Bioequivalence studies on some selected brands of ciprofloxacin hydrochloride tablets in the Nigerian market with ciproflox® as innovator brand
Osonwa Uduma E., Agboke Ayodeji A., Amadi Rosemary C., Okorie, Ogbonna, Opurum Christian C
Pages: 80-84

In-vitro bioequivalence study of 8 brands of metformin tablets in Iran market
Parvin Zakeri-Milani, Peyman Nayyeri-Maleki, Saeed Ghanbarzadeh, Mahboob Nemati, Hadi Valizadeh
DOI: 10.7324/JAPS.2012.2834Pages: 194-197

Design of dissolution media for in-vitro bioequivalence testing of Lamivudine
Nagiat T Hwisa, Shanta Kumari Adiki, Prakash Katakam, Babu Rao Chandu
DOI: 10.7324/JAPS.2013.3617Pages: 106-110

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: Impact on generic drug substitution
Nitika Kaushal, Sachin Kumar Singh, Monica Gulati, Yogyata Vaidya, Munish Kaushik
DOI: 10.7324/JAPS.2016.60430Pages: 206-222

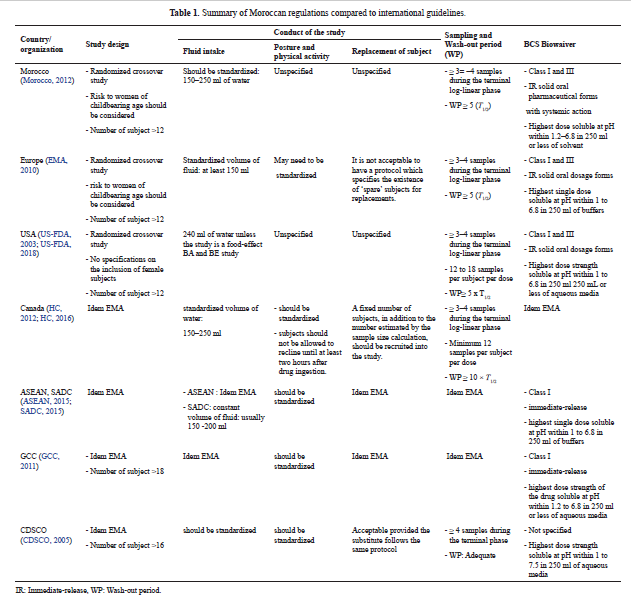
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035
Factors affecting purchasing behaviors of generic drugs versus originator counterparts in Jordan
Maha N. Abu Hajleh, Ali AL-Samydai, Zahraa Aloosi, Raghad Abuhamdan, Sumaiah Al.Naimat, Lina Abdelfattah, Lidia Al-Halaseh
DOI: 10.7324/JAPS.2021.110902Pages: 009-017

Model integrated evidence approach for rational and safe formulation development: case of alfuzosin prolonged-release tablets
Sivacharan Kollipara, Rajkumar Boddu, Suribabu Bonda, Hariharan Venugopal, Chandra Deb, Pavan Kumar Mittapalli, Anand Arumugam, Sohel Mohammed Khan, Venkat Ramana Naidu
DOI: 10.7324/JAPS.2025.231215Pages: 253-264
_.webp)