-methoxy-5-(pyrrolidin-1-ylmethyl)-benzylidene]-6-(4-methyl- benzylidene)-cyclohexanone (3c)
Red caramel-like solid, yield 52.08%, mp 82°C–84°C. FT-IR (KBr) cm−1: 2,937–2,833 (C-H aliphatic), 1,654 (carbonyl), 1,566, 1,415 (C=C), 1,255 (C-N), and 1,155 (C-O-C). 1H-NMR (CDCl3, 500 MHz), δ: 1.80 ppm (4H, t, J = 6 Hz, CH2CH2 pyrrolidine), 1.86 ppm (2H, p, J = 6 Hz, CH2CH2CH2 cyclohexanone), 2.37 ppm (3H, s, 4-CH3Ar), 2.68 ppm (4H, t, J = 6 Hz, two CH2-N pyrrolidine), 2.89 and 2.95 ppm (4H, t overlap, J = 5 Hz, two CH2CH2C cyclohexanone), 3.88 ppm (3H, s, 3-CH3O), 3.90 ppm (2H, s, ArCH2-N), 6.82 ppm (1H, s, H phenyl), 6.98 ppm (1H, d, J = 2 Hz, H phenyl), 7.20 ppm (2H, d, J = 8 Hz, two H phenyl), 7.36 ppm (2H, d, J = 8 Hz, two H phenyl), 7.72 and 7.76 ppm (1H, s, and 1H, s 2H methylidene). 13C-NMR (CDCl3, 125 MHz), δ: 21.5 ppm (1C, 4-CH3Ar), , 23.8 ppm (2C, CH2CH2 pyrrolidine), 23.2, 28.6, and 28.8 ppm (3C, three CH2 cyclohexanone), 53.6 ppm (2C, CH2N- pyrrolidine), 56.1 ppm (1C, ArCH2N), 58.6 ppm (1C, 3-CH3-O), 113.5, 122.3, 123.6, 126.7, 129.2, 130.5, 137.7, and 138.8 ppm (8C, CAr), 133.4, 133.6, 135.7, and 136.6 ppm (4C, -C=C- methylidene), 147.8 and 149.0 ppm (2C, C-O), 190.3 ppm (1C, carbonyl) (Silverstein et al., 2005). Calcd masses for C27H31NO3 : 417.2304, HR-ESI-MS (m/z) found 418.2379 ([M+H]+).
2-[4-Hydroxy-3-methoxy-5-(4-methylpiperazin-1-ylmethyl)-benzylidene]-6-(4-methyl-benzylidene)-cyclohexanone (3d)
Orange powder, yield 67.76%, mp 134°C–136°C. FT{IR (KBr) cm−1: 2,937–2,837 (C-H aliphatic), 1,658 (carbonyl), 1,602, 1,562, and 1,492 (C=C), 1,253 (C-N) and 1,157 (C-O-C). 1H-NMR (CDCl3, 500 MHz), δ: 1.79 ppm (2H, p, J = 6 Hz, CH2CH2CH2 cyclohexanone), 2.29 ppm (3H, s, 4-CH3-N methylpiperazine), 2.36 ppm (3H, s, 4-CH3-Ar); 2.60 ppm (8H, m, two -N-CH2CH2-N methylpiperazine), 2.89 and 2.92 ppm (4H, t, J = 6 Hz, CH2CH2C cyclohexanone), 3.75 ppm (2H, s, ArCH2-N), 3.89 ppm (3H, s, 3-CH3-O), 6.81 ppm (1H, d, J = 2 Hz, H phenyl), 6.96 ppm (1H, d, J = 2 Hz, H phenyl); 7.20 ppm (2H, d, J = 8 Hz, two H phenyl); 7.37 ppm (2H, d, J = 8 Hz, two H phenyl), 7.69 and 7.75 ppm (1H, s, and 1H, s, two H methylidene). 13C-NMR (CDCl3, 125 MHz), δ: 21.5 ppm (1C, 4-CH3-Ar), 23.5, 28.5, and 28.8 ppm (3C, three CH2 cyclohexanone), 45.9 ppm (1C, 4-CH3-N-piperazine), 52.5 and 54.9 ppm (4C, -N-CH2CH2-N- piperazine), 56.1 ppm (1C, ArCH2-N), 61.2 ppm (1C, 3-CH3-O), 113.7, 121.1, 123.9, 127.2, 129.2, 130.5, 137.5, and 138.8 ppm (8C, CAr), 133.4, 133.8, 135.6, and 136.7 ppm (4C, -C=C- methylidene), 147.8 and 148.47 ppm (2C, C-O), 190.2 ppm (1C, carbonyl) (Silverstein et al., 2005). Calcd masses for C28H34N2O3 : 446.2569, HR-ESI-MS (m/z) found 447.2652 ([M+H]+).
Cytotoxicity evaluation
Screening
The synthesized compounds (3a–f) was screened for their cytotoxic activity against five cancer cell lines: estrogen-dependent breast carcinoma (MCF-7), Colon carcinoma (WiDr), cervix carcinoma (HeLa), lung carcinoma (A549), and hepatoma (PLC/PRF/5) and one normal cell lines: normal liver (Chang Liver) using the methyl thiazolyl tetrazolium (MTT) method conducted according to the protocol of MTT Assay for cell viability reported earlier (Stockert et al., 2012). The cell lines were purchased from American Type Culture Collection, the cells were grown with a density of 5,000 cells in 100 µl growth media consisting of Roswell Park Memorial Institute 1640, Dulbecco's Modified Eagle's Medium (D-MEM), Fetal Bovine Serum (FBS) 5%, Penicillin 100 U/ml, and Streptomycin 100 µg/ml. After 50% confluent cell (24 hours), the tested compounds and 5-fluorouracil (positive control) solutions were added to each well to the final concentration of 12.5 µg/ml. The MTT test was carried out on day 3. The culture medium was replaced by complete D-MEM and then added 10 µl of a fresh solution of MTT (5 mg/ml). After the cells were incubated for 4 hours at 37°C, the medium was removed and the culture was washed with phosphate buffer saline. The dissolved formazan product in ethanol was measured spectrophotometrically at 595 nm. The experiment was conducted in triplicate. The formula used to calculate the percentage of proliferation inhibition:
At, Ab, and Ac = Absorbance of test, blank, and control solution
The compounds showed growth inhibition against cancer cells more than 80% and the ratio between the inhibition to cancer and normal cells more than 1.5 were continued to determine the IC50 values.
IC50 determination
The selected cancer cells and Chang cells were grown with a density of 5,000 cells in 100 µl growing media consisting of D-MEM, FBS 5%, Penicillin 100 U/ml, and Streptomycin 100 µg/ml. After the cell reaches 50% confluent (24 hours), a series of concentrations of selected compounds, 5-fluorouracil and curcumin solutions was added to each well to the final concentration of 1.56–100 µg/ml. Furthermore, the MTT test was carried out as described in the screening.
.png) | Scheme 1. Synthesis of the target compounds [Click here to view] |
The IC50 values were obtained by analyzing the relationship between the concentrations of the tested compounds and their percent (%) inhibitions using GraphPad Prism 7 (La Jolla, CA, www.graphpad.com). The ratio between the IC50 value of the compounds in normal cells and selected cancer cells shows the value of the selectivity index (SI).
RESULTS AND DISCUSSION
Chemistry
A series of new aminomethyl derivatives of methyl-substituted asymmetrical curcumin mono-carbonyl (3a–d) were synthesized stepwise summarized in Scheme 1 in a good yield. The FTIR spectra of 3a–d showed the appearance of C-O-C and C-N bands at 1,155–1,271 cm−1 and the disappearance of OH phenolic group. In the 1H-NMR spectra, the two singlet peaks at 2.34–2.37 and 3.85–3.90 ppm (3H) correspond to protons of methyl groups of Ar-CH3 and Ar-OCH3, respectively. While the protons of methylene group linking the amine to the phenyl ring appeared as a singlet peak at 3.72–3.90 ppm. The two protons of the two methylidene chain (1H, respectively) appeared as two singlet peaks and more downfield in range of 7.64–7.71 ppm indicated that the structures of the synthesized compounds are asymmetrical and E-configuration (Silverstein et al., 2005). Furthermore, the structures were completed with 13C-NMR and HR-MS data, which showed the full conformity of the structures assigned.
Cytotoxicity and selectivity
The synthesized compounds were screened against five cancer cell lines: MCF-7, WiDr, HeLa, A549, and PLC/PRF/5 and one normal cell lines: Chang Liver using MTT assay at a final concentration of 12.5 µg/ml. The results showed that all the synthesized compounds (3a–f) exhibited high cells growth inhibition (more than 80%) against WiDr cells lines, but only compounds 3a–e had high cytotoxic activity against MCF-7 cells lines, and only compound 3b showed high cytotoxic activity against HeLa, A549, and PLC/PRF/5 cell lines. Unfortunately, compound 3b and 3c exhibited high cells growth inhibition against Chang Liver (normal liver) cells lines (Table 1). Based on the above screening’s results, then further anticancer potential evaluation only performed for compounds 3a, 3d, 3e, and 3f by IC50 values determination. Compounds 3a, 3d, and 3e were evaluated against MCF-7 and WiDr cells lines, while compound 3f was evaluated against WiDr cells lines. Curcumin and 5-fluorouracil were used as compared and positive control. The compounds also were tested against Chang Liver cell lines to evaluate their selectivity. The results showed that all the compounds possessed better cytotoxic activity against MCF-7 and WiDr cells lines than curcumin and 5-fluorouracil (Table 2, Fig. 2). The low cytotoxic activity of 5-fluorouracil indicated that MCF-7 and WiDr cells lines have been resistance to the compound (Chibaudel et al., 2008). Compounds 3a, 3d, and 3e exhibited moderate-to-high cytotoxicity against MCF-7 cells lines, (IC50 values = 4.18, 18.29, and 15.85 µM), but no one of the compounds showed high selectivity index (SI= 0.43, 1.38, and 0.94). These results were consistent to reported previously (Prasetyaningrum et al., 2018). Compounds 3a, 3d, 3e, and 3f exhibited high cytotoxicity against WiDr cells lines (IC50 values = 3.98, 5.70, 5.55, and 2.97 µM), but compound 3a was not selective (SI = 0.45), while compounds 3d, 3e, and 3f showed moderate-to-high selectivity index (SI = 4.43, 2.69, and 2.04).
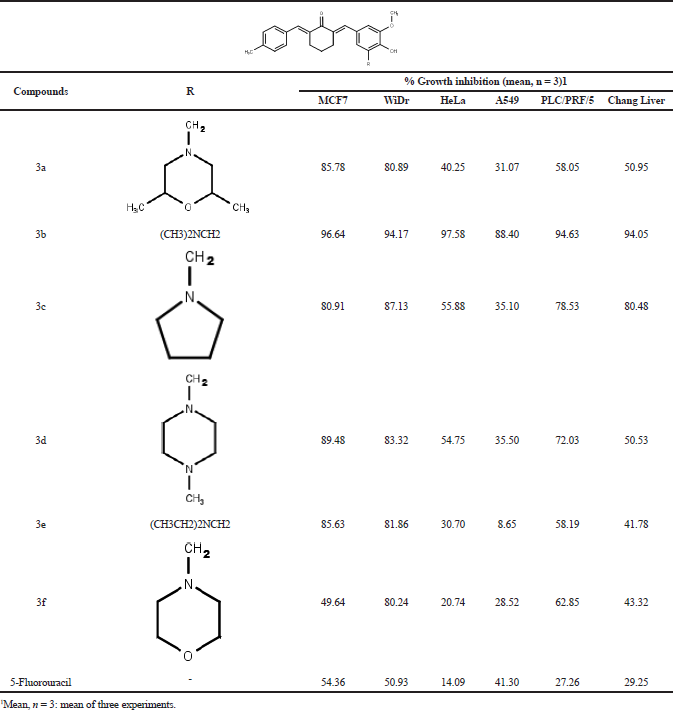 | Table 1. The percentage of growth inhibition (% GI) of the various cell lines due to the synthesized compounds (3a–f) at 12,5 µg/ml. [Click here to view] |
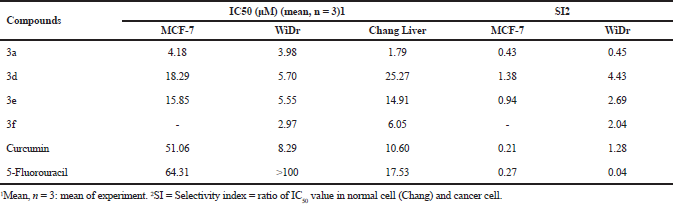 | Table 2. The cytotoxicity (IC50 values) of compound 3a, 3d, 3e, 3f, curcumin, and 5-fluorouracil against MCF-7, WiDr, and Chang Liver cells [Click here to view] |
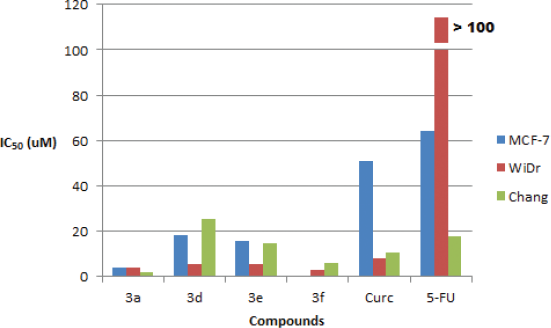 | Figure 2. Cytotoxicity of compounds 3a, 3b, 3e, and 3f, curcumin (Curc) as a comparative compound, and 5-fluorouracil (5-FU) as a positive control, against MCF-7, WiDr, and Chang Liver cells. 3f was not tested against MCF-7 cells [Click here to view] |
The standard used previously for pure compounds considered to be further tested as anticancer agents in preclinical tests using experimental animals should possess IC50 values equal or less than 10 µM (4 ppm) in cell cultures with SI value more than 2 (Burger and Fiebig, 2004). Therefore, compounds 3d, 3e, and 3f were potential as an anticancer agent for colorectal carcinoma and fulfilled the requirements for further evaluated in vivo pre-clinical studies. The compounds should also be further study to explore their mechanism action for justifying their cytotoxic activity.
CONCLUSION
A series of new aminomethyl derivatives of methyl-substituted asymetrical curcumin mono-carbonyl was successfully synthesized. The synthesized compounds exhibited low to high cytotoxicity against MCF-7, WiDr, HeLa, A549, and PLC/PRF/5 cells. Further evaluations showed that compound 3d, 3e, and 3f exhibited a potent and selective cytotoxic agent (IC50 < 10 µM, SI > 2) against colorectal carcinoma (WiDr) cells. The compounds should be considered for further evaluation for investigating their mechanism of action and their effectivity in vivo pre-clinical studies.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Research, Technology, and Higher Education, Republic of Indonesia for the ï¬nancial support (PDUPT Research Grant, 2018), and to Chemistry Study Program, Institut Teknologi Bandung (ITB), Indonesia for recording NMR and mass spectra.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem, 2004; 12(14):3871–83. CrossRef
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol, 2008; 76:1590–161. CrossRef
Bala S, Sharma N, Kajal A, Kamboj S, Saini V. Mannich bases: an important pharmacophore in present scenario. Int J Med Chem, 2014; 2014:1–15. CrossRef
Biersack B, Ahmed K, Padhye S, Schobert R. Recent developments concerning the application of the Mannich reaction for drug design. Expert Opin Drug Discov, 2018; 13(1):34–9. CrossRef
Burger AM, Fiebig HH. Preclinical screening for new anticancer agents. In: Figg WD, McLeod HL (eds.). Handbook of anticancer pharmacokinetics and pharmacodynamics, cancer drug discovery and development, Humana Press Inc., Totowa, NJ, pp 36–7, 2004.
Chibaudel B, Tournigand C, André T, de Gramont A. Therapeutic strategy in unresectable metastatic colorectal cancer. Ther Adv Med Oncol, 2012; 4(2):75–89. CrossRef
Dimmock JR, Advikolanu KM, Scott HE, Duffy MJ, Reid RS, Quail JW, Jia Z, Hickie RA, Allen TM, Rutledge JM. Evaluation of cytotoxicity of some Mannich bases of various aryl and arylidene ketones and their corresponding arylhydrazones. J Pharm Sci, 1992; 81(12):1147–52. CrossRef
GraphPad Software, Inc., 2017. Available via www.graphpad.com (Accessed 13 October 2017).
Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharm, 2017; 174:1325–48. CrossRef
Liang G, Shao L, Wang Y, Zhao C, Chu Y, Xiao J, Zhao Y, Li X, Yang S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem, 2009; 17:2623–31. CrossRef
Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Synthesis and biological analysis of new curcumin. Mol Cancer Ther, 2006; 5(10):2563–71. CrossRef
Prasetyaningrum PW, Bahtiar A, Hayun H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci Pharm, 2018; 86(2):25. CrossRef
Press Release No. 263. International Agency for Research Cancer (IARC), World Health Organization, Geneva, Switzerland, 2018. Available via https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf. (Accessed 26 December 2018).
Putri TN, Bachtiar A, Hayun H. Synthesis, antioxidant, and anti-inflammatory activity of morpholine Mannich base of AMACs ((2E, 6E)-2-({4-hydroxy-3-[morpholin-4-yl-) methyl]phenyl}methylidene)-6-(phenylmethylidene)cyclohexan-1-one) and its analogs. J App Pharm Sci, 2018; 8(05):019–25.
Roche VF. Cancer and chemotherapy. In: Lemke TL, Williams DA, Roche VF, Zito SW (eds.). Foye’s principles of medicinal chemistry. 11th edition, Lippincott Wiliams and Wilkins, Baltimore, MD, pp 1199–266, 2013.
Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem, 2015; 89:743–816. CrossRef
Silverstein RM, Webster FX, Kiemle DJ. Spectrometric identification of organic compounds. 7th edition, John Wiley & Sons, Inc., New York, NY, 2005.
Song Y, Wang P, Wu JJ, Zhou X, Zhang XL, Weng LH, Cao X, Liang F. Biological studies of photoinducible phenol quaternary ammonium derivatives. Bioorg Med Chem Lett, 2006; 16:1660–4. CrossRef
Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1.Cell Cycle, 2007; 6(23):2953-61. CrossRef
Stockert JC, Blázquez-Castroa A, Canete M, Horobinb RW, Villanuevaa A. MTT assay for cell viability: intracellular localization of the formazan product is in lipid droplets. Acta Histochem, 2012; 114:785–96. CrossRef
Subramaniapillai, SG. Mannich reaction: a versatile and convenient approach to bioactive skeletons. J Chem Sci, 2013; 125(3):467–82. CrossRef
Untung J, Iskandarsyah I, Hayun H. 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)-2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxyphenyl} ethenyl]-1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol. Molbank, 2017; 3:M949. CrossRef
Yerdelen KO, Gul HI, Sakagami H, Umemura N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem, 2015; 30(3):383–8. CrossRef