Noveon AA1 as enhancer of HPMC as a direct compression matrix for controlled release
Sara Laguna-López, Leopoldo Villafuerte-Robles
DOI: 10.7324/JAPS.2014.41111Pages: 062-068

Influence of different types of lactose on powder flow and tablets dissolution
Karen Velázquez-González, Eduardo Ramírez-Flores, Leopoldo Villafuerte-Robles
DOI: 10.7324/JAPS.2015.50916Pages: 089-096

Functionality of Benecel K4M/Carbopol 971P NF matrices in direct compression tablets for controlled release
Leopoldo Villafuerte-Robles, Zeltzin Michelet Aguilar-Hernández
DOI: 10.7324/JAPS.2016.60901Pages: 001-008

In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia
Ahmed F. Hanafy
DOI: 10.7324/JAPS.2016.60909Pages: 063-068

Physicochemical Characterization of Physical Mixture and Solid Dispersion of Diclofenac Potassium with Mannitol
Yong K. Han, Sonia N. Faudone, Gustavo Zitto, Silvina L. Bonafede, María A. Rosasco, Adriana I. Segall
DOI: 10.7324/JAPS.2017.70130Pages: 204-208

Xanthine Oxidase Inhibitor Febuxostat: Quality Comparisons and Release Kinetic Profile
Saquib M. Qureshi, Farya Zafar, Huma Ali, Shazia Alam, Yusra Shafiq, Sohail Khan, Saba A. Baloch, Kashif Maroof
DOI: 10.7324/JAPS.2017.70231Pages: 223-227

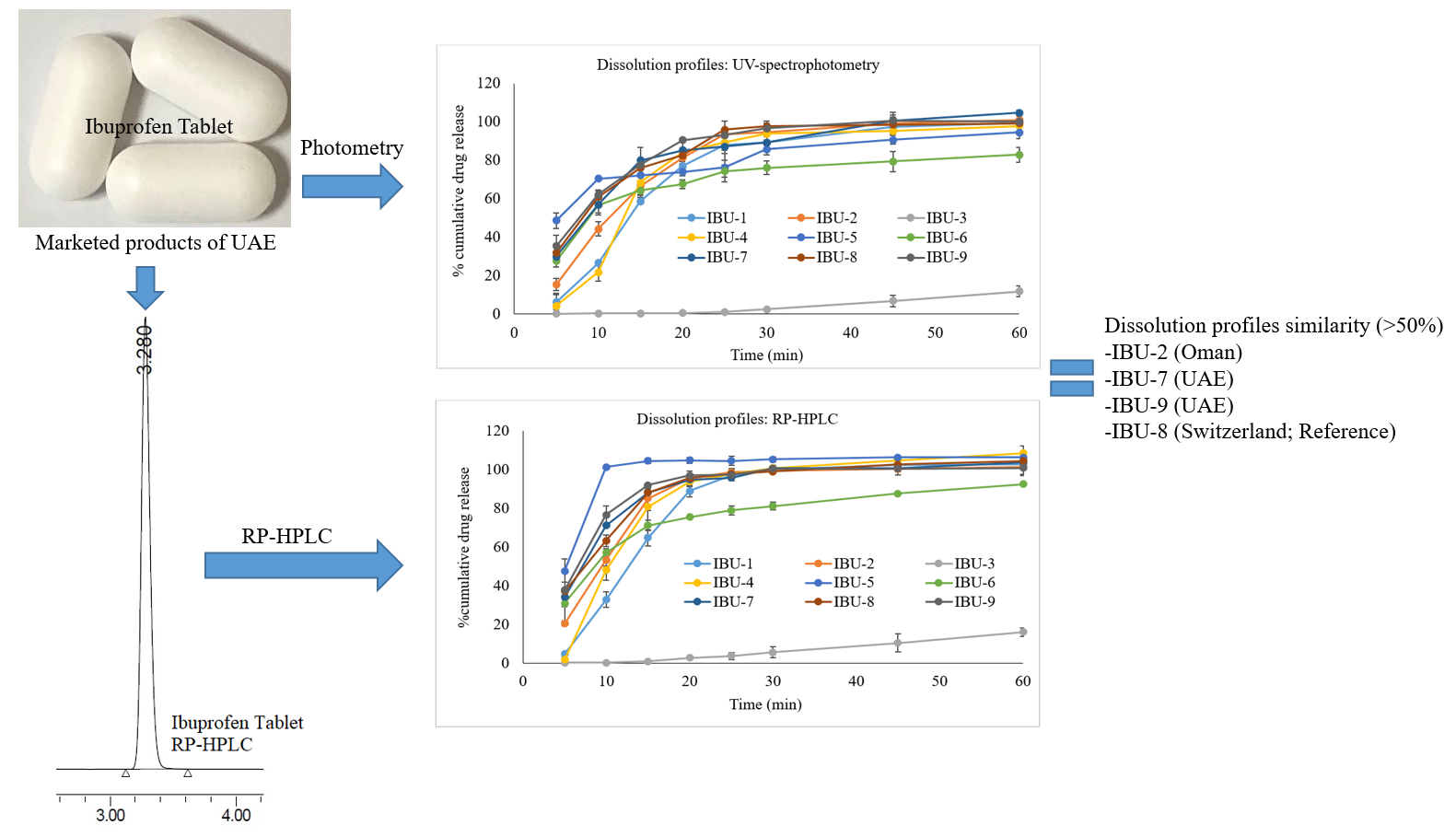
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
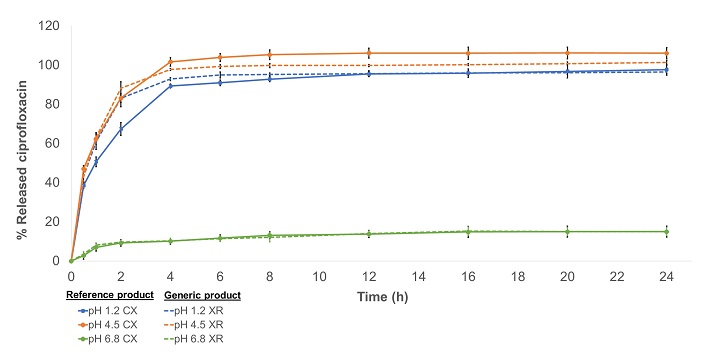
Comparison between the dissolution profiles of prolonged-release ciprofloxacin tablets available in the Colombian market
Andrés Vicente De la Cruz Gómez, Raynni Marcela Ramos Iglesias, Tatiana Sugey Ruiz Afanador, Indira Beatriz Pájaro Bolívar, Gina Paola Domínguez Moré
DOI: 10.7324/JAPS.2022.120322Pages: 209–217