Generic competition and drug prices in the Malaysian off-patent pharmaceutical market
Omotayo Fatokun, Mohamed Izham Mohamed Ibrahim, Mohamed Azmi Hassali
Pages: 33-37

Community pharmacist's perceptions towards the quality of locally manufactured generic medicines: A descriptive study from Malaysia
Mohamed Azmi Hassali, Asrul Akmal Shafie, Chee Ping Chong, Fahad Saleem, Muhammad Atif, Ginnie Chua, Noman Ul Haq
Pages: 56-60

Current Situation of Availability and CostEffectiveness Analysis of Selected Drugs inAnantapur, AP, India
Meenu Singh, Y. Pragzna, Dharma Dev Bommi
DOI: 10.7324/JAPS.2012.2616Pages: 179-185

Generic Substitution in Malaysia: Recommendations from a Systematic Review
Mohamed Azmi Hassali, Jayabalan Thambyappa, Fahad Saleem, Noman ul Haq, Hisham Aljadhey
DOI: 10.7324/JAPS.2012.2827Pages: 159-164

Economic evaluation of antibiotic prescriptions: a cost minimization analysis
L. Ramesh
DOI: 10.7324/JAPS.2013.3627Pages: 160-163

An Exploratory Study on the Drug Utilization Pattern in Glaucoma Patients at A Tertiary Care Hospital
Pooja Prajwal, Mohandas Rai, H N Gopalakrishna, Ramya Kateel
DOI: 10.7324/JAPS.2013.31027Pages: 151-155

Evaluation of the quality and stability of amoxicillin oral suspension
Blanca Elena Ortega Markman, Maria Regina Walter Koschtschak, Elizabeth Wu Meihuey, Paulo Cesar Pires Rosa
DOI: 10.7324/JAPS.2014.40706Pages: 038-040

Assessment of Medical and Pharmacy Students’ Knowledge & Perceptions about Generic Medicines’ Prices & Quality in Kabul- Afghanistan
Mohammad Bashaar, Mohamed Azmi Hassali, Fahad Saleem, Asrul Akmal Shafie
DOI: 10.7324/JAPS.2015.50816Pages: 100-104

Pilot Study of Quality of Diclofenac Generic Products Using Validated In-House Method: Indian Drug Regulatory Concern
Ahmed Nawaz Khan, Roop Krishen Khar, Malairaman Udayabanu
DOI: 10.7324/JAPS.2015.501226Pages: 147-153

A descriptive cross-sectional study to evaluate the Generic Drug User Fee Act: A boon or loss to the Indian generic pharmaceutical industry
Sarika Prasanna Pardhe
DOI: 10.7324/JAPS.2019.90206Pages: 044-051
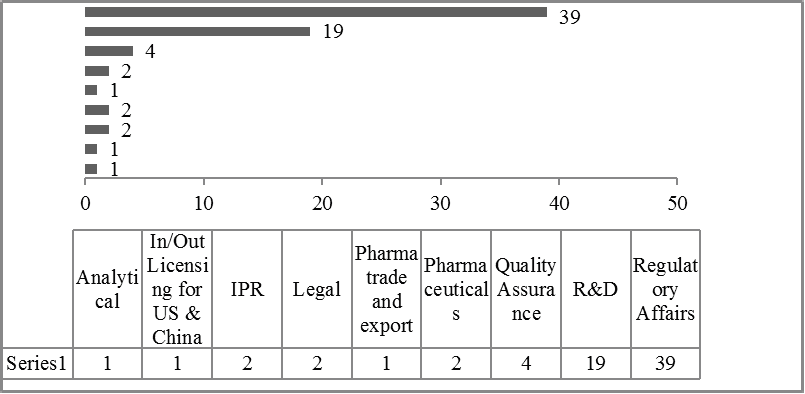
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035
Validation of methodology for assay, pharmaceutical equivalence, and comparative dissolution profile for tablets containing amlodipine besylate
Renata Micheli Martinez, Jenifer Freitas da Silva, Larissa Regina Jorge, Rhye Lessa Ishikawa, Ana Paula Novelli, Talita Laiane Cardoso Cezar, Sandra Regina Georgetti, Marcela Maria Baracat, Rúbia Casagrande
DOI: 10.7324/JAPS.2019.91112Pages: 093-100

Factors affecting purchasing behaviors of generic drugs versus originator counterparts in Jordan
Maha N. Abu Hajleh, Ali AL-Samydai, Zahraa Aloosi, Raghad Abuhamdan, Sumaiah Al.Naimat, Lina Abdelfattah, Lidia Al-Halaseh
DOI: 10.7324/JAPS.2021.110902Pages: 009-017

A deep dive into the development of complex generics: A comprehensive review
Amatha Sreedevi, Prashant B. Musmade, Krishnamurthy Bhat, Sreedhar Dharmagadda, Manthan D. Janodia, Bhavana B. Bhat, Virendra S. Ligade
DOI: 10.7324/JAPS.2024.204337Pages: 001-014
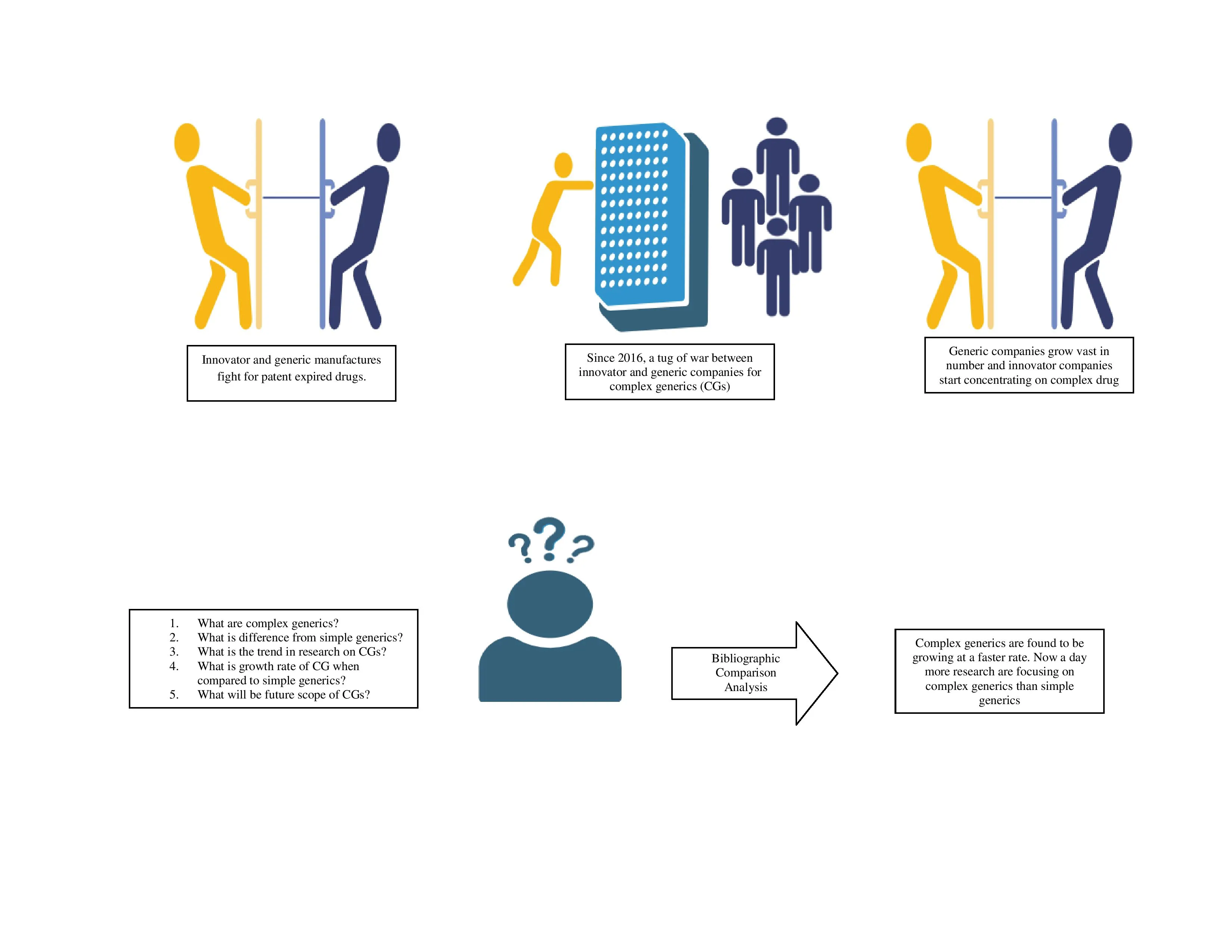
Exploring the market dynamics of complex generics in United States and India
Amatha Sreedevi, Vani Lakshmi Ramesh, Manthan D. Janodia, Praveen Kumar, Virendra S. Ligade
DOI: 10.7324/JAPS.2025.v15.i12.15Pages:
_.jpg)