INTRODUCTION
Diabetes mellitus represents a complex array of metabolic disorders characterized by hyperglycemia and disturbances in the metabolism of carbohydrates, lipids, and proteins. This condition can lead to significant macrovascular complications, including coronary heart disease, cerebrovascular accidents, and peripheral vascular disease. Furthermore, diabetes may induce microvascular complications such as neuropathy, which, if left untreated, can diminish nerve sensitivity. This reduction in sensitivity subsequently heightens the risk of developing gangrenous injuries [1].
Diabetic wounds are lesions that exhibit partial or full-thickness damage to the skin, potentially extending into underlying tissues, including tendons, muscles, bones, and joints. Inadequately treated diabetic wounds may progress to necrosis and ultimately result in gangrene. The wound-healing process is traditionally divided into four phases: hemostasis, inflammation, proliferation, and maturation. These phases are regulated by cellular components, the extracellular matrix, cytokines, and growth factors. However, diabetic wounds frequently fail to advance through these standard phases, rendering them susceptible to chronicity [2,3].
The coconut tree (Cocos nucifera L.) is extensively cultivated and utilized throughout Indonesia; however, the coconut shell remains underutilized within local communities. Composed of cellulose, hemicellulose, and lignin, coconut shells can be converted into liquid smoke through pyrolysis. This liquid smoke is abundant in phenolic compounds exhibiting potent antioxidant properties, effectively neutralizing deleterious free radicals, such as reactive oxygen species (ROS) [3,4].
Furthermore, coconut shell liquid smoke has shown promising results in inhibiting the production of nitric oxide (NO), the secretion of tumor necrosis factor-alpha (TNF-α)—an inflammatory cytokine—and the activation of nuclear factor kappa beta (NF-κB). As a result, it stimulates fibroblast proliferation, enhances collagen synthesis, promotes re-epithelialization, and facilitates angiogenesis. These mechanisms underscore the potential of coconut shell liquid smoke as a topical agent for wound treatment, significantly promoting the growth of fibroblasts, collagen, and capillaries. This discovery opens up new possibilities for wound care, offering hope for more effective treatments in the future [5].
The present study selected hydrogel due to its advantageous properties in wound management. Hydrogel assists in maintaining hydration within the wound environment, absorbs exudate, facilitates necrotic tissue removal, and safeguards the wound against microbial contamination. Collectively, these characteristics contribute to the acceleration of the wound-healing process [6].
This study aims to evaluate the efficacy of hydrogel infused with DLS derived from coconut shells in enhancing healing in diabetic wounds, which are characteristically associated with delayed recovery. Previous studies have substantiated the effectiveness of DLS from coconut shells in treating burn wounds. This study will utilize immunohistochemical and histopathological analyses to examine the effects of wound healing on excisional wounds in diabetes-induced Wistar rats.
MATERIALS AND METHODS
Equipment and materials
The equipment used in this experiment included an analytical balance (Mettler Toledo), cover slides, a digital camera (Olympus DP20), a distillation set, a glucometer, glucostrips (Easy Touch), image analysis software (Olympus DP2-BSW), laboratory glassware, microscopes (Olympus BX51, Olympus CX31, Zeiss Primostar), a microtome (Leica Biosystems), a plastic container for tissue, a pyrolysis set, surgical scalpels with blade no. 11, a tissue cassette, and a tissue mould.
The materials utilized in this experiment consisted of bovine serum albumin 5%, citrate buffer, coconut shell liquid smoke hydrogel, Dako labeled streptavidin-biotin (LSAB) System-horseradish peroxidase (HRP), Diaminobenzidine (DAB), Entellan, ethanol, NF-κB Rel A Rabbit antibody (ABclonal), Hematoxylin-Eosin, 3% H2O2, ketamine HCl, liquid paraffin, 0.9% NaCl, nicotinamide, phosphate-buffered saline (PBS) (pH 7.4), Strep Avidin HRP (SA-HRP), streptozotocin, a syringe, TNF-α Rabbit antibody (ABclonal), Vaseline, and xylene.
The coconut shells (C. nucifera L.) were purchased from a local market in Medan, Indonesia, in June 2022. Their identification and verification, confirming they belong to the genus Cocos, were conducted by Herbarium Medanense in Medan, Indonesia, under reference number 207/MEDA/2022.
Pyrolysis, distillation, and characterization of liquid smoke
The coconut shells were thoroughly cleaned and cut into small pieces. These fragmented coconut shells were then placed into a pyrolysis furnace with a heating stove and an outer reactor. A pipeline connected the furnace to cooling tubes, which condensed the fumes produced during the combustion of the coconut shells, resulting in grade 3 liquid smoke. The pyrolysis process was conducted at a temperature of 400°C for 255 minutes. The yield percentage of the liquid smoke was calculated using the following formula:
The grade 3 liquid smoke was distilled at 150°C to produce the grade 2 liquid smoke. This process was designed to eliminate tar and benzopyrene, recognized carcinogens, from the liquid smoke, ensuring its safety [4,7]. The yield percentage of the DLSwas then calculated using the following formula:
The grade 1 liquid smoke was analyzed using gas chromatography-mass spectrometry (GC-MS) and Fourier transform infrared spectroscopy (FTIR) to determine the compounds’ characteristics.
Formulation of hydrogel
Table 1 outlines the hydrogel formulation. To prepare the hydrogel, start by heating distilled water to 70°C. In a mortar, stir up HPMC, nipagin, and nipasol in the heated distilled water to create the gel base. Mix chitosan with grade 1 liquid smoke in a second mortar until a homogeneous mixture is achieved, then add glycerin. Once this mixture is ready, incorporate the gel base from the first mortar into the second mortar and gently stir until fully homogenized. Finally, distilled water is added to reach a total weight of 100% for the hydrogel.
Table 1. Formula of the hydrogel.
| Ingredients | Concentration (%) | |||
|---|---|---|---|---|
| F0 | F1 | F2 | F3 | |
| Grade 1 liquid smoke | - | 25 | 50 | 75 |
| Chitosan | 2.5 | 2.5 | 2.5 | 2.5 |
| Hydroxypropyl Methylcellulose (HPMC) | 2 | 2 | 2 | 2 |
| Glycerin | 5 | 5 | 5 | 5 |
| Nipagin | 0.18 | 0.18 | 0.18 | 0.18 |
| Nipasol | 0.01 | 0.01 | 0.01 | 0.01 |
| Distilled water ad | 100 | 100 | 100 | 100 |
Animals
Ethical approval for this study was obtained from the Ethical Clearance of Animal Research Committee, Faculty of Mathematics and Natural Sciences, University of Sumatra Utara, Medan, under registered number 0430/KEPH-FMIPA/2023.
The experimental subjects were male Wistar rats aged 2 to 3 months, weighing 150 to 300 g. The animal experiments were conducted in the Pharmacology and Toxicology Laboratory, Faculty of Pharmacy, University of Sumatra Utara, Medan. The Wistar rats underwent a week of acclimatization before undergoing treatment in cages, during which unlimited food and water were provided.
Diabetic induction in the rats
A total of 72 Wistar rats were administered nicotinamide at a dosage of 100mg/kg through intraperitoneal injection for 15 minutes, followed by the administration of streptozotocin at a dosage of 50 mg/kg through intraperitoneal injection. Nicotinamide was administered at a dosage of 100 mg/kg, followed 15 minutes later by streptozotocin at a dosage of 50 mg/kg. Nicotinamide was prepared by dissolving it in a 0.9% NaCl solution to achieve a concentration of 5 g per 100 ml. Streptozotocin was dissolved in a 0.1 M citrate buffer (pH 4.5) to achieve a concentration of 1 g per 100 ml. Before induction, the Wistar rats underwent an overnight fasting period. Diabetes mellitus was confirmed seven days after streptozotocin administration, based on fasting blood glucose levels exceeding 126 mg/dl, as measured with a glucometer [8,9].
Wound inflicted in the diabetic rats
Once the Wistar rats were diagnosed with diabetes mellitus, a precise incision measuring 2 cm in length and 2 mm in depth was made along the dorsal region of 18 rats using a surgical scalpel blade no. 11. Before the incision procedure, the rats were anesthetized with ketamine at a dosage of 10 mg/kg.
Wound treatment in the diabetic rats
Following the incision, therapeutic topical treatments were applied to the wounds of the Wistar rats. The topical treatments included a hydrogel base, Bioplacenton gel, and coconut shell liquid smoke hydrogel at concentrations of 25%, 50%, and 75%.
Immunohistochemistry and histopathology analysis
Biopsy procedures were performed 3, 7, and 14 days after treatment. The biopsied samples were immediately immersed in a 10% neutral buffered formalin solution to prevent tissue degradation. The fixed tissues were subsequently subjected to immunohistochemical analysis to evaluate the expression levels of NF-κB and TNF-α, as well as histopathological analysis to assess parameters such as macrophage activity, re-epithelialization, fibroblast proliferation, collagen synthesis, neutrophil and lymphocyte infiltration, and angiogenesis [10,11].
Statistical analysis
The data were presented as mean values and corresponding standard deviations for each measurement. Statistical analysis was conducted using SPSS software, which included One-Way Analysis of Variance and Kruskal–Wallis tests. For post hoc analysis, the Mann-Whitney U test was performed.
RESULT AND DISCUSSION
Yield of distilled coconut shell liquid smoke
Table 2 presents the yields of grade 3, grade 2, and grade 1 liquid smoke from 60 kg of coconut shells through pyrolysis. The process yielded 7,850 ml of grade 3 liquid smoke, which corresponds to a yield of 13.08% (b/b). This grade 3 liquid smoke was then distilled to produce 6,000 ml of grade 2 liquid smoke, achieving a yield of 76.43% (v/v). Furthermore, the grade 2 liquid smoke was redistilled, resulting in 3,480 mL of grade 1 liquid smoke, yielding 58.30% (v/v).
Table 2. The yield of Grade 3, Grade 2, and Grade 1 coconut shell liquid smoke.
| Grade of the liquid smoke | Yield (%) |
|---|---|
| Grade 3 | 13.08%b/b |
| Grade 2 | 76.43%v/v |
| Grade 1 | 58.30%v/v |
GC-MS and FTIR results of distilled coconut shell liquid smoke
Table 3 provides the results of the GC-MS analysis of the liquid smoke compounds derived from coconut shells (C. nucifera L.). This includes compounds with a Similarity Index (SI) greater than 95% and their respective Molecular Weights (MWs). The FTIR overlay results are illustrated in Figure 1.
Table 3. The GC-MS results of liquid smoke compounds in coconut shells (Cocos nucifera L.).
| No | Compounds | Similarity Index | Molecular Weight |
|---|---|---|---|
| 1 | Propylene Sulfide | 97 % | 74.15 g/mol |
| 2 | Diethyl Ether | 96 % | 74.12 g/mol |
| 3 | Urea | 98 % | 60.056 g/mol |
| 4 | Acetic Acid | 98 % | 60.05 g/mol |
| 5 | Propionic Acid | 97 % | 74.08 g/mol |
| 6 | 1-Hidroxy-2-Butanone | 97 % | 88.11 g/mol |
| 7 | 2-Cyclopenten-1-one | 98 % | 82.1 g/mol |
| 8 | Phenol | 98 % | 94.11 g/mol |
| 9 | Guaiacol | 98 % | 124.14 g/mol |
| 10 | 4-Methoxyphenol | 96 % | 124.14 g/mol |
| 11 | 2-Propanone | 98 % | 58.08 g/mol |
| 12 | Butyric Acid | 95 % | 88.11 g/mol |
| 13 | 2-Methyl-2-cyclopentenone | 96 % | 96.13 g/mol |
The FTIR functional group analysis of the different grades of liquid smoke revealed minimal differences across the grades. Functional groups such as phenol, carboxyl, ethanol, and aromatic compounds consistently evaporated during each purification stage because their vapor points were below 125°C. The FTIR overlay results are displayed in Figure 1.
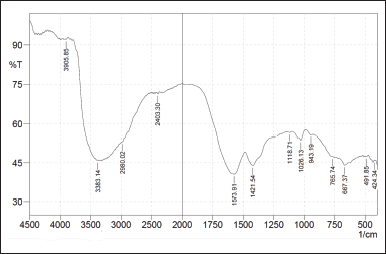 | Figure 1. Results of the FTIR functional group analysis in grade 1 liquid smoke [Click here to view] |
Notably, the FTIR functional group analysis of grade 1 liquid smoke exhibited an absorption peak at a wave number of 3383.14 cm-1, which is likely associated with the OH group. The absorption peak supports this hypothesis at 1,118.17 cm-1, corresponding to the C-O alcohol group. Additionally, a sharp absorption observed at 1,573.91 cm-1 is likely attributed to aromatic C=C bonds, further corroborated by absorptions at 2,980.02 cm-1, indicating sp² C-H bonds [12].
Wound healing percentage
Figure 2 illustrates the wound healing progression of incisive dorsal wounds treated with DLS hydrogel at concentrations of 25%, 50%, and 75%, as well as the gel base. The treatment’s healing process was monitored on Day 1, Day 3, Day 7, Day 14, and Day 21.
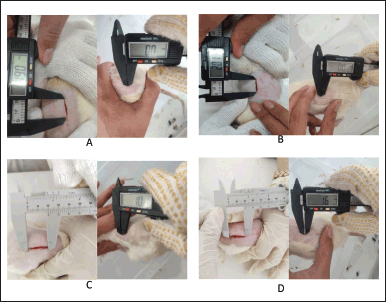 | Figure 2. Wound healing process using hydrogel on day-1 and day-21 for concentrations of 25% (A), 50% (B), 75% (C), and hydrogel base (D). [Click here to view] |
The experimental results indicate that the hydrogel containing DLS exhibits significant wound-healing potential in diabetic rats. Among the tested concentrations, the best wound-healing percentage was observed with hydrogel treatments at 25%, followed by 75% and 50%. The lowest concentration (25%) yielded the most favorable outcomes, likely due to its lower levels of acetic acid than the higher concentrations. Elevated levels of acetic acid in higher hydrogel concentrations can lead to skin irritation and a burning sensation. While higher concentrations may provide wound-healing effects, they can also increase skin irritation, which can delay healing [13]. Table 4 and Figure 3 present each treatment’s average wound healing percentages.
Table 4. Mean percentage of wound healing.
| Duration of treatment | Sample groups | |||
|---|---|---|---|---|
| Hydrogel 25% | Hydrogel 50% | Hydrogel 75% | Negative Control | |
| Day-3 | 25.29% | 5.26% | 12.62% | 19.86% |
| Day-7 | 32.09% | 30.44% | 21.52% | 42.05% |
| Day-14 | 95.90% | 87.71% | 57.67% | 94.83% |
| Day-21 | 100% | 98.61% | 97.78% | 97.33% |
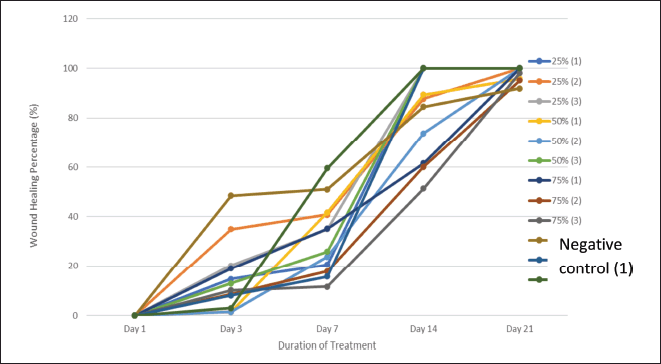 | Figure 3. Percentage of wound healing. [Click here to view] |
Based on the results of the GC-MS analysis in this study, as well as findings from previous research, the bioactive compounds in liquid smoke derived from coconut shell distillation that is potentially involved in wound healing include phenol, guaiacol, 4-methoxyphenol, propionic acid, and butyric acid. These compounds exhibit antioxidant, anti-inflammatory, and regenerative properties, contributing to wound healing.
Phenol and guaiacol act as membrane integrity agonists and NADPH oxidase inhibitors, reducing oxidative stress and promoting angiogenesis. 4-Methoxyphenol and propionic acid have been identified as inhibitors of MMP-9 expression, preventing excessive extracellular matrix degradation and enhancing fibroblast activity. Additionally, butyric acid plays a crucial role in modulating macrophage colony-stimulating factors, facilitating the transition of macrophages from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype—a key mechanism in wound healing. Previous studies also demonstrate that these compounds exhibit significant anti-inflammatory activity in vitro, particularly through mechanisms such as inhibiting protein denaturation and modulation of the inflammatory response [3,14].
Immunohistochemistry analysis
The immunohistochemical analysis yielded data based on an Immunoreactive Score (IRS), computed by multiplying the percentage of positively stained cells by the intensity score of the staining. Figures 4 and 5 depict NF-κB and TNF-α expression levels, as evidenced by the brown coloration prevalent in the cytoplasm, resulting from applying the DAB chromogen.
 | Figure 4. Expression of NF-κB. [Click here to view] |
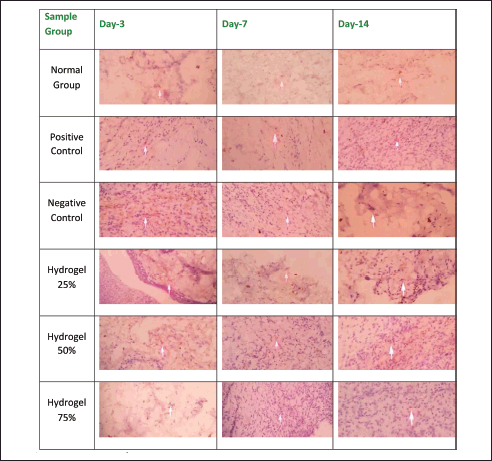 | Figure 5. Expression of TNF-α. [Click here to view] |
Table 5 presents the IRS scores corresponding to NF-κB and TNF-α expressions. Notably, the negative control group exhibited the highest NF-κB and TNF-α expression levels compared to the other experimental groups. A marked increase in expression was observed from Day 3 to Day 7 and Day 14 of treatment. This elevation in expression levels is ascribed to the prolonged inflammation in the negative control group, which was subjected solely to the hydrogel base. In contrast, the groups treated with hydrogel concentrations of 25%, 50%, and 75% exhibited a gradual reduction in both NF-κB and TNF-α expression levels. The most significant decrease in NF-κB and TNF-α expressions was observed in the group treated with 25% hydrogel, resulting in outcomes that closely mirrored those of the positive control group treated with Bioplacenton gel.
Table 5. Immunohistochemistry analysis.
| Sample groups | Normal group | Positive control | Negative control | Hydrogel 25% | Hydrogel 50% | Hydrogel 75% | |
|---|---|---|---|---|---|---|---|
| NF-κB IRS Score | Day-3 | 0.33 ± 0.33c | 1 ± 0.00c | 12 ± 0.00ab | 1 ± 0.00c | 9 ± 0.00abc | 9 ± 0.00abc |
| Day-7 | 0.33 ± 0.33c | 1 ± 0.00c | 10 ± 1.00ab | 1 ± 0.00c | 7.33 ± 1.66ab | 2 ± 1.00c | |
| Day-14 | 0 ± 0.00c | 0 ± 0.00c | 12 ± 0.00ab | 0 ± 0.00c | 1 ± 0.00abc | 1 ± 0.00abc | |
| TNF-α IRS Score | Day-3 | 0.67 ± 0.33b | 6 ± 0.00ac | 1 ± 0.00b | 4 ± 0.00abc | 6 ± 0.00ac | 4 ± 0.00abc |
| Day-7 | 0.33 ± 0.33c | 1 ± 0.00c | 2 ± 0.00ab | 4 ± 0.00abc | 2 ± 0.00ab | 2 ± 0.00ab | |
| Day-14 | 0.33 ± 0.33c | 1 ± 0.00c | 4 ± 0.00ab | 1 ± 0.00c | 1 ± 0.00c | 1 ± 0.00c | |
Information:
a. Significant difference is observed compared to normal groups (p < 0.05)
b. Significant difference is observed compared to positive control (p < 0.05)
c. Significant difference is observed compared to negative control (p < 0.05)
The findings substantiate the therapeutic efficacy of coconut shell liquid smoke hydrogel in promoting wound healing in diabetic rats. Coconut shell liquid smoke contains phenolic compounds, including guaiacol, furfural, and 4-ethyl-2-methoxyphenol, which possess hydroxyl groups capable of binding to superoxide and peroxynitrite free radicals. This binding mechanism inhibits the activation of the NF-κB kinase inhibitor (IKK), thereby preventing the degradation of the inhibitor of NF-κB (IκB) and subsequently suppressing both NF-κB and TNF-α expressions.
Results from the experiment demonstrate that the hydrogel containing DLS possesses significant wound-healing potential in diabetic rats. Among the tested concentrations, the highest wound healing percentages were realized with hydrogel treatments at 25%, followed by 75% and 50%, respectively. The 25% hydrogel concentration yielded the most favorable results; higher concentrations of DLS hydrogel included elevated acetic acid and phenols levels. Excessive concentrations of these compounds can induce skin irritation, prolonged inflammation, and tissue necrosis, impeding wound healing [15,16].
Excessively high concentrations of acids and phenolic compounds within the hydrogel, specifically at the 50% and 75% levels, resulted in irritation and inflammation, ultimately leading to necrosis of the wounded tissue. The presence of necrotic tissue prompts adjacent cells to activate inflammatory responses aimed at debris removal and facilitation of healing. Consequently, the group treated with 75% hydrogel demonstrated a more pronounced reduction in NF-κB and TNF-α expression levels than the 50% hydrogel group [12,13].
Histopathology analysis
The histopathological analysis results are presented through the enumeration of macrophages, fibroblasts, neutrophils, and lymphocytes, expressed as cell counts; re-epithelialization is quantified according to epidermal thickness (µm); and angiogenesis and collagen levels are assessed based on blood vessel count and collagen density, respectively.
Table 6 summarizes the histopathological findings based on criteria evaluating the impact of wound healing in diabetic mice. The negative control group exhibited a significant and sustained increase in macrophage count from Day 3 to Day 14, contrasting sharply with the positive control group outcomes. The hydrogel group with a concentration of 25% demonstrated a notable reduction in macrophage numbers. Conversely, the 50% and 75% hydrogel groups revealed a significant increase in macrophage presence within the tissues on Days 3, 7, and 14. The elevated macrophage count indicates persistent inflammation within the injured tissue, which suggests inadequate wound healing [17].
Table 6. Histopathology analysis.
| Sample Groups | Normal Group | Positive Control | Negative Control | Hydrogel 25% | Hydrogel 50% | Hydrogel 75% | |
|---|---|---|---|---|---|---|---|
| Number of Macrophage Cells | D-3 | 0.26 ± 0.11 | 1.26 ± 0.11 | 1.2 ± 0.2 | 1.26 ± 0.3a | 1.4 ± 0.4a | 0.6 ± 0.00abc |
| D-7 | 0.46 ± 0.11 | 1 ± 0.2 | 1.13 ± 0.3 | 2.46 ± 1.2a | 11.13 ± 2.35a | 10.53 ± 2.87a | |
| D-14 | 0.4 ± 0.00 | 0.46 ± 0.06 | 1.26 ± 0.24 | 1.73 ± .26 | 15.26 ± 1.76ab | 14.46 ± 0.54ab | |
| Number of Neutrophil Cells | D-3 | 0.86 ± 0.66bc | 23.00 ± 1.44ac | 213.60 ± 9.72ab | 200.46 ± 4.86ab | 153.80 ± 1.44abc | 131.46 ± 1.53abc |
| D-7 | 0.10 ± 0.11bc | 17.86 ± 0.24a | 16.60 ± 0.91a | 15.80 ± 1.31ac | 138.93 ± 12.33abc | 153.26 ± 7.62abc | |
| D-14 | 1.00 ± 0.11c | 1.33 ± 0.06c | 45.13 ± 23.23ab | 22.06 ± 8.27ab | 227.20 ± 7.68ab | 353.46 ± 18.12ab | |
| Number of Lymphocyte Cells | D-3 | 0.53 ± 0.66bc | 24.73 ± 0.87ac | 140.53 ± 8.91ab | 135.60 ± 3.89ab | 120.80 ± 1.60ab | 122.80 ± 2.38ab |
| D-7 | 0.53 ± 0.66bc | 5.53 ± 0.59ac | 15.00 ± 0.91ab | 4.86 ± 0.63ac | 52.46 ± 1.73abc | 52.80 ± 2.90abc | |
| D-14 | 0.60 ± 0.11c | 0.73 ± 0.66c | 20.00 ± 3.12abc | 5.46 ± 3.39abc | 182.13 ± 11.44abc | 132.80 ± 11.86abc | |
| Re-epithelialization Layer Thickness | D-3 | 36.24 ± 5.97 | 47.50 ± 27.68 | 24.31 ± 4.60 | 41.30 ± 10.53 | 36.03 ± 4.10 | 44.43 ± 9.57 |
| D-7 | 30.73 ± 0.43b | 59.03 ± 7.87a | 53.94 ± 33.99 | 44.09 ± 11.05a | 37.17 ± 22.73 | 18.50 ± 1.70bc | |
| D-14 | 38.27 ± 1.60 | 30.74 ± 1.87 | 45.07 ± 30.59 | 26.92 ± 6.06 | 40.84 ± 17.31 | 27.72 ± 5.76 | |
| Number of Fibroblast Cells | D-3 | 7.27 ± 0.64bc | 20.93 ± 1.29a | 23.07 ± 1.45a | 23.33 ± 0.64ab | 15.93 ± 0.99abc | 13.33 ± 1.70abc |
| D-7 | 12.93 ± 1.17b | 37.60 ± 5.72a | 24.13 ± 7.70 | 31.60 ± 4.45a | 16.13 ± 5.28b | 14.87 ± 1.81b | |
| D-14 | 12.33 ± 2.39c | 9.73 ± 0.58c | 24.87 ± 2.83ab | 44.73 ± 5.51abc | 58.73 ± 2.20abc | 11.60 ± 3.61c | |
| Collagen Density Score | D-3 | 4 ± 0.00bc | 3 ± 0.00a | 3 ± 0.00a | 2.8 ± 0.20a | 4 ± 0.00bc | 3 ± 0.00a |
| D-7 | 4 ± 0.00bc | 3 ± 0.00a | 3 ± 0.00a | 2.8 ± 0.20a | 4 ± 0.00bc | 3 ± 0.00a | |
| D-14 | 4 ± 0.00c | 4 ± 0.00c | 3 ± 0.00ab | 2.73 ± 0.12abc | 4 ± 0.00c | 4 ± 0.00c | |
| Number of Angiogenesis | D-3 | 0.53 ± 0.66bc | 3.40 ± 0.11ac | 2.60 ± 0.11ab | 2.20 ± 0.00abc | 1.20 ± 0.00abc | 0.73 ± 0.66bc |
| D-7 | 1.00 ± 0.11 | 1.13 ± 0.13 | 0.93 ± 0.17 | 1.40 ± 0.30 | 2.66 ± 0.56abc | 1.93 ± 0.46 | |
| D-14 | 0.86 ± 0.13 | 0.60 ± 0.00 | 1.20 ± 0.34 | 2.40 ± 0.46ab | 13.06 ± 0.66abc | 4.53 ± 0.37abc | |
Compared to the other experimental groups, the negative control group displayed a prolonged inflammatory response, evidenced by a gradual increase in epidermal thickness from Day 3 to Day 14. Conversely, the 25% and 75% hydrogel groups exhibited an increase in epidermal thickness from Day 3 to Day 7, followed by a gradual decrease from Day 7 to Day 14. The 25% hydrogel group exhibited the most favorable conditions for re-epithelialization. Similar trends were observed concerning fibroblast count and collagen density, with the 25% hydrogel group yielding optimal results that closely resembled those of the positive control group treated with Bioplacenton gel.
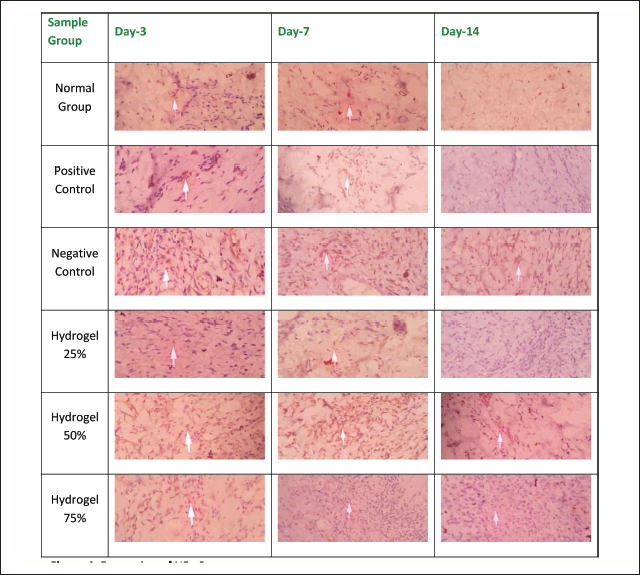
Figures 6 and 7 illustrate the histopathological outcomes regarding neutrophil and lymphocyte levels, as the arrows indicate. These results were derived from hematoxylin-eosin staining techniques. The histopathological analysis indicates that the wound healing process is enhanced when neutrophil levels decrease and lymphocyte levels increase, thereby facilitating sustained inhibition of bacterial proliferation. Conversely, an increase in neutrophil count adversely affects wound healing and tissue repair, while a decrease in neutrophils promotes prolonged epithelial maintenance. In contrast, a reduction in lymphocyte count may facilitate the proliferation of bacteria. The negative control group exhibited a significant increase in angiogenesis from Day 3 to Day 14, akin to the positive control group. In the 25% hydrogel group, a notable reduction in neutrophil count was observed alongside an enhancement in angiogenesis. Conversely, the 50% hydrogel group demonstrated a marked increase in neutrophils and a decrease in lymphocyte count between Days 3 and 14. These findings suggest that the tissues subjected to the 25% hydrogel treatment undergo effective healing and can be favorably compared to the positive control group treated with Bioplacenton gel [18,19].
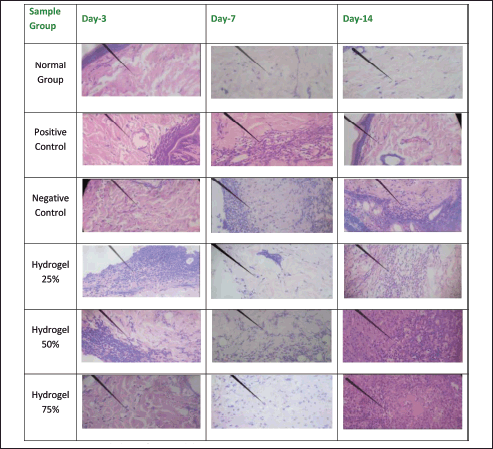 | Figure 6. Histopathology of neutrophils. [Click here to view] |
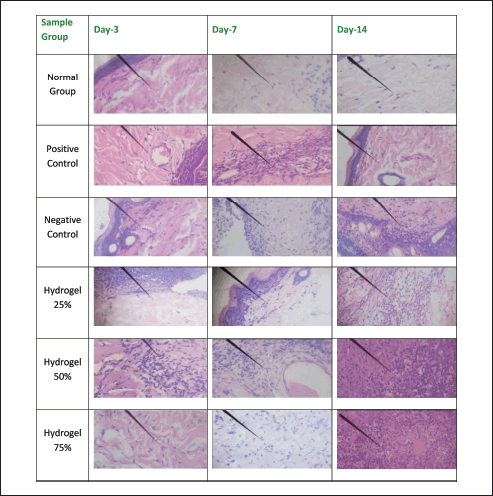 | Figure 7. Histopathology of lymphocyte. [Click here to view] |
Figures 8–12 present the histopathological results related to macrophage presence, re-epithelialization, fibroblast and collagen density, and angiogenesis, as indicated by the arrows. These histopathological data, also obtained via hematoxylin-eosin staining, reveal that the optimal conditions for re-epithelialization are observed in the 25% hydrogel group. Similar favorable outcomes were noted for both fibroblast and collagen density parameters, with the 25% hydrogel group exhibiting results that closely approximate those of the positive control group treated with Bioplacenton gel.
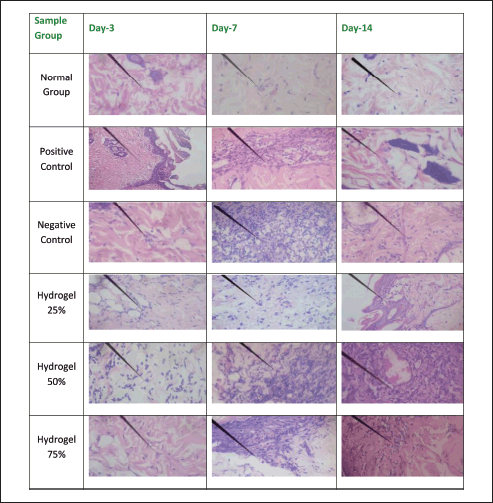 | Figure 8. Histopathology of macrophage. [Click here to view] |
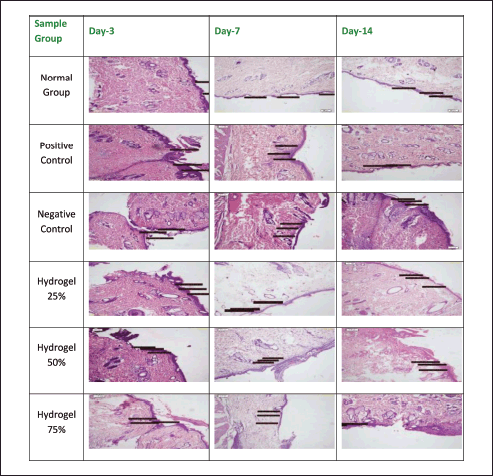 | Figure 9. Histopathology of Re-epithelialization. [Click here to view] |
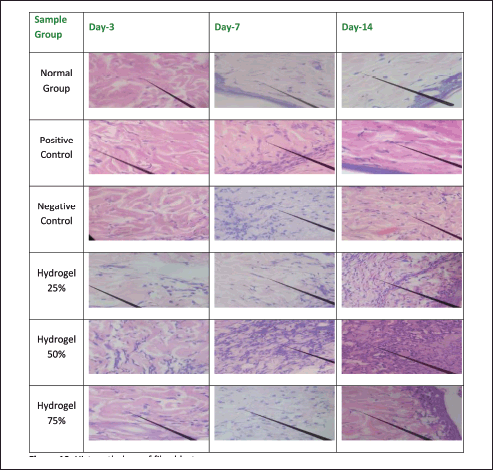 | Figure 10. Histopathology of fibroblasts. [Click here to view] |
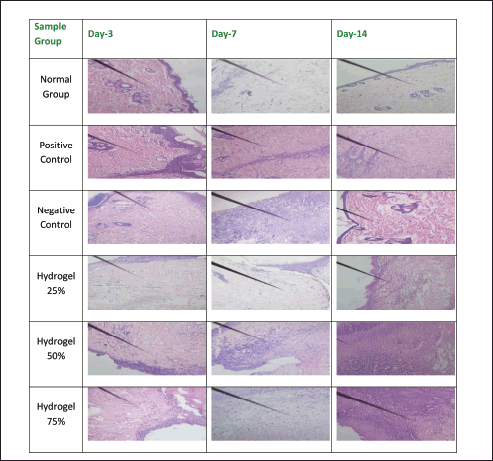 | Figure 11. Histopathology of collagen. [Click here to view] |
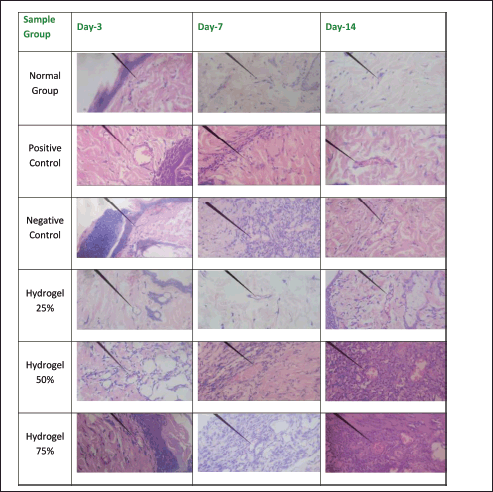 | Figure 12. Histopathology of angiogenesis. [Click here to view] |
Angiogenesis is characterized by the formation of spherical neocapillaries, which are distinctly defined by a white lumen containing small red-stained dots indicative of erythrocytes, with the nuclei appearing as purple-stained structures at the periphery. Regarding the negative control group, angiogenesis progressively increased from Day 3 to Day 14. There was a significant angiogenesis surge in the 50% hydrogel group on Days 3, 7, and 14. These findings collectively indicate that the tissue healing process is progressing and closely resembles the healing trajectory observed in the positive control group treated with Bioplacenton gel [12].
As per the immunohistochemistry analysis, it is noteworthy that the 50% and 75% hydrogel concentrations contain excessively high levels of acids and phenols, which contribute to tissue irritation and inflammation, ultimately resulting in necrosis in the affected areas. The formation of necrotic tissue is attributed to inadequate blood supply, which restricts the delivery of essential nutrients and oxygen necessary for optimal wound healing. Consequently, this pathological state obstructs the re-epithelialization process. Thus, the elevated concentrations of acids and phenols present in coconut shell liquid smoke are deemed toxic and detrimental to the healing process [13].
CONCLUSION
Hydrogel, a concentrated form of liquid smoke, is believed to have biological properties that aid wound healing. Among the tested concentrations, the 25% DLS hydrogel showed the most effective wound-healing results in diabetic rats. The effectiveness is due to its moderate acetic acid content, which makes it suitable for topical application without causing skin irritation or prolonged inflammation.
ACKNOWLEDGMENTS
We extend our gratitude to Universitas Sumatera Utara for the financial support provided through the Indonesian Collaborative Research program (7092.1/UN5.1.R/PPM/2023), which enabled the successful completion of this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details are provided in the ‘Materials and Methods’ section.
DATA AVAILABILITY
All data produced or analyzed during this study are included in the published articles listed under references.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Li W, Zhang X, Chen R, Li Y, Miao J, Liu G, et al. HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J Ethnopharmacol. 2020;254:112740. CrossRef
2. Li Y, Chen J, Cao L, Li L, Wang F, Liao Z, et al. Characterisation of a novel polysaccharide isolated from Phyllanthus emblica L. and analysis of its antioxidant activities. J Food Sci Technol. 2018;55(7):6. CrossRef
3. Masfria, Lucida H, Atifah Y, Syahputra H, Sihombing HM. Inhibition activity of liquid smoke Cocos nucifera L. on dpp-iv and age-rage in silico and in vitro: antidiabetic and anti-inflammatory activity. Int J Appl Pharm. 2024;16(5):275–82. CrossRef
4. Masfria, Syahputra H, Wahyuni HS, Zebua NF, Rahmansyah W. Production, characterisation, and potential phenol content of various grades liquid smoke made from coconut shell waste (Cocos nucifera L.). In: IOP Conference Series: Earth and Environmental Science; 2024. Vol, 1352. London: Institute of Physics. CrossRef
5. Yokoyama T, Masuda H, Suzuki M, Nogi K. Basic properties and measuring methods of nanoparticles, nanoparticle technology handbook. Amsterdam: Elsevier; pp: 3–47; 2018.
6. Swarbrick J. Encyclopedia of pharmaceutical technology. London: Routledge; 2013. CrossRef
7. Maulina S, Sinaga FA. Improving the quality of liquid smoke from oil palm fronds through adsorption and distillation processes. In: IOP Conference Series: Materials Science and Engineering. Vol. 801; 2020. CrossRef
8. Fitriani yeyen nor, INHS C, Yuliati N, Aryantini D. Formulation and evaluation of physical stability of suspension cilembu (Ipomoea batatas L.) with Suspending Agent CMC Na and PGS As Antihypercholesterol. J Farm Sains Dan Terapan. 2015;2:1.
9. Jang JG, Kang JH, Joe KB, Sakthiabirami K, Jang KJ, Jun MJ, et al. Evaluation of physical properties of zirconia suspension with added silane coupling agent for additive manufacturing processes. Materials. 2022;15(4):1337. CrossRef
10. Surboyo MDC, Arundina I, Rahayu RP, Mansur D, Bramantoro T. Potential of distilled liquid smoke derived from coconut (Cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent. 2019;13(2):527. CrossRef
11. Agrawal N, Jedge P, Iyer S, Shah J, Dsouza J, Chougale S. Experimental chest x-ray scoring system for determining patient outcomes in COVID-19 patients. Indian J Crit Care Med. 2021;25(SUPPL 1):509–13.
12. Vasilenko T, Slezák M, Novotný M, Kovác I, Durkác J, Tomková I, et al. Pre-and/or postsurgical administration of estradiol benzoate increases skin flap viability in female rats. Aesthetic Plast Surg. 2013;37(5):1003–9. CrossRef
13. Rabat Sarpooshi H, Vaheb M, Tabarayee Y, Vidinsky B, Vasilenko T, Mozes S, et al. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: Standardisation of semi-quantitative and quantitative histological assessments. Vet Med (Praha). 2016;1(4):652–9.
14. Masfria, Syahputra H, Wahyuni HS, Zebua NF, Elizabeth, Marcelynn. Analysis of total flavonoid and antioxidant activity of coconut shell liquid smoke (Cocos nucifera L.) as an antibacterial. Pharm Educ. 2024;24(2):39–45. CrossRef
15. Surboyo MDC, Mahdani FY, Ernawati DS, Sarasati A, Rezkita F. The macrophage responses during diabetic oral ulcer healing by liquid coconut shell smoke: an immunohistochemical analysis. Eur J Dent. 2020;14(3):410–4. CrossRef
16. Agrawal KS, Sarda AV, Shrotriya R, Bachhav M, Puri V, Nataraj G. Acetic acid dressings: Finding the Holy Grail for infected wound management. Indian J Plastic Surg. 2017;50(3):273–80. CrossRef
17. Maassen S, Coenen B, Ioannidis M, Harber K, Grijpstra P, Van den Bossche J, et al. Itaconate promotes a wound resolving phenotype in pro-inflammatory macrophages. Redox Biol. 2023;59:102591. CrossRef
18. Somboonwong J, Kankaisre M, Tantisira B, Tantisira MH. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: an experimental animal study. BMC Complement Altern Med. 2012;12:103. CrossRef
19. Assar DH, Elhabashi N, Mokhbatly AAA, Ragab AE, Elbialy ZI, Rizk SA, et al. Wound healing potential of licorice extract in rat model: antioxidants, histopathological, immunohistochemical and gene expression evidences. Biomed Pharmacother. 2021;143:112151. CrossRef