INTRODUCTION
The Ziziphus genus, belonging to the Rhamnaceae family, comprises over 100 species widely distributed in tropical and subtropical regions. Ziziphus is known to provide numerous benefits, serving as a food source, traditional medicine, and environmental protector. Many species of this genus have been utilized as traditional medicines for millennia, and remain popular today for treating various conditions, including fever, diabetes, and skin infections [1,2].
Prominent species of this genus commonly employed in folk medicine and extensively researched are Ziziphus jujuba, Ziziphus spina-christi, and Ziziphus mauritiana [3–9]. These three plants are recognized for their antihyperglycemic properties, and their effectiveness is often linked to their antioxidant abilities [10–12]. The leaves of these plants were compared and evaluated with LCMS-based metabolomics and examined against antioxidant, anti-inflammatory, and antidiabetic tests. The results of this study revealed that Z. spina-christi leaves as particularly effective in antioxidant and α-glucosidase inhibition, while Z. mauritiana leaves exhibited high COX-1 enzyme inhibitory activity [9]. Nevertheless, the understanding of metabolite profiles on various organs of these plants remains ambiguous.
In this report, we employed 1H NMR-based metabolomics for evaluating the metabolite profiles of diverse organs (fruits, seeds, and leaves) of Z. spina-christi, Z. mauritiana, and Z. jujuba. The evaluation was also carried out with bioactivity examination, including assessments of radical-scavenging abilities against DPPH and CUPRAC radicals, along with the evaluation of their potential to inhibit α-glucosidase and dipeptidyl peptidase IV (DPP-IV), enzymes crucial in diabetes. This study provided a comprehensive exploration of the metabolite profiles and bioactivities of Ziziphus species, contributing valuable insights into their potential therapeutic applications and highlighting the significance of different plant organs in traditional medicine and nutritional contexts.
MATERIALS AND METHODS
Chemicals and reagents
Deuterium oxide (D2O, CID: 24602), methanol-d4 (CD3OD, CID: 71568), 3-(trimethylsilyl)- 2,2,3,3-tetradeuteropropionic acid sodium salt (TSP, CID: 23688921), trizma® hydrochloride (CID: 93573), acetic acid (CID: 176), sitagliptin (CID: 4369359), methanol (CH3OH, CID: 887), ethanol (CH3CH2OH, CID: 702), cupric chloride (CuCl2, CID: 24014), neocuproine (C14H12N2, CID: 65237), ascorbic acid (C6H8O6, CID: 54670067), 1,1-diphenyl-2-picrylhydrazyl (DPPH, CID: 74358), α-glucosidase from Saccharomyces cerevisiae, 4-nitrophenyl-α-D-glucopyranoside (p-NPG, CID:92930), Gly-Pro p-nitroanilide hydrochloride (GPPN, CID: 16219380), and DPP-IV human were acquired from Sigma-Aldrich (St. Louis, USA), Acarbose hydrate (CID: 91659006) was obtained from TCI (Tokyo, Japan).
Sample and extract preparation
The utilized samples encompassed leaves, fruits, and seeds sourced from Z. jujuba, Z. mauritiana, and Z. spina-christi. Ziziphus jujuba was procured from Miaoli, Taiwan, whereas Z. mauritiana was obtained from Pekanbaru, Indonesia, and Z. spina-christi was collected from Depok, Indonesia. All samples underwent a drying process using a freeze dryer and were subsequently pulverized. The preparation of samples for NMR analysis was conducted using a powdered form. Each sample was subjected to extraction with a CD3OD-D2O mixture containing 1 mM TSP in a ratio of 7:3. This extraction process involved sonication at 60°C for a duration of 30 minutes. Subsequent steps included filtration and centrifugation (12,000 rpm, 5 minutes), with the resulting supernatant being transferred to the NMR tube for analysis.
For the bioactivity assay, the sample preparation involved using the powdered form of the material. Extraction was carried out through sonication at 60°C for 30 minutes, utilizing a mixture of ethanol 96% and water (7:3). The resulting filtrate was concentrated using a rotary evaporator at 50°C. The extract obtained from this process was destined for assessing antioxidant activity and the inhibition of the alpha glucosidase enzyme.
1H-NMR spectroscopic analysis
The 1H-NMR spectra were captured using an NMR Variant Unity INOVA-500 Spectrometer from Agilent Technologies (Santa Clara, United States). For these measurements, the presaturation program was employed, with an acquisition time of 2.72 seconds, a delay time of 2.00 seconds, 64 K data points, a spectral width of 8012 Hz, and 128 scans. Post-acquisition, the NMR FID data underwent further processing through ACD/Labs 12.0 software (Toronto, Canada) [13]. All 1H NMR signals across the samples were both identified and cross-referenced, utilizing databases including www.hmdb.ca and www.foodb.ca, in addition to pertinent scientific articles for signal characteristic comparison and verification.
Bioactivities of Ziziphus
Determination of antioxidant capacity with DPPH and CUPRAC methods
The method for assessing antioxidant activity was adapted from the work of Celep et al. [14]. The ascorbic acid solution was prepared carefully with varying concentrations of methanol, then mixed with the DPPH solution, and then incubated for 30 minutes in a light-protected environment at room temperature [14]. Ascorbic acid served as a positive control, with concentrations of 5 µg/ml, and extract concentrations of 100 µg/ml. Subsequent to the incubation period, the absorbance of the solution was gauged using UV-vis spectrophotometry, specifically at a wavelength of 517 nm. The acquired absorbance values were subsequently converted to absorption percentages for each respective concentration. A calibration curve was established for the absorption percentage of ascorbic acid, ensuring a minimum R2 value of 0.99. The extract capacity to scavenge DPPH radicals was measured using the same method as for vitamin C. The percent inhibition value of each extract at a concentration of 100 ug/ml was analyzed alongside the processed data from the ¹H-NMR spectrum using principal component analysis (PCA).
The CUPRAC method was employed for evaluating antioxidant activity, following modifications based on Özyürek and Bektas’s technique [15]. A CuCl2.H2O solution was prepared, achieving a concentration of 1,705 µg/ml, along with a neocuproin solution with a concentration of 1,562 µg/ml. Additionally, an ammonium acetate buffer at pH 7.0 was prepared. Neocuproin solution (1:1) was mixed with the CuCl2 solution. Around 6,122 μl of this mixture was then combined with ammonium acetate buffer to yield a total volume of 100 ml (designated as the CUPRAC mixture). Ascorbic acid served as a positive control, with concentrations of 1 µg/ml, and extract concentrations of 100 µg/ml. For analysis, ascorbic acid solutions were prepared with various concentrations in ammonium acetate buffer and then mixed with CUPRAC solution. Next, the absorption was measured using a UV-vis spectrophotometer at a wavelength of 450 nm.
The determination of the increase in capacity for each concentration was accomplished through the establishment of a calibration curve, exhibiting a minimum regression equation with R2 = 0.99. To evaluate the antioxidant activity of the extract, a procedure similar to that used for vitamin C measurement was employed. This involved selecting a concentration with absorbance within the range of 0.2–0.8, followed by calculating the corresponding equivalent value of ascorbic acid (mg ascorbic acid equivalent/g sample). The percentage inhibition value for each sample at a concentration of 100 μg/ml was analyzed alongside the processed data from the ¹H NMR spectrum using PCA.
α-glucosidase and DPP-IV inhibitory assays
The α-glucosidase inhibitory assay was conducted as reported by Nor et al. [16] with slight modification. In 96-well microplates, 30 µl of the sample was mixed with 36 μl of a 0.1 M phosphate buffer solution at pH 6.8, and 17 µl of a 6 mM p-nitrophenyl-a-D-glucopyranoside, and then incubated at 37°C for 5 minutes. Around 17 µl of α-glucosidase enzyme at a concentration of 0.2 units/ml was added into the mixture and then incubated for 15 minutes at 37°C to facilitate the completion of the reaction. The chemical reaction was terminated by the addition of 100 µl of 200 mM Na2CO3 to each well. The extracts and acarbose concentrations used in this assay were 100 µg/ml. Afterward, the absorbance of the mixture was measured with a microplate reader set at 405 nm (ThermoFisher). Each measurement was carried out in triplicate to ensure accuracy. The positive control was acarbose, whereas the negative control was the mixture without the sample [16].
The in-vitro DPP IV inhibitory assay was carried out based on the method employed by Zhang et al. [17]. In this assay, 35 µl of the sample in 96-well microplates was combined with 30 µl of the enzyme DPP-IV (0.05 µg/ml). The mixture was then incubated for 15 minutes at 37°C, reacted with 100 µl of GPPN substrate at a concentration of 0.5 mM, and then incubated for 90 minutes at 37°C. The reaction was terminated by the addition of 35 µl of 25% acetic acid. The absorbance of the mixture was measured at a wavelength of 377 nm using a microplate reader (ThermoFisher). All extracts were tested at a concentration of 1,000 µg/ml, and sitagliptin was used at a concentration of 1 µg/ml. All reagents in this study were dissolved in Trizma-HCl buffer with a pH of 6.8, and each measurement was conducted in triplicate to ensure accuracy. Sitagliptin (Merck) was employed as a positive control in this study.
Statistical analysis
Bioactivity data, including antioxidant activity and inhibition of α-glucosidase and DPP-IV enzymes, were analyzed using ANOVA with Minitab 21 (Minitab Inc., USA). NMR data from all samples were analyzed using multivariate PCA with SIMCA version 12.0 software (Umetrics, Sweden).
RESULT AND DISCUSSION
Metabolite identification
The identification of metabolites present in all examined Ziziphus samples was achieved by analyzing their distinctive signals in the 1H-NMR spectra, as depicted in Figure 1 and Table 1. The outcomes of this identification process were cross-referenced with the data documented within databases (www.hmdb.ca and www.foodb.ca) as well as pertinent scientific literature [18–21].
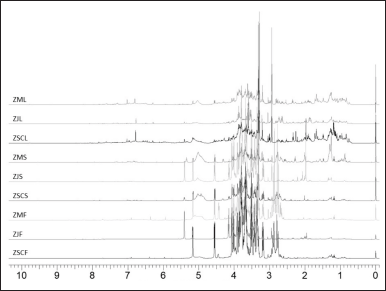 | Figure 1. 1H-NMR spectrum of fruits (F), seeds (S), and leaves (L) of Z. spina-christi (ZSC), Z. jujuba (ZJ), and Z. mauritiana (ZM). [Click here to view] |
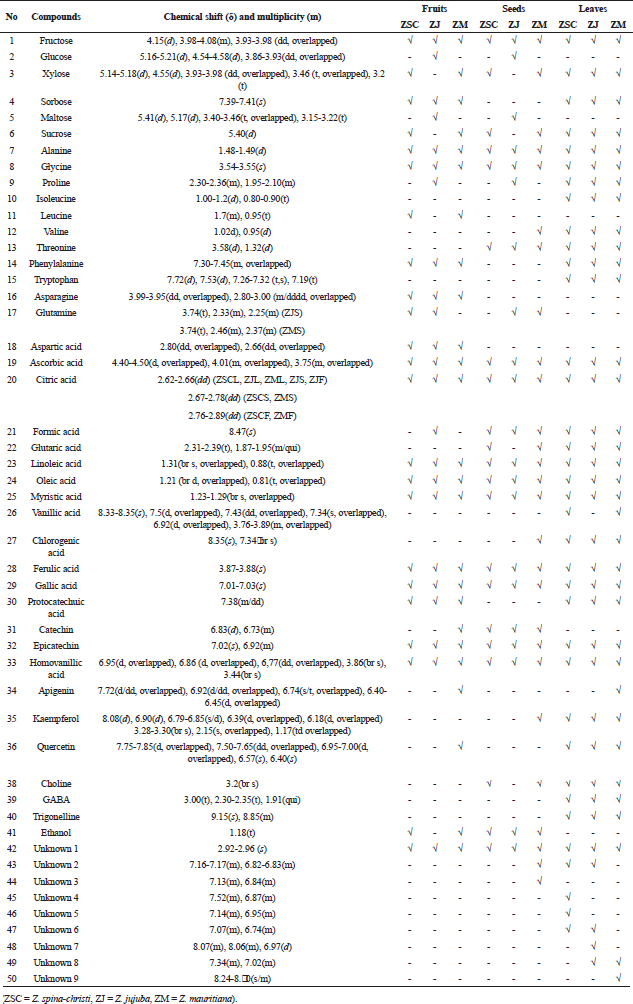 | Table 1. Characteristic signals of the identified compounds in 1H-NMR spectra of Ziziphus samples. [Click here to view] |
The metabolite identification in the 1H-NMR spectra successfully annotated a total of 50 metabolites, comprising 41 known compounds and 9 unknown compounds. The unknown compounds belong to the phenolic group, characterized by signals appearing in the chemical shift range of δ 6.00–9.00 ppm. Among the identified metabolites, there were sugar, amino acids, fatty acids, organic acids, phenolic acids, phenolic compounds, and other compounds.
Sugar compounds detected in the δ 3.00–5.00 ppm area include fructose, glucose, xylose, sorbose, maltose, and sucrose. Fructose was identified in all samples, marked by a signal at δ 4.15(d) ppm. Glucose and maltose were detected only in Z. jujuba fruit and seeds, with signals at δ 5.16–5.21(d) ppm and δ 4.55(d) ppm for glucose, and δ 5.41(d) ppm for maltose. Xylose and sucrose were detected in almost all samples except Z. jujuba fruit and seeds. Sorbose signal was detected at δ 7.39–7.41(s) ppm in the fruit and leaves of the three species. The signals of alanine at δ 1.48–1.49(d) ppm and glycine at δ 3.54–3.55(s) ppm, belonging to amino acids, were detected in all samples. Isoleucine and tryptophan, the other amino acids, were only detected in the leaves; leucine, asparagine, and aspartic acid were only detected in the fruit; and glutamine was only detected in the seeds.
The organic acids found in all samples were ascorbic acid and citric acid. There was a slight variation in the position of the citric acid signal in each sample, including δ 2.62–2.66(dd) ppm in all leaf samples and the fruit and seeds of Z. jujuba. In the seeds of Z. spina-christi and Z. mauritiana, the signal shifted slightly to δ 2.67–2.78(dd) ppm, and in Z. spina-christi and Z. mauritiana fruit, it shifted further to δ 2.76–2.89(dd) ppm. The signals of fatty acids, including oleic acid, linoleic acid, and myristic acid were detected in all samples at δ 0.80–1.33 ppm. Phenolic acids detected in all samples included ferulic acid at δ 3.87–3.88(s) ppm and gallic acid at δ 7.01–7.03(s) ppm.
Catechin and epicatechin having the signals at δ 6.83(d) and 7.02(s) ppm, respectively, were phenolic compounds detected in all samples. Phenolic compounds in the form of flavonoids, including apigenin, kaempferol, quercetin, and rutin, were dominantly identified in all leaf samples. Some signals of detected flavonoids in the δ 6.00–8.00 ppm area were found to be overlapping as described in Table 1. However, each annotated flavonoid still had non-overlapped signals, including the peaks at δ 3.28–3.30 (brs) ppm for kaempferol, δ 6.57 (s) and 6.40 (s) ppm for quercetin, and δ 6.50 (d), 6.30(d), and 5.16 (d) ppm for rutin.
Bioactivities of Ziziphus samples
The antioxidant activity of Ziziphus samples was evaluated by measuring the percentage of DPPH inhibition and the percentage of CUPRAC increase. Ascorbic acid served as a positive control, with concentrations of 5 and 1 µg/ml for DPPH and CUPRAC tests, respectively. The inhibitory percentage of α-glucosidase enzyme was measured at a concentration of 100 µg/ml for all samples, while DPP-IV inhibition was assessed at a concentration of 1,000 µg/ml. Acarbose with a concentration of 100 µg/ml was used as a positive control in an inhibitory test of the α-glucosidase enzyme. Sitagliptin (1 µg/ml) was employed as a positive control in the inhibitory assay of the DPP-IV enzyme. The results of the antioxidant and the enzyme inhibitory assays are shown in Table 2.
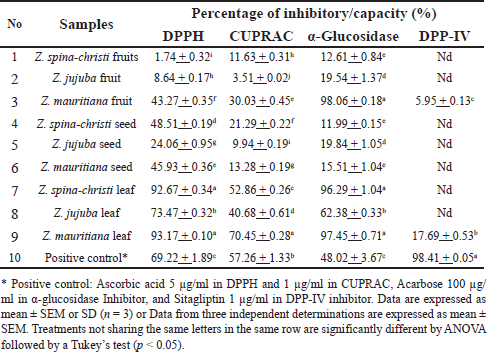 | Table 2. Antioxidant properties and enzyme inhibitory activities of Ziziphus samples. [Click here to view] |
The antioxidant activity of Zizhipus samples was assessed using DPPH and CUPRAC methods. The results revealed that Z. mauritiana leaves consistently had the highest activity percentages. However, the ability of Z. mauritiana and Z. spina-christi leaves to inhibit DPPH radicals were relatively similar, with no significant difference (p > 0.05), followed by Z. jujuba leaves. A similar pattern was observed for CUPRAC antioxidant capacity, with Z. mauritiana leaves exhibiting the highest activity, followed by Z. spina-christi and Z. jujuba leaves. Compared to the other fruit samples, the extract of Z. mauritiana fruit had the highest percentage of CUPRAC (30.03% ± 0.45%) and DPPH (43.27% ± 0.35%) activities. Meanwhile, the extract of Z. spina-christi seeds was detected having the highest percentage of CUPRAC (21.29% _ 0.22%) and DPPH (48.50% ± 0.19%) activities, when compared to the other seed samples.
As antidiabetic agents, the 3 Zizhipus leaf extracts demonstrated excellent activity in inhibiting the α-glucosidase enzyme. Among them, Z. mauritiana (97.45% ± 0.71%) and Z. spina-christi (96.30% ± 1.04%) leaves displayed significantly higher inhibition rates compared to the Z. jujuba leaves (62.38% ± 0.33%). Surprisingly, the fruit of Z. mauritiana exhibited the highest inhibitory percentage of α-glucosidase (98.06% ± 0.18%) compared to the other samples. These four Zizhipus samples showed higher inhibition activity compared to acarbose at the same test concentration of 100 µg/ml. In contrast, other extract samples at the same concentration exhibited less than 20% inhibition of α-glucosidase. Regarding the inhibition of the DPP-IV enzyme, none of the samples could be considered potent, since they all displayed very low inhibitory activity. At a concentration of 1,000 µg/ml, only the leaf and fruit extracts of Z. mauritiana demonstrated potential inhibitory activity against the DPP-IV enzyme, with inhibition values of 17.69% ± 0.53% and 5.95% ± 0.13%, respectively. The other samples did not exhibit any notable inhibitory potential at this concentration. In contrast, sitagliptin (the positive control), at a significantly lower concentration of 1 µg/ml, achieved a much higher inhibition of 98.41% ± 0.05%.
Classification of samples by PCA and hierarchical cluster analysis (HCA) dendogram
Multivariate analysis was applied on the bucket data derived from 1H NMR spectra, along with the percentages of antioxidant properties, and the inhibition percentage of the α-glucosidase enzyme. PCA analysis was employed with Pareto scaling, yielding values R2X = 0.999 and Q2 = 0.982. The PCA analysis successfully distinguished each group of tested samples based on their primary components. This separation is illustrated in the dendrogram and score scatter plot (Fig. 2). Meanwhile, the compounds contributing to the differentiation were depicted in the loading scatter plot and biplot in Figure 2.
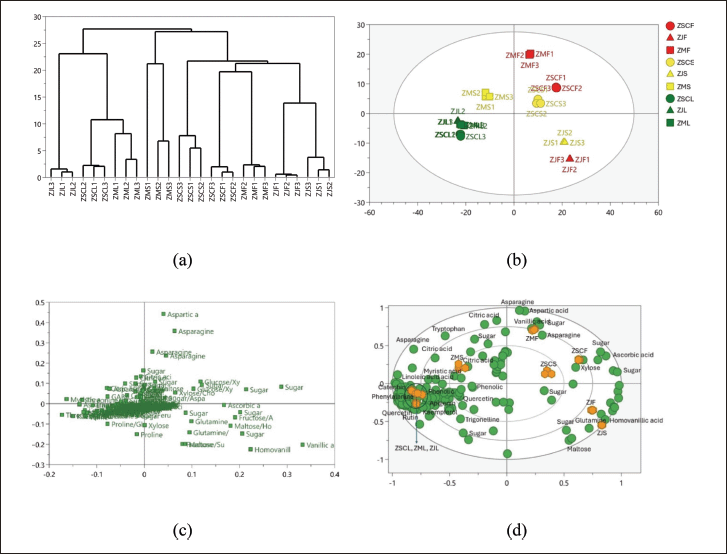 | Figure 2. The HCA and PCA of Ziziphus: (a) HCA, (b) score plot, (c) loading scatter plot, (d) biplot. ZSCF: Z. spina-christi fruits; ZJF: Z. jujuba fruits; ZMF: Z. mauritiana fruits; ZSCS: Z. spina-christi seeds; ZJS: Z. jujuba seeds; ZMS: Z. mauritiana seeds; ZSCL: Z. spina-christi leaves; ZJL: Z. jujuba leaves; ZML: Z. mauritiana leaves. [Click here to view] |
The PCA revealed distinct spatial patterns in the chemical profiles of the Ziziphus samples. All leaf samples were clustered in a single region, indicating a high degree of chemical similarity among them. Meanwhile, the fruit and seed samples were dispersed across various regions of the score plot. This dispersion suggests a greater variation in the chemical composition of the fruits and seeds, when compared to the leaves. The grouping based on the first principal component (PC1), which accounted for 55.4% of the total variance, showed that the leaves and the seeds of Z. mauritiana were located in the same quadrant, highlighting their chemical relatedness. The other samples, including the fruits and seeds of Z. jujuba and Z. spina-christi, were positioned in separate quadrants, reflecting their distinct chemical profiles. The second principal component (PC2), contributing 16.9% to the total variance, further differentiated the samples. For instance, PC2 successfully distinguished all the Ziziphus fruits based on their species.
These PCA findings were further confirmed by the results of HCA, which provided a clear visualization of the relationships among the samples. The HCA dendrogram revealed that all leaf samples were grouped together on a single branch, underscoring their close chemical kinship. In contrast, the other samples were segregated into distinct branches, emphasizing the differences in their chemical compositions. Notably, the Z. jujuba fruit and seed samples shared a branch, reflecting their chemical affinity. However, the seeds of Z. mauritiana and Z. spina-christi were distinctly separated from the other groups, indicating unique chemical profiles that set them apart from the other samples.
To identify compounds responsible for the separation, we evaluated the corresponding loading plot and Biplot (Fig. 2c and d). Loading plot analysis revealed that some metabolites were detected contributing to the classification, including aspartic acid (bucket at δ 2.74–2.83 ppm), asparagine (bucket at δ 2.83–2.88 ppm), vanillic acid (bucket at δ 8.30–8.35 ppm), homovanillic acid (bucket at δ 3.81–3.87 ppm), threonine (bucket at δ 3.57–3.61 ppm), oleic acid (bucket at δ 0.80–0.85 ppm), catechin (bucket at δ 6.72–6.74 ppm) kaempferol (bucket at δ 6.79–6.85 ppm), quercetin (bucket at δ 7.59–7.65 ppm), and rutin (bucket at δ 6.25–6.31 ppm). As seen in the biplot, the leaves of the three Ziziphus species and the seeds of Z. mauritiana, were distinguished by the presence of a rich array of bioactive compounds, including phenolic compounds, flavonoids, fatty acids, and several amino acids. In the PCA score plot and corresponding biplot, key metabolites such as oleic acid, linoleic acid (bucket at δ 0.85–0.91 ppm), gallic acid (bucket at δ 6.99–7.05 ppm), catechin, apigenin (bucket at δ 6.43–6.47 ppm), quercetin, rutin, and kaempferol were prominently detected within the leaf region, highlighting the abundance of these compounds in the leaf samples. Within the same quadrant, Z. mauritiana seeds were associated with the presence of citric acid and phenolic compounds, underscoring the biochemical similarities between these seeds and the leaf samples.
In contrast, the remaining seed and fruit samples were distinctly separated from the previous samples. These remaining seed and fruit samples were characterized by the presence of various sugar components, including glucose (bucket at δ 5.14–5.20 ppm), fructose (bucket at δ 4.12–4.18 ppm), and maltose (bucket at δ 3.41–3.46 ppm), indicating a significant difference in their metabolomic profiles. Additionally, these samples were also marked by the detection of ascorbic acid (bucket at δ 4.04–4.10 ppm), aspartic acid (bucket at δ 2.74–2.80 ppm), and glutamine (bucket at δ 3.72–3.74 ppm), further emphasizing the chemical divergence between the leaf and seed samples of Z. mauritiana and the other Ziziphus species. The separation in the score plot reflects the distinct metabolic pathways and compound synthesis occurring in the different plant parts and species.
Furthermore, we also compared the metabolite profiles of the same organs with different Zizhipus species using PCA (the score and loading plots of the PCA models are provided in Supplementary Data 1) as depicted in the corresponding biplots (Fig. 3). First, we evaluated the metabolite profiles of Zizhiphus fruit samples, yielding a PCA model with a total variance (R2X) of 0.995 and a cross-validation value (Q2) of 0.985, indicating clear sample separation and strong model validity. The correlated biplot (Fig. 3a) classified the fruit samples based on their species and revealed that Z. jujuba fruits were distinguished by the presence of maltose, fructose, glutamine, and homovanilic acid. Meanwhile, formic acid and trigonelline were found as distinctive compounds for Z. spina-christi fruits, whereas unknown phenolic components were detected as characteristic of Z. mauritiana fruits. These unknown phenolic components might contribute to the bioactivities of Z. mauritiana leaves having higher antioxidant capabilities and inhibiting α-glucosidase enzyme more effectively than the other two fruits.
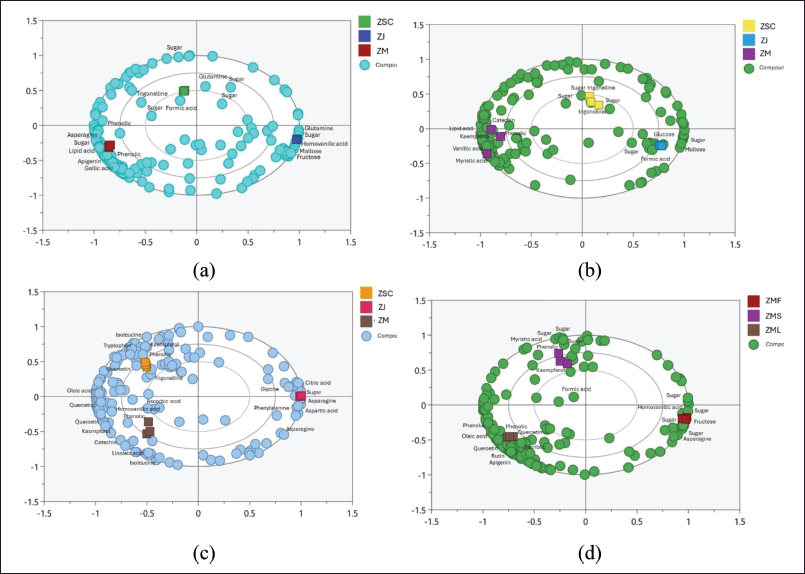 | Figure 3. Biplots of PCA analysis of fruits (a), seeds (b), leaves (C), and (d) Z. mauritiana’s all parts [Click here to view] |
Comparison of Zizhipus seed samples yielded a PCA model (R2X = 0.992 and Q2 = 0.972) with 3 well-separated clusters according to their species (Fig. 3b). Zizhipus mauritiana seeds contained higher catechin, kaempferol, fatty acids, and phenolics. The presence of catechin and kaempferol makes Z. mauritiana seeds particularly interesting for further research due to their potential bioactivity. Conversely, unknown sugar compounds are prevalent in Z. jujuba and Z. mauritiana seeds, alongside compounds like trigonelline and formic acid.
The next PCA model was created to compare metabolite profiles of Ziziphus leaf samples. This PCA model had a total variance (R2X) of 0.992 and a cross-validation value (Q2) of 0.966, resulting in clear separation among the Ziziphus leaf samples. Evaluating the corresponding biplot (Fig. 3c) revealed that Z. jujuba leaves were characterized by unknown sugar compounds, asparagine (bucket at δ 2.92–2.96 ppm), glycine (bucket at δ 353–3.57 ppm), aspartic acid (bucket at δ 278–2.80 ppm), and citric acid (bucket at δ 2.67–2.69 ppm). In contrast, Z. mauritiana and Z. spina-christi leaves were characterized by numerous phenolic and flavonoid components. Kaempferol, quercetin, isoleucine (bucket at δ 1.13–1.16 ppm), and tryptophan (bucket at δ 7.15–7.20 ppm) were identified as distinguished metabolites for Z. spina-christi leaves. Meanwhile, Z. mauritiana leaves were represented with catechin, quercetin, kaempferol, and unknown phenolics. Additionally, oleic acid, linoleic acid, and ascorbic acid were detected as other discriminant compounds for Z. mauritiana and Z. spina-christi leaves.
We also compared metabolite profiles of different organs of Z. mauritiana, yielding a PCA model with a total variance (R2X) of 0.991 and a cross-validation value (Q2) of 0.971. This model successfully distinguished the Z. mauritiana samples based on the organ type as depicted in Figure 3d. The corresponding biplot revealed that the Z. mauritiana fruits were characterized by abundant fructose and other unknown sugar compounds. Moreover, the fruit was also distinguished by asparagine (amino acid) and homovanlc acid (phenolic compound), indicating its diverse metabolic composition. Ziziphus mauritiana seeds were represented with kaempferol, myristic acid, and other unknown sugar compounds. Meanwhile, Z. mauritiana leaves were predominantly characterized by a high concentration of phenolic compounds, notably quercetin, apigenin, and rutin, which are well-known for their bioactive properties. Additionally, oleic acid, a monounsaturated fatty acid, was also detected as a discriminant compound in the leaf samples, further contributing to the distinctive metabolomic signature of Z. mauritiana leaves.
Correlation between biological activities and metabolite content of Ziziphus
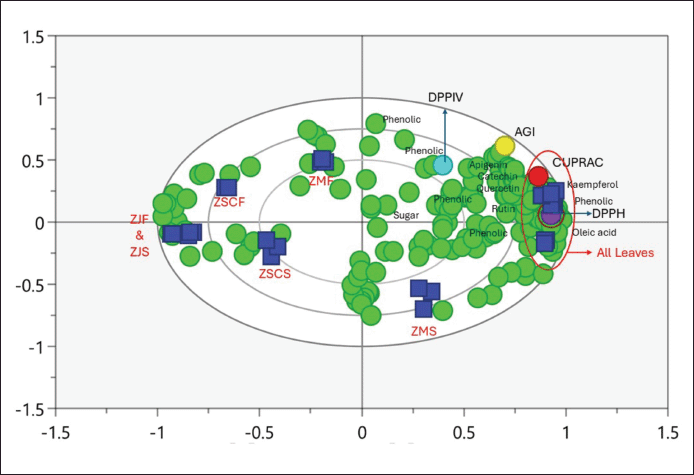
To assess the correlation between identified compounds and bioactivity, a partial least squares (PLSs) analysis was applied. Several compounds predicted to influence the activities were evaluated for their correlation coefficients, with the data displayed in biplot Figure 4 and Table 3. The PLS analysis demonstrated high reliability, indicated by R2X of 0.975, R2Y of 0.994, and Q2 of 0.973.
The components detected in the biplots include oleic acid, catechin, apigenin, kaempferol, and quercetin, along with their respective correlation coefficients, which are detailed in Table 3.
 | Figure 4. Biplot of PLS correlation of metabolites and bioactivities. [Click here to view] |
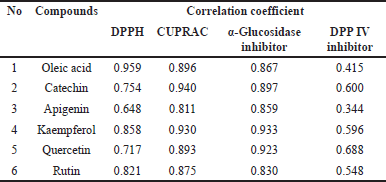 | Table 3. Identified compounds that correlate with activities. [Click here to view] |
Based on the obtained correlation values, oleic acid, kaempferol, and rutin exhibited correlation indices greater than 0.800 in the DPPH test, indicating a strong antioxidant capacity to reduce the DPPH radical. Meanwhile, all evaluated compounds showed correlation indices exceeding 0.800 in both the CUPRAC test and a-glucosidase inhibitory assay. It indicated that oleic acid, catechin, apigenin, kaempferol, quercetin, and rutin positively contributed to the antioxidant capacity and the inhibition of the a-glucosidase enzyme of the Zizhipus samples. Moreover, these results suggested that the components strongly correlated with these activities are phenolic and flavonoid compounds which are abundant in all Zizhipus leaves. However, none of these compounds showed a high correlation with DPP IV enzyme inhibition.
The PLS analysis presented in Figure 4 reveals that leaves are the organ most strongly associated with significant antioxidant activity and α-glucosidase inhibition. This is evident from the tight clustering of leaf sample data with bioactivity indicators, suggesting that the metabolites present in the leaves are key contributors to these bioactivities. In contrast, other plant organs, such as fruits and seeds, are positioned farther away from the leaf and bioactivity clusters, indicating a weaker or less direct correlation with these beneficial properties. The proximity of leaf samples to bioactivity indicators underscores the importance of leaf metabolites in driving these effects.
Table 3 provides a detailed overview of the specific metabolites found near the leaf samples, including oleic acid, catechin, apigenin, kaempferol, quercetin, and rutin. These compounds are well-known for their antioxidant properties and enzyme inhibition capabilities, further supporting the observed bioactivity in leaf samples. The presence of these bioactive compounds in the leaves suggests that this organ may play a pivotal role in the overall health benefits attributed to the plant. The findings emphasize the potential of leaf-derived extracts in developing natural remedies or functional foods aimed at combating oxidative stress and regulating blood sugar levels.
To assess the accuracy of the PLS model in correlating bioactivity with components, permutation tests were calculated for each bioactivity assessed: antioxidant activity using the DPPH and CUPRAC methods, and alpha-glucosidase enzyme inhibition activity. The DPP IV enzyme inhibition data was not analyzed due to its extremely low inhibition values, which made it non-informative for meaningful analysis. However, the validation of the antioxidant and enzyme inhibition models provided insightful results. For the DPPH method, the model yielded an R2 value of 0.347 and a Q2 value of −1.21. Similarly, the CUPRAC method produced an R2 of 0.341 and a Q2 of −1.23. The model for α-glucosidase inhibitor activity generated an R2 of 0.297 and a Q² of −1.32. These results show that all three models have R2 values greater than Q2, indicating good accuracy and suitability for correlation analysis. The permutation values can be seen in Figure 5.
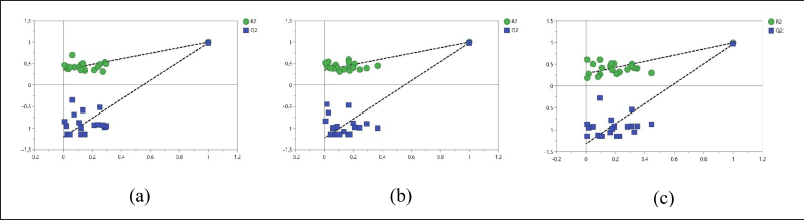 | Figure 5. Validate model of (a) DPPH, (b) CUPRAC, and (c) AGI. [Click here to view] |
DISCUSSION
In this work, a total of 50 metabolites were successfully annotated in Ziziphus samples. This metabolite annotation was validated by comparing it with the data obtained from the public databases (www.hmdb.ca and www.foodb.ca) and correlated literature [18–21]. Among those, nine metabolites were unknown compounds and predicted as phenolic compounds. These unknown compounds were exclusively found in the leaves of all samples, and in the seeds of Z. mauritiana as well. It is noteworthy that secondary metabolites, particularly phenolic compounds, dominated in the leaf organs. Consequently, leaves stand out as the primary focus for investigating and isolating bioactive components within the genus Ziziphus [1]. Moreover, all identified flavonoids were found in every leaf sample of Ziziphus, confirming that the leaves are the primary sources of flavonoids in this genus, and aligning with the previous reports [9,22].
Ziziphus species are recognized for their dominant polysaccharide content, with extensive research concentrating on the Z. jujuba fruit. Polysaccharides contribute to various biological activities and health benefits, highlighting their importance in the phytochemistry of Ziziphus. [23–26]. Unfortunately, in this work, we were unable to identify polysaccharides in all Ziziphus samples, which may be due to the soft extraction method employed using deuterated solvents.
Regarding the inhibition of the DPP-IV enzyme, none of the samples could be considered potent, as they all displayed very low inhibitory activity. At a concentration of 1,000 µg/ml, only the leaf and fruit extracts of Z. mauritiana demonstrated any potential inhibitory activity against the DPP-IV enzyme, with inhibition values of 17.69% ± 0.53% and 5.95% ± 0.13%, respectively. The other samples did not exhibit any notable inhibitory potential at this concentration. Among the nine Ziziphus samples tested for DPP-IV inhibition, only the fruit and leaf extracts of Z. mauritiana exhibited measurable inhibitory activity at 1,000 µg/ml. These findings indicate that the three Ziziphus species studied do not possess strong potential for DPP-IV inhibition. The observed activity in Z. mauritiana leaf and fruit extracts may be attributed to the presence of unknown bioactive compounds identified through metabolite annotation, which were not detected in Z. spina-christi and Z. jujuba. These compounds may belong to the phenolic and anthocyanin groups [27]. Additionally, curcumin, syringic acid, and resveratrol have been reported to exhibit high affinity for DPP-IV enzymes [28,29]. In contrast, sitagliptin (the positive control), at a significantly lower concentration of 1 µg/ml, achieved a much higher inhibition of 98.41% ± 0.05%.
Based on the correlation analysis between bioactivities and metabolite contents using multivariate data examination, six compounds exhibited a robust relationship with antioxidant properties and alpha-glucosidase inhibitory activity. These compounds are oleic acid, catechin, apigenin, kaempferol, quercetin, and rutin. The structures of these compounds can be visualized in Figure 6. Furthermore, this correlation highlighted their potential dual benefits, emphasizing their importance in both antioxidant activity and the inhibition of the alpha-glucosidase enzyme.
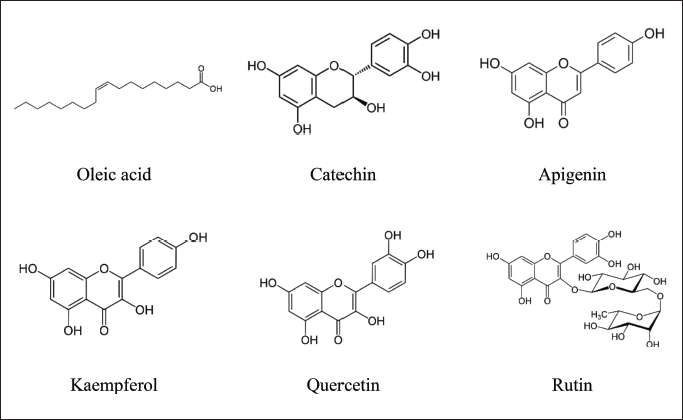 | Figure 6. Metabolites contained in Ziziphus samples that contribute to antioxidant bioactivity and inhibition of the alpha glucosidase enzyme. [Click here to view] |
At least, there are two metabolomic studies conducted on the same plants using different analytical instruments. The first metabolomic study by Sakna et al. [9] successfully detected 102 metabolites from the leaves of the three Ziziphus species using UHPLC/PDA/ESI-MS. This study also identified quercetin-3-O-(2-pentosyl)-rhamnoside as a marker metabolite in the leaves of Z. jujuba. The research further indicated that the leaves of Z. spina-christi and Z. mauritiana exhibited better antioxidant, anti-inflammatory, and antidiabetic activities compared to Z. jujuba [9]. However, this study did not highlight the active components responsible for these pharmacological activities. The differences in bioactivity results between the study by Sakna et al. [9] and our study can be attributed to variations in growing locations and other environmental factors. Additionally, the similarity in bioactivity between Z. spina-christi and Z. mauritiana may be due to their comparable metabolite compositions.
A similar study by Dawood et al. [30] employed UPLC-MS/MS to analyze the metabolomes of the seeds, ripe fruits, and leaves of Z. spina-christi and Z. lotus and assessed their bioactivities in inhibiting α-amylase and α-glucosidase enzymes. The study revealed that the fruits and leaves of Z. spina-christi exhibited the highest α-glucosidase inhibitory activity, while the fruits of Z. lotus showed the highest α-amylase inhibitory activity. The compounds contributing to these activities were identified as caffeic acid, ferulic acid, betulinic acid, hemsine, zizyotin, apetaline, quercetrin, jujubogenin, and zizyphursolic acid [30]. Interestingly, in our report here, quercetin was also identified as a compound having a positive correlation with the α-glucosidase inhibitory activity of Zizhipus samples. Besides quercetin, our study also revealed that oleic acid, catechin, apigenin, kaempferol, and rutin contributed positively to the α-glucosidase inhibitory activity of Zizhipus samples. Thus, our results shed more light on the bioactive compounds of Zizhipus plants.
The identification of oleic acid as a metabolite having positive correlations with antioxidant properties and α-glucosidase inhibitory activity of Zizhipus samples is in accordance with the previous reports [31,32]. The antioxidant mechanism of this compound is by reducing reactive oxygen species. Besides that, oleic acid is widely recognized for its numerous health benefits, including cancer prevention, cardiovascular protection, and anti-inflammatory effects [33,34]. Its benefits extend to hypoglycemic, hypotensive, cardioprotective, hepatoprotective, anti-inflammatory, and antimicrobial activities, which are likely due to its molecular structure that enhances its antioxidant capacity [35–39].
In this research, flavonoids including apigenin, catechin, kaempferol, and rutin, contributed positively to the antioxidant property and α-glucosidase inhibitory activity of Zizhipus samples. This result is in agreement with the previous works [40–43]. These compounds have shown considerable potential in reducing oxidative stress and regulating blood glucose levels [44–46]. These flavonoids work by inhibiting carbohydrate digestion, enhancing glucose uptake, and protecting pancreatic beta cells from hyperglycemia-induced damage. The antioxidant properties of these compounds, supported by their structural features, contribute to their effectiveness in various assays, making them valuable in managing hyperglycemia and maintaining overall metabolic health [47–49].
CONCLUSION
In this study, the 1H NMR-based metabolomics approach, coupled with multivariate analysis, effectively distinguished the fruit, seed, and leaf samples from Z. spina-christi, Z. jujuba, and Z. mauritiana based on their metabolite contents. Integrating antioxidant bioactivity tests, as well as assessments of alpha-glucosidase and DPP IV enzyme inhibition, this metabolomic approach successfully identified compounds contributing to these activities. The assay results revealed that Z. mauritiana leaves had the highest antioxidant, and α-glucosidase inhibitory activities compared to other samples. Correlation analysis successfully revealed compounds that contributed to these activities, including apigenin, catechin, kaempferol, quercetin, rutin, and oleic acid. These findings shed more light on the potential bioactive compounds in Ziziphus species, providing valuable insights for further exploration and utilization in various health-related applications.
ACKNOWLEDGMENTS
We are grateful to the Education Financing Service Center (BPPT), Ministry of Education, Culture, Research and Technology of the Republic of Indonesia (Kemdikbudristek RI) and Indonesia Endowment Fund for Education Agency (LPDP), Ministry of Finance of the Republic of Indonesia, for providing a scholarship to Ihsanul Hafiz via Beasiswa Pendidikan Indonesia (BPI) Program.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research obtained financial support from LPDP via the BPI Scholarship Program 2021.
CONFLICT OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. El Maaiden E, El Kharrasi Y, Qarah NAS, Khalid A, Moustaid K, Nasser B. Genus Ziziphus: a comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J Ethnopharmacol. 2020;259:112950. CrossRef
2. Yahia Y, Benabderrahim MA, Tlili N, Bagues M. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS One. 2020;15(5):e0232599. CrossRef
3. Li X, Li X, Zhou B, Man S, Gao W, Jing S. Study on the bioactive constituents and in vitro antioxidant and in vivo anti- inflammatory activities of extracts from the fruits of Ziziphus Jujuba Mill. cv. Jinsixiaozao Hort. Food Sci Technol Res. 2017;23(3):417–26. CrossRef
4. Zandiehvakili G, Khadivi A. Identification of the promising Ziziphus spinachristi (L.) Willd. genotypes using pomological and chemical proprieties. Food Sci Nutr. 2021;9:5698–711. CrossRef
5. Asgarpanah J, Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina-christi (L.) Willd. Afr J Pharm Pharmacol. 2012;6(31):2332–9. CrossRef
6. Andrade JC, Santos ATL, Silva ARP, Freitas MA, Afzal MI, Gonçalo MIP, et al. Phytochemical characterization of the Ziziphus joazeiro Mart. metabolites by UPLC-QTOF and antifungal activity evaluation. Cell Mol Biol. 2020;66(4):127–32. CrossRef
7. El Cadi H, Bouzidi HEL, Selama G, El Cadi A, Ramdan B, Oulad Y, et al. Physico-chemical and phytochemical characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” fruit crude crude extract and fractions. Molecules. 2020;25:5237. CrossRef
8. Ashraf A, Sarfraz RA, Anwar F, Shahid SA, Alkharfy KM. Chemical composition and biological activities of leaves of Ziziphus mauritiana L. native to Pakistan. Pakistan J Bot. 2015;47(1):367–76.
9. Sakna ST, Mocan A, Sultani HN, El-fiky NM. Metabolites profiling of Ziziphus leaf taxa via UHPLC/PDA/ESI-MS in relation to their biological activities. Food Chem. 2019;293:233–46. CrossRef
10. Niamat R, Khan MA, Khan KY, Ahmed M, Mazari P, Ali B, et al. A review on Zizyphus as antidiabetic. J Appl Pharm Sci. 2012;02(03):177–9. CrossRef
11. Mohankumar R, Eugine S, Prakash L, Irfan N, Mohanraj S, Kumarappan C. Evaluation of analgesic, anti-inflammatory, and antipyretic activities of Ziziphus mauritania Lam leaves in animal models. Pharmacol Res Mod Chinese Med. 2022;4:100153. CrossRef
12. Sakna ST, Maghraby YR, Abdelfattah MS, Farag MS. Phytochemical diversity and pharmacological effects of triterpenes from genus Ziziphus: a comprehensive review. Phytochem Rev. 2023;22:1611–36. CrossRef
13. Febrina L, Happyana N, Maolana Y. Metabolite profiles and antidiabetic activity of the green beans of Luwak (civet) coffees. Food Chem. 2021;355:129496. CrossRef
14. Celep E, Charehsaz M, Akyüz S, Türköz E, Yesilada E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res Int. 2015;78:209–15. CrossRef
15. Özyürek M, Bektasoglu B, Güclü K, Güngör N, Apak R. Simultaneous total antioxidant capacity assay of lipophilic and hydrophilic antioxidants in the same acetone – water solution containing 2% methyl-B-cyclodextrin using the cupric reducing antioxidant capacity (CUPRAC) method. Analytica Chimica Acta. 2008;630(1):28–39. CrossRef
16. Nor I, Ruslan K, Hartati R, Insanu M. The a-glucosidase inhibitory activity of avicularin and 4-O-methyl gallic acid isolated from Syzygium myrtifolium leaves. Saudi Pharm J. 2023;31(8):101677. CrossRef
17. Zhang Y, Chen R, Zuo F, Ma H, Zhang Y, Chen S. Comparison of dipeptidyl peptidase IV-inhibitory activity of peptides from bovine and caprine milk casein by in silico and in vitro analyses. Int Dairy J. 2016;53:37–44. CrossRef
18. Savova MS, Vasileva LV, Mladenova SG, Amirova KM, Ferrante C, Orlando G, et al. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed Pharmacother. 2021;141:111934. CrossRef
19. Gogna N, Hamid N, Dorai K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1 H NMR spectroscopy and multivariate statistical analysis. J Pharm Biomed Anal. 2015;115:74–85. CrossRef
20. Abreu AC, Marin P, Aguilera-Saes LM, Tristan AI, Pena A, Oliveira I, et al. Effect of a shading mesh on the metabolic, nutritional, and defense profiles of harvested greenhouse-grown organic tomato fruits and leaves revealed by NMR metabolomics. J Agric Food Chem. 2019;67(46):12972–85. CrossRef
21. Kim H, Jung S, Hyun S, Yang S, Lee J, Auh J, et al. Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation stage by 1 H NMR using multiple solvent systems. FRIN. 2011;44(7):1977–87. CrossRef
22. Moon KM, Hwang Y, Yang J, Yeul J, Lee B. Spinosin is a flavonoid in the seed of Ziziphus jujuba that prevents skin pigmentation in a human skin model. J Funct Foods. 2019;54:449–56. CrossRef
23. Ji X, Peng Q, Yuan Y, Shen J, Xie X, Wang M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chem. 2017;227:349–57. CrossRef
24. Yang Y, Qiu Z, Li L, Vidyarthi SK, Zheng Z, Zhang R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: a comparison. Carbohydr Polym. 2021;261(2020):117879. CrossRef
25. Chun HL, Su JG, Jin YL. Characterization of the chemical composition and in vitro anti-inflammation assessment of a novel lotus (Nelumbo nucifera Gaertn) plumule polysaccharide. Food Chem. 2011;125(3):930–5. CrossRef
26. Ji X, Hou C, Yan Y, Shi M, Liu Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int J Biol Macromol. 2020;149:1008–18. CrossRef
27. Amin MS, Saputri FC, Mun’im A. Inhibition of dipeptidyl peptidase 4 (DPP IV) activity by some Indonesia edible plants. Pharmacogn J. 2019;11(2):231–6. doi: CrossRef
28. Huang PK, Lin SR, Chang CH, Tsai MJ, Lee DN, Wang CF. Natural phenolic compounds potentiate hypoglycemia via inhibition of dipeptidyl peptidase IV. Sci Rep. 2019;9:15585. CrossRef
29. Lin SR, Chang CH, Tsai MJ, Cheng H, Chen JC, et al. The perceptions of natural compounds against dipeptidyl peptidase 4 in diabetes: from in silico to in vivo. Ther Adv Chronic Dis. 2019;10:1–16. CrossRef
30. Dawood HM, Shawky E, Zayed ME, Elsayed M, Ghareeb DA, Darwish RS. Metabolomics and chemometrics approaches unravel the metabolic diversity and in-vitro antidiabetic potential of two Ziziphus species. Ind Crop Prod. 2024;212:118288. CrossRef
31. Menendez JA, Papadimitropoulou A, Vellon L, Lupu R. A genomic explanation connecting “Mediterranean diet”, olive oil and cancer: Oleic acid, the main monounsaturated Fatty acid of olive oil, induces formation of inhibitory “PEA3 transcription factor-PEA3 DNA binding site” complexes at the Her-2/neu (erbB-2. Eur J Cancer. 2006;42(15):2425–32. CrossRef
32. Perona JS, Cabello-moruno R, Ruiz-gutierrez V. The role of virgin olive oil components in the modulation of endothelial function. J Nutr Biochem. 2006;17:429–45. CrossRef
33. Tutunchi H, Ostadrahimi A, Saghafi-asl M. The effects of diets enriched in monounsaturated oleic acid on the management and prevention of obesity: a systematic review of human intervention studies. Adv Nutr. 2020;11(4):864–77. CrossRef
34. Visioli F, Poli A, Galli C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev. 2002;22(1):65–75. CrossRef
35. Gill CIR, Boyd A, MecDermott E, McCann M, Servili M, Selvaggini R, et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer. 2005;117(1):1–7. CrossRef
36. Berbert AA, Kondo CRM, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Appl Nutr Investig. 2005;21:131–6. CrossRef
37. Mishra S, Chattopadhyay A, Naaz S, Ghosh AK, Das AR, Bandyopadhyay D. Oleic acid ameliorates adrenaline induced dysfunction of rat heart mitochondria by binding with adrenaline: an isothermal titration calorimetry study. Life Sci. 2019;218:96–111. CrossRef
38. Bitler CM, Viale TM, Damaj B, Crea R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J Nutr. 2005;135(6):1475–9. CrossRef
39. Owen RW, Haubner R, Wurtele G, Hull WE, Spiegelhalder B, Bartsch H. Olives and olive oil in cancer prevention. Eur J Cancer Prev. 2004;13(4):319–26. CrossRef
40. Yen FS, Qin CS, Tan S, Xuan S, Ying PJ, Le HY, et al. Hypoglycemic effects of plant flavonoids: a review. Evidence-Based Complement Altern Med. 2021;2021:2057333. CrossRef
41. Grzesik M, Napar K, Bartosz G, Sadowska-bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem. 2018;241:480–92. CrossRef
42. Dhanya R, Arya AD, Nisha P, Jayamurthy P. Quercetin, a lead compound against Type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front Pharmacol. 2017;8:1–9. CrossRef
43. Eid HM, Nachar A, Thong F, Sweeney G, Haddad PS. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn Mag. 2015;11(41):74–81. CrossRef
44. Mechchate H, Es-safi I, Haddad H, Bekkari H, Grafov A, Bousta D. Combination of catechin, epicatechin, and rutin: optimization of a novel complete antidiabetic formulation using a mixture design approach. J Nutr Biochem. 2021;88:108520. CrossRef
45. Tian C, Liu X, Chang Y, Wang R, Lv T, Cui C, et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South African J Bot. 2021;137:257–64. CrossRef
46. Kim J, Kang M, Choi H, Jeong S, Lee Y, Kim J. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5(2):107–11. CrossRef
47. Eid HM, Haddad PS. The antidiabetic potential of quercetin: underlying mechanisms. Curr Med Chem. 2017;24(4):355–64. CrossRef
48. Suan L. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol. 2013;150(3):805–17. CrossRef
49. Santulli G, Lacampagne A, Marks AR, Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest. 2015;125(5):1968–78. CrossRef