Development and validation of RP-HPLC method for pitavastatin calcium in bulk and formulation using experimental design
Vinodkumar D. Ramani, Girish K. Jani, Ashim Kumar Sen, Girish U. Sailor, Vijaykumar B. Sutariya
DOI: 10.7324/JAPS.2019.91010Pages: 075-083

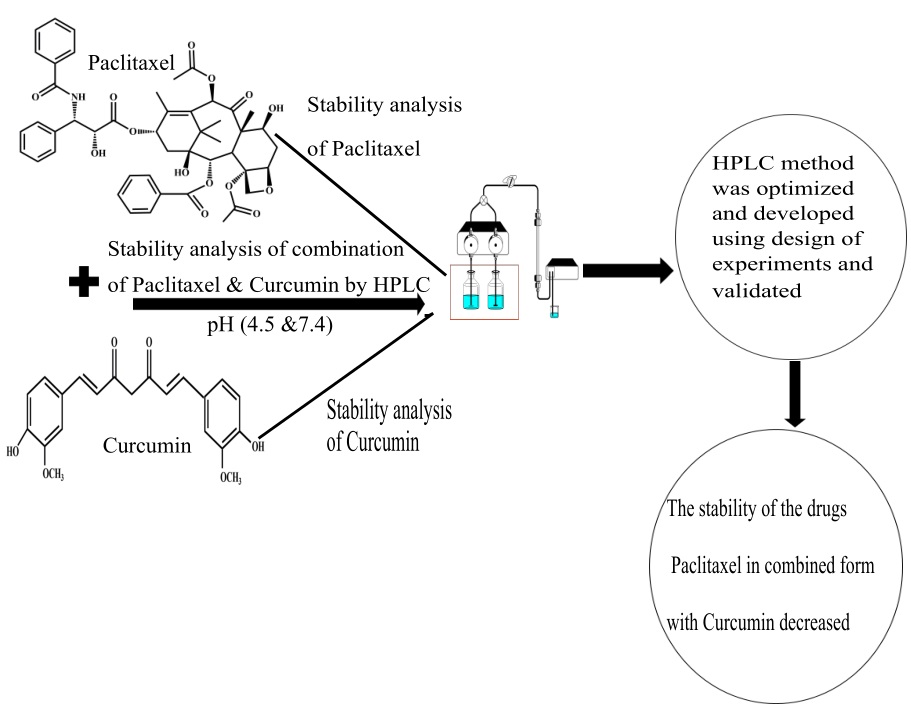
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
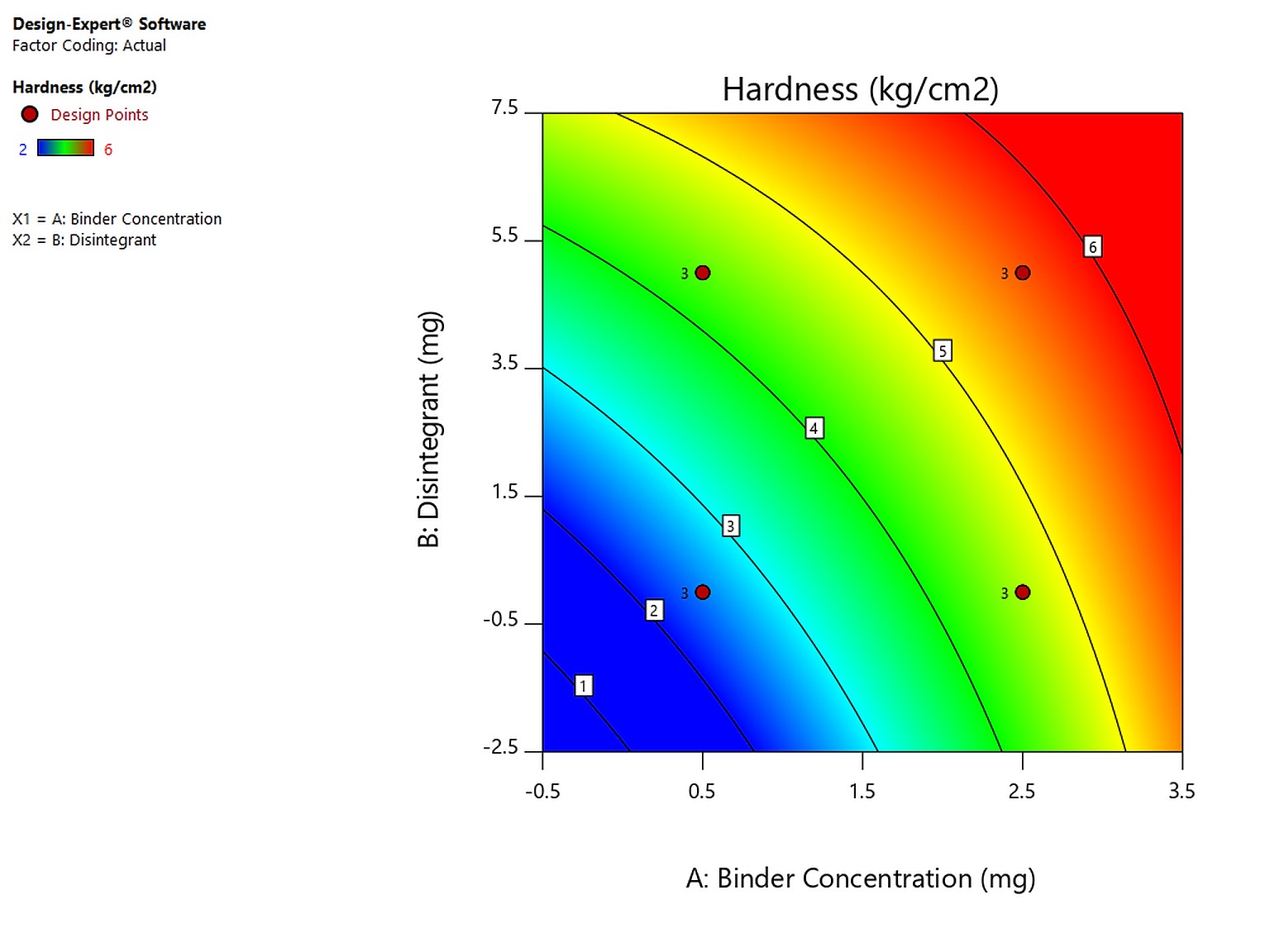
The development and process optimization of atorvastatin calcium and Naringin bilayer tablet to improve the bioavailability of atorvastatin calcium by two-level factorial design using Design-Expert®
Dhanish Joseph, Suseem Sundaram Renjitham
DOI: 10.7324/JAPS.2021.110608Pages: 070-077
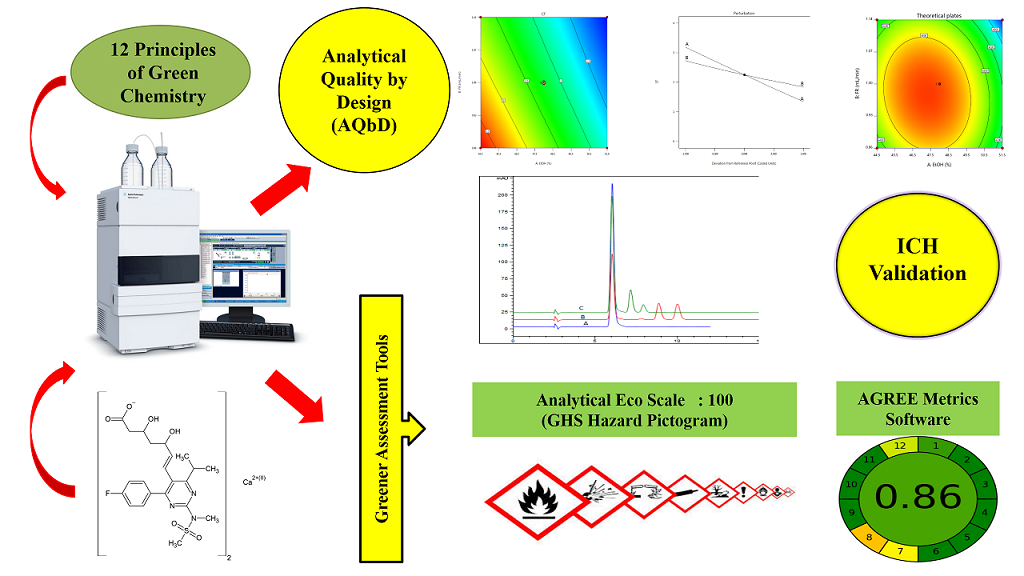
Analytical quality by design approach for estimating rosuvastatin calcium in pharmaceutical formulation by green HPLC method: Ecologically evaluated and stability-indicating
Seetharaman Rathinam, Lakshmi Karunanidhi Santhana
DOI: 10.7324/JAPS.2021.1101119Pages: 150-160
Design of experiment based formulation optimization of chitosan-coated nano-liposomes of progesterone for effective oral delivery
Prachi Dehariya, Reena Soni, Suresh Kumar Paswan, Prakash Kumar Soni
DOI: 10.7324/JAPS.2023.142351Pages: 256-270
.jpg)
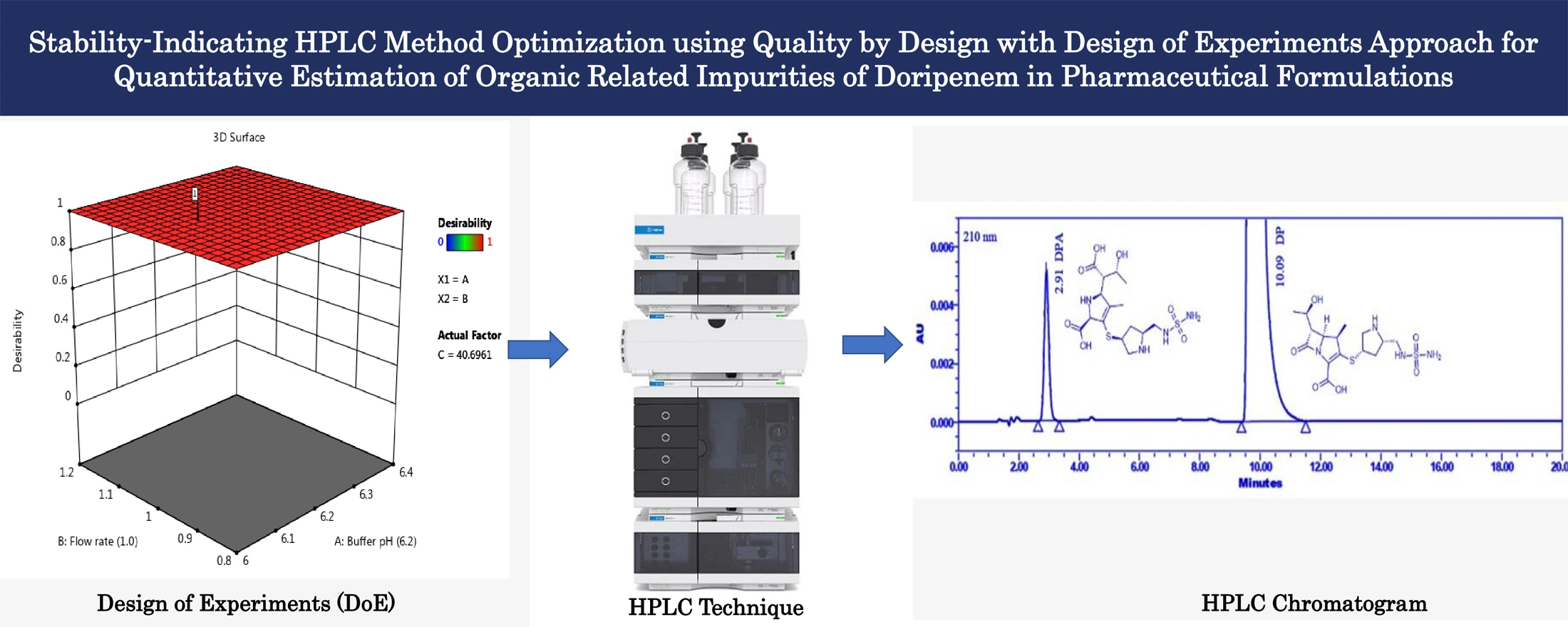
Stability-indicating HPLC method optimization using quality by design with design of experiments approach for quantitative estimation of organic related impurities of Doripenem in pharmaceutical formulations
N. V. V. D. Praveen Boppy, Sharath Babu Haridasyam, Niroja Vadagam, Naveen Sara, Karthik Sara, Eswarlal Tamma
DOI: 10.7324/JAPS.2024.190386Pages: 114-126