INTRODUCTION
Nerium oleander L. (synonyms are Nerium indicum and Nerium odorum) belongs to the family Apocynaceae [1–3]. Known as the common oleander, the plant is an evergreen shrub, producing clear, thick, and gummy sap. Leaves of N. oleander are linear, leathery, and dark green to greyish green, with distinct light-yellowish veins. Flowers are in clusters at the tip of twigs, scented, white, pink, or red in color, with five petals forming a central corolla tube (Fig. 1). There are varieties with pink double flowers. Fruits are narrow pods containing many silky-haired seeds [1–3].
The leaves and flowers of N. oleander are used in folk medicine for the treatment of many diseases such as heart failure, hemorrhoids, indigestion, leprosy, malaria, ringworms, snakebites, and ulcers [4]. In India, the leaves and roots are used to treat leprosy, piles, ringworms, and ulcers [5].
Major classes of chemical constituents of N. oleander are cardenolides, pregnanes, triterpenoids, flavonoids, iridoids, alkaloids, and steroids [6–9]. Cardenolides are 23-carbon steroids consisting of a steroid nucleus, a five-membered lactone moiety at C17 of ring D, and a hydroxyl ( ? OH) group at C3 at ring A, and C14 at the junction of rings C and D (Fig. 2) [10,11]. Glycones of cardenolides have a sugar moiety at C3 of ring A comprising one to three monosaccharide units.
From the leaves of N. oleander, cardenolides included adynerin, 5α-adynerin, deacetyloleandrin, digitoxigenin, folinerin, neriagenin, neriaside, odorosides A, B, G, and H, oleandrin, and oleaside A [4]. Oleandrin, odorosides A, B, G, H, and K, gentiobiosyl odoroside A, and odorobioside G have also been reported Wen [11]. New cardenolides were 14-carbonyl-neriaside, 21-hydroxy-neriaside, 16-hydroxy-oleaside A, and 5α-oleaside A. From the stems and twigs of N. oleander, four new cardenolide monoglycosides N-1 to N-4 were isolated, together with nine known cardenolides and cardenolide monoglycosides [12], and three new cardenolides B-1, B-2, and oleagenin [13].
 | Figure 1. White (left), pink (middle), and red (right) flowers of N. oleander. [Click here to view] |
Flavonoids such as apigenin 7-O-galactoside, isorhamnetin 3-O-galactoside, luteolin 4’- methyl ether, and luteolin 7-O-glucuronide have been reported from the aerial parts of N. oleander [14]. From the leaves, phenolic compounds such as quercetin, kaempferol, rutin, chlorogenic acid, caffeoylquinic acids, and dicaffeoylquinic acids have been reported [15].
Cardenolides from stems and twigs of N. oleander have been reported to exhibit cytotoxic, anti-inflammatory, and multi-drug resistance (MDR)-reversal activities [12,16]. Recently, pharmacological properties from different plant parts of N. oleander have been reported by Pandey et al. [17]. From the leaves, anti-inflammatory, immunomodulatory, anti-leukemia, diuretic, larvicidal, antimicrobial, anti-diabetic, antinociceptive, and antioxidant activities have been reported. Flowers possess anticancer, hepatoprotective, anti-inflammatory, and antioxidant activities. Roots and bark have antimicrobial activities.
In this overview, the chemistry, resources, anticancer effects, other pharmacological properties, and clinical trials of oleandrin from N. oleander are reviewed. To date, there are two reviews on the anticancer effects of oleandrin [18,19]. The current overview focuses on the other pharmacological properties of oleandrin, including those of two commercial drugs of oleandrin, namely, Anvirzel and PBI-05204. The anticancer effects and mechanisms of oleandrin serve as an update while the other pharmacological properties of oleandrin present new and interesting findings. Sources of information used in this overview were Google, Google Scholar, ScienceDirect, PubMed, Wiley, Springer, J-Stage, and PubChem.
CHEMISTRY
Oleandrin (synonyms are foliandrin, folinerin, neriolin, and neriostene) has a molecular formula of C32H48O9 and a molecular weight of 576.7 g/mol [20]. Oleandrin is a cardenolide. Cardenolides are 23-carbon steroids consisting of a steroid nucleus, a five-membered unsaturated lactone moiety at C17 of ring D, a glucose moiety at C3 of ring A, and an OH group at C14 at the junction of rings C and D (Fig. 2) [10]. Oleandrin contains a central steroid nucleus with a lactone structure at C17 (encircled in blue), a dideoxy arabinose or L-oleandrose group at C3 (encircled in red), and an acetyloxy group at C16 (encircled in purple) (Fig. 3) [20,21]. The dideoxy arabinose group is a 2-deoxy sugar molecule while the acetyloxy group has a −O−C(=O)−CH3 structure. Oleandrin when deglycosylated (without the L-oleandrose group), oleandrigenin is formed. Oleandrigenin is, therefore, an aglycone of oleandrin.
RESOURCES
A study was conducted on the quantity of oleandrin in dried plant parts of N. oleander sampled in May 2012 from different regions of Syria [22]. Results on the amount of oleandrin ranged from 0.18 to 0.31 mg/g in leaves, 0.12−0.23 mg/g in the stem, and 0.34−0.64 mg/g in the root. The amount of oleandrin was generally the lowest in the flower (0.07−0.13 mg/g). Ranking of oleandrin quantity from different plant parts of N. oleander was therefore root > leaf > stem > flower. In Brazil, the amount of oleandrin in the leaves did not show a statistically significant difference between varieties having red flowers (4.31 mg/g), pink flowers (4.16 mg/g), and white flowers (6.20 mg/g) [23]. The mean amount was 4.89 mg/g. In India, the quantity of oleander in the leaves of N. oleander ranged from 368 µg/g in the winter season to 704 µg/g in the rainy season [24]. Amounts in the stem were much lower ranging from 58 to 71 µg/g. A recent study on the quantity of oleandrin from dried leaf samples collected from five provinces in eastern Algeria ranged from 0.01 to 0.48 mg/g [25]. The mean amount was 0.12 mg/g. As reported earlier by Pedroza et al. [23], the difference in the quantity of oleandrin from the leaves of cultivars with pink, red, and white flowers was statistically not significant. An early study in Egypt compared the quantity of oleandrin in different plant parts (leaf, flower, stem, and root) of N. oleander plants during the flowering stage [26]. Data of varieties were 0.18%, 0.17%, 0.10%, and 0.08% with red flowers, and 0.15%, 0.14%, 0.08%, and 0.07% with white flowers, respectively, suggesting that the amounts of oleandrin of the red flower varieties were slightly higher than those of the white flower varieties.
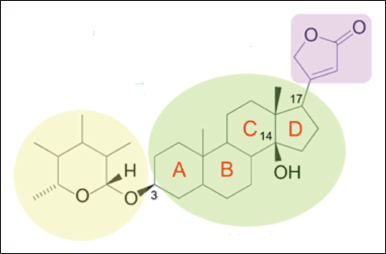 | Figure 2. General molecular structure of a cardenolide. [Click here to view] |
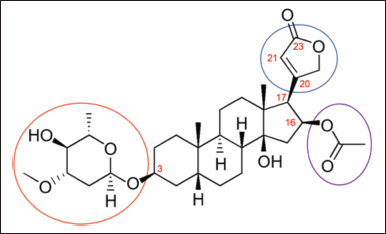 | Figure 3. Molecular structure of oleandrin. [Click here to view] |
ANTICANCER PROPERTIES
Cytotoxicity
Several studies have reported on the cytotoxicity of oleandrin in IC50 values against different types of cancer cells. Against Panc-1, MiaPaca, and BxPC3 pancreatic cancer cells, the cytotoxicity of oleandrin was 5.6, 15.6, and 210 nmol/l, respectively [27]. The cytotoxicity of oleandrin against undifferentiated CaCO-2 colon cancer cells (8.25 nM) was more than 3.0 times stronger than that of differentiated cells (>25 nM) [28]. Against a panel of six types of cancer cells, the IC50 values of oleandrin were 0.02, 0.04, 0.07, 0.10, 0.03, and 0.33 µM against HCT116 colon, HT29 colon, SW620 colon, RKO colon, GT gastric and HeLa cervical cancer cells, respectively [4]. Against a panel of breast cancer cells, the IC50 values of oleandrin at 24 h and 48 h were 14.5 and 6.07 nM for MCF-7, 6.13 and 1.42 nM for SK-BR-3, and 24.6 and 11.5 nM for MDA-MB-231, respectively [29]. Oleandrin inhibited SW480 colon cancer cells (0.02 μM) [30]. Cytotoxicity against non-cancer NCM460 colon epithelial cells (0.56 μM) was 28 times weaker. Oleandrin induced apoptosis in Jurkat leukemia, U-937 lymphoma, HL-60 leukemia, HeLa cervical, and MCF-7 breast cancer cells but not in SP-2, J774, C2C12, P338D1, and NIH3T3 murine cancer cells. Raghavendra et al. [31]. investigated the anticancer mechanisms against the human cancer cells included the expression of Fas ligand, dephosphorylation of Akt, and alteration of membrane fluidity.
Studies have demonstrated that the anticancer properties of oleandrin involved the inhibition of cell proliferation, reduction in cell viability, induction of apoptosis, and promotion of cell cycle arrest. In a review by Kanwal et al. [18], cancer types affected by oleandrin were lung, prostate, colon, pancreatic, melanoma, osteosarcoma, breast, cervical, leukemia, and brain cancers. In another review, the types of cancer cells affected by oleandrin included breast, lung, pancreatic, colon, prostate, colorectal, oral, ovarian, glioma, melanoma, glioblastoma, and lymphoma cancer [19]. Oleandrin induces apoptosis via modulation of multiple cellular signaling pathways such as nuclear factor kappa B cells (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt). In another review, the mechanisms of oleandrin included inhibition of Wnt/β-catenin signaling; reduction of NF-kB and Jun N-terminal kinase (JNK); activation of activator protein-1 (AP-1); inhibition of 12-O-tetradecanoylphorbol-13-acetate (TPA); enhancement of apoptosis; and promotion of autophagic cell death [32].
Against PANC-1 pancreatic cancer cells, the anticancer properties of oleandrin involved inhibition of tumor cell proliferation that the reduction of pAkt and increased pERK are important anticancer targets [33]. Against BRO melanoma cells, oleandrin modulated tumor necrosis factor (TNF-α), degradation of IκB-α, and release of cytochrome c and poly (ADP-ribose) polymerase (PARP) cleavage [34]. Oleandrin halted the NF-κB activity and inhibited TNF-mediated AP-1 activation.
In Table 1, including data from Kanwal et al. [18] and Francischini et al. [19], oleandrin has anticancer effects on a total of 19 types of cancer cells, suggesting the very diverse anticancer activities of oleandrin. All cancer types are represented by single studies. They include pancreatic, leukemia, cervical, osteosarcoma, glioblastoma, liver, melanoma, prostate, ovarian, oral, uterus, endometrial carcinoma, lymphoma, glioma, and rhabdomyosarcoma cancer cells (Table 1). Exceptions are colon (3), lung (2), breast (2), and osteosarcoma (2) cancer cells that are represented by more than one study. The data from Table 1 agree with the observation by Manna et al. [35] that the anticancer effects of oleandrin are not cell-type specific.
Table 1. A summary of anticancer properties of oleandrin.
| Cancer type, effect, and mechanism | References |
|---|---|
| Colon cancer (digestive system) | |
| Oleandrin caused autophagic cell death and altered ERK phosphorylation in undifferentiated CaCO-2 colon cancer cells but not in differentiated cells. The IC50 values for cytotoxicity were 8.25 and >25 nM, respectively. | [28] |
| Oleandrin induced apoptosis in SW480 colon cancer cells via the mitochondrial pathway. IC50 value for cytotoxicity against SW480 cells was 0.02 μM, whereas against NCM460 normal colon cells, cytotoxicity was 0.56 μM or 28 times weaker. | [30] |
| Oleandrin suppressed HCT-116 colon cancer cells by inducing apoptosis and by suppressing GRP78 (a chaperone protein within the endoplasmic reticulum). It also impeded tumor growth in a xenograft model by suppressing GRP78 expression. | [37] |
| Lung cancer (respiratory system) | |
| Oleandrin induced DNA damage responses in A549 and H1299 lung cancer cells by suppressing the expression of Rad51, a key protein involved in HR. | [38] |
| Against A549 and H1299 lung cancer cells, oleandrin enhanced radiotherapy sensitivity, suppressed ATM/ATR -mediated DNA damage response, and reduced DDR ability. | [39] |
| Breast cancer (breast) | |
| Oleandrin induced apoptosis in MCF-7, MDA-MB-231, and SK-BR-3 breast cancer cells via activation of endoplasmic reticulum stress. Anticancer properties did not affect normal mammary epithelial cells. | [29] |
| Oleandrin exhibited anticancer effects against MDA-MB-231 and RT-R MDA-MB-231 breast cancer cells by inhibiting invasion via suppression of the STAT-3 signaling pathway. Cytotoxic IC50 values were 72 and 183 nM, respectively. | [36] |
| Osteosarcoma (bone) | |
| Oleandrin inhibited U2OS and SaOS-2 osteosarcoma cells by increasing ROS, decreasing MMP, and activating the intrinsic and extrinsic apoptotic pathways. | [40] |
| Oleandrin synergistically sensitized U-2OS and MG-63 osteosarcoma cells to cisplatin by preventing degradation of CTR1 and down-regulating the expression of proteome-related genes. | [41] |
| Skin cancer (skin) | |
| Oleandrin inhibited TPA-induced tumors in the skin of mice by increasing the expression of PI3K, phosphorylation of Akt, and activation of NF-κB. | [42] |
| Oleandrin inhibited A375 melanoma cells by down-regulating the TLR pathway and suppressing the expression of associated miRNA. The IC50 value of oleandrin against A375 cells was 47 nM. | [43] |
| Other cancer cells | |
| Oleandrin suppressed HeLa cervical cancer cells (genital system) via potentiation of apoptosis, and activation of NF-κB and AP-1. | [44] |
| Oleandrin induced autophagic cell death in PANC-1 pancreatic cancer cells (digestive system) by reducing pAkt and increasing pERK expression. | [33] |
| Oleandrin reduced GL261 and GBM glioblastoma (brain and CNS) growth by inhibiting cell proliferation, reducing tumor size, and increasing the survival of mice with tumors. | [45] |
| Oleandrin inhibited HepG2 liver cancer cells (digestive system) by stimulating GLUT1 endocytosis via intracellular Na+K+-ATPase α3-isoform. | [46] |
| Oleandrin inhibited Ishikawa endometrial carcinoma cells (genital system) with an IC50 value of 75.3 nM by suppressing colony formation, invasion, and migration via the EMT pathway. | [47] |
Akt = protein kinase B, API-1 = activator protein-1, ATM = Ataxia-telangiectasia mutated kinase, ATR = Ataxia telangiectasia and Rad3-related kinase, CNS = central nervous system, CTR1 = copper transporter 1, DDR = DNA damage repair, DNA = deoxyribonucleic acid, EMT = epithelial-mesenchymal transition, ER = endoplasmic reticulum, ERK = extracellular signal-regulated kinase, FasL = Fas ligand, GBM = glioblastoma multiforme, GLUT1 = glucose transporter 1, HR = homologous recombination, MMP = mitochondrial membrane potential, NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells, pAkt = phosphorylated Akt, pERK = protein kinase RNA-like ER kinase, RNA = ribonucleic acid, ROS = reactive oxygen species, RT-R = radiotherapy-resistant, STAT-3 = signal transducer and activator of transcription 3, TLR = toll-like receptor, and TPA = 12-O-tetradecanoylphorbol-13-acetate.
Anticancer structure activity relationship (SAR)
The SAR of oleandrin suggests that anticancer activity of oleandrin is in part due to the presence of a sugar moiety at C3 [18]. The absence of a sugar moiety at C3 as in odoroside A, an oleandrin derivative, displayed markedly weaker anticancer activity. Against MDA-MB-231 breast cancer cells and radiotherapy-resistant (RT-R) MDA-MB-231 breast cancer cells, the cytotoxicity of oleandrin was 72 and 183 nM, respectively [36]. Odoroside A without the C3 sugar moiety was cytotoxic to MDA-MB-231 breast cancer cells (163 nM) but not cytotoxic to RT-R MDA-MB-231 breast cancer cells.
Anvirzel and PBI-05204
Anvirzel and PBI-05204 are two commercial anticancer drugs from N. oleander. Oleandrin is the principal bioactive component with contents of 2.5 µg/mg [48] and 3.0% [49], respectively. Anvirzel is available as a lyophilized powdered extract from Ozelle Pharmaceuticals, Inc. in San Antonio, Texas, USA. PBI-05204, a supercritical CO2 extract can be purchased from Universal Biologicals in Cambridge, UK.
Anvirzel and oleandrin were highly cytotoxic to human cancer cells (PC-3M prostate cancer cells) but not murine cancer cells (K1735-X21 melanoma cells) [50]. Cytotoxicity against PC-3M cells occurred at low drug concentration, and mediated via loss and fragmentation of DNA, and cell cycle arrest. In another study, Anvirzel and oleandrin inhibited the growth of PC3 and DU145 prostate cancer cells by reducing the release of fibroblast growth factor-2 [51]. The anticancer efficacy of Anvirzel was tested against 37 cancer cell lines by Apostolou et al. [52]. At concentrations of 0.01–0.05 ng/ml, the efficacy of Anvirzel was evident in the prostate (VCaP, LNCaP, and PC3), lung (COLO699N and CALU-1), colon (LoVo and SW480), uterus (COLO684), and breast (T47D) cancer cells. Cytotoxicity was the strongest at incubation of 72 h. Against CALU-1 lung cancer cells, cytotoxicity of Anvirzel was more effective in combination with carboplatin and docetaxel than in monotherapy [53]. In another study, the efficacy of Anvirzel was tested against breast (MCF-7), colon (HCT-15), lung (CALU-1), prostate (PC3), melanoma (A375), and pancreatic (PANC-1) cancer cells in combination with cisplatin [54]. When used in combination, the anticancer activity was more effective than in monotherapy even at low concentrations. Against U87 glioma cells, Anvirzel markedly inhibited cell growth via inhibition of glycogen synthase kinase-3 (GSK-3), nitric oxide synthase (NOS) and hypoxia-inducible factor 1-alpha (HIF1-α) and by activation of extracellular signal-regulated kinase (ERK) [55].
PBI-05204 inhibited the growth of PANC-1 cancer cells by targeting the PI3K/Akt and mammalian target of rapamycin (mTOR) pathways [56]. PBI-05204 inhibited the growth of U87MG, U251, and T98G glioblastoma cells by reducing Akt/mTOR activities, and by modulating the renewal of glioma stem cells (GSC) [57]. PBI-05204 also inhibited the growth of these glioblastoma cells in mice xenograft models. In a related study, PBI-05204 enhanced the anti-tumor efficacy of radiotherapy in the treatment of U251, A172, U87MG, and T98G glioblastoma cells [57]. Mechanisms involved apoptotic cell death, down-regulation of the PI3K/mTOR pathway, and reduction of stemness in glioblastoma multiforme (GBM) cells and in glioma tumor-initiating cells.
PBI-05204 sensitized alveolar and embryonal rhabdomyosarcoma (RMS) cells to radiotherapy [58]. RMS is a soft tissue sarcoma in children and adolescents. Against fusion negative (FN)-RMS and fusion positive (FP)-RMS cells, PBI-05204 induced cell death, cell cycle arrest, and counteracted cell migration and invasion. The role of PBI-05204 in enhancing the radio-sensitizing proprieties of tumors in mice xenograft experiments was also demonstrated. In another study by Chakraborty et al. [59], PBI-05204 suppressed the growth of glioblastoma stem cells (GBM9, GSC28, and TS543), by inhibiting GRP78 (a protein primarily found in the endoplasmic reticulum) and inducing necroptosis (a programmed cell death that resembles necrosis).
In 2000, a Phase I clinical study using Anvirzel for patients with advanced solid tumors was approved by the U.S. Food and Drug Administration (FDA) [60]. Phase I and Phase II clinical trials of PBI-05204 for treating cancer have been conducted [61,62]. A brief summary of the clinical trials of Anvirzel and PBI-05204 is shown in Table 2.
Table 2. A brief summary of clinical trials on Anvirzel and PBI-05204.
Title: Phase I trial of Anvirzel in patients with advanced solid tumors [60]. Summary: Objective: The current Phase I trial study reported on the maximum tolerated dose (MTD) and safety of Anvirzel in patients with advanced solid tumors. Methods: Patients were divided into randomized groups and were given injections at doses of 0.1, 0.2, and 0.4 ml/m2/day with subsequent patients receiving 0.8 or 1.2 ml/m2/day. Eighteen patients completed at least one treatment cycle of three weeks. Results: Most patients developed mild pain at the injection site (78%). Other symptoms included fatigue, nausea, and shortness of breath. Conclusion: Anvirzel can be safely administered at doses up to 1.2 ml/m2/day. The recommended Phase II dose level was 0.8 ml/m2/day. Title: First study of PBI-05204 in patients with advanced solid tumors [61]. Summary: Objective: The study aimed to determine the safety, pharmacokinetics, and pharmacodynamics of PBI-05204 in patients with advanced cancer. Methods: Forty-six patients received PBI-05204 orally. The dose was escalated 100% until grade two toxicity was observed. Plasma PK and mTOR effector protein expressions were evaluated. Results: Dose-limiting toxicity was observed at 0.338 mg/kg/day. Common adverse effects were fatigue, nausea, and diarrhea. The MTD was 0.225 mg/kg. Seven patients had stable disease for more than four months. Conclusion: The recommended Phase II dose was 0.225 mg/kg/day. Title: Phase II study on PBI-05204 in patients with advanced PANC-1 adenocarcinoma [62]. Summary: Objective: A phase II, single-arm, open-label study to determine the efficacy of PBI-05204 in patients with refractory mPDA therapy was conducted. The primary endpoint was overall survival (OS), with the hypothesis that 50% of patients would be alive at 4.5 months. Methods: Patients received oral PBI-05204 daily. Ten patients were alive at 4.5 months. Common symptoms were fatigue, vomiting, nausea, decreased appetite, and diarrhea. Conclusion: PBI-05204 did not meet its primary end-point for OS. A Phase II trial is being designed. |
OTHER PHARMACOLOGICAL PROPERTIES
Neuroprotective
The neuroprotective properties of oleandrin and PBI-05204 were studied using a brain slice assay and an in vivo novel model of neuroprotection, both using rat pups [49]. In the brain slice assay, oleandrin and PBI-05204 exerted neuroprotection to neural tissues damaged by oxygen and glucose deprivation. In an in vivo novel model, oleandrin was shown to penetrate the blood–brain barrier suggesting that oleandrin can be used for treatment of ischemic stroke and other neurological disorders. An earlier study by Ni et al. [63] reported that the lipophilic property of oleandrin allowed it to permeate the blood–brain barrier as reflected by the presence of oleandrin in brain tissues of mice 30 min after intra-peritoneal injection.
Van Kanegan et al. [64] reported that oleandrin in PBI-05204 protected rat brain slices damaged by oxygen-glucose deprivation (OGD) mediated by the brain-derived neurotrophic factor (BDNF) and apoptotic mechanisms. A follow-up study on the neuroprotective properties of PBI-05204 found that PBI-04711 (a fraction of PBI-05204) exerted broad neuroprotection that may ameliorate neurodegenerative disorders such as Alzheimer’s disease and dementia [65].
Oleandrin administered alone or in combination with temozolomide increased the survival of glioma-implanted mice by releasing BDNF and activating tropomyosin receptor kinase B (TrkB), a neuroprotective target, suggesting the potential use of oleandrin in the treatment of neurodegenerative, neurooncological, and other neurological disorders [66]. Oleandrin possessed neuroprotection in rotenone-exposed zebrafish as Parkinson’s disease model by improving mitochondrial function and reducing oxidative stress [67]. A recent study showed superior neuroprotective properties in a derivative of oleandrin [68]. The content of C4’-dehydro-oleandrin in the brain was four times higher than in the heart using mice and human neural cells. In addition, the oleandrin derivative also exhibited promising pharmacological properties such as better brain bioavailability and lower toxicity than oleandrin. In another recent study, Nguyen et al. [69] reported that oleandrin had neuroprotective effects on rat cortical neurons by rescuing loss in neuron and neurite viability, up-regulating miR-132 and miR-212, and ameliorating neurodegeneration.
Anti-inflammatory
An early study by Manna et al. [70] reported that the anti-inflammatory properties of oleandrin used in the treatment of cancer cells. Mechanisms involved inhibition of NF-κB and activator protein 1 (AP-1) by blocking tumor necrosis factor (TNF). A follow-up study by the same group of scientists showed that oleandrin suppressed interleukin 8 (IL-8) in neutrophils and macrophages by down-regulating IL-8 receptors through altering membrane fluidity [35]. Oleandrin also inhibited IL-8-induced NF-κB, epidermal growth factor (EGF), nerve growth factor (NGF), and formyl peptide (FMLP), suggesting that oleandrin might be a significant inhibitor of inflammation.
Anti-osteolysis
The anti-osteolysis activities of oleandrin have been recently reported. In a mouse skull model, oleandrin inhibited receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis and bone resorption [71]. Mechanisms involved the suppression of nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) via the NF-κB/PI3K signaling pathway. Oleandrin also has the ability to suppress osteolysis or osteoclast by reducing bone loss in osteoporotic mice [72]. Inhibition of osteoclast differentiation involved the low-density lipoprotein receptor-related protein 4 (LRP4)/MAPK/NF-κB signaling pathway in the treatment of osteoporosis.
Anti-viral
Studies have shown that oleandrin possesses anti-viral properties [73]. Oleandrin in Anvirzel had the ability to inhibit the replication of human immunodeficiency virus (HIV) [74]. The anti-viral mechanisms demonstrated that oleandrin diminished the HIV envelope glycoprotein expression by reducing viral infectivity in order to induce anti-HIV effects. In human T-cell leukemia virus type-1, oleandrin inhibited viral infectivity by forming virological synapses and reducing viral transmission [75]. Against SARS-CoV-2, the coronavirus that causes COVID-19, administration at oleandrin (in pure form or as ingredient of PBI-06150) at 0.05 and 0.10 μg/ml exhibited strong anti-viral activity with an 800-fold and 3,000-fold reduction in viral production, respectively [76]. Oleandrin and PBI-06150 activated innate immune cells, enhanced anti-viral immune responses through activation of natural killer cells and interferon-gamma, and modulated immune responses under inflammation [77].
Anti-allergy
A study reported that oleandrin possessed anti-allergic effects by down-regulating key cytokines secreted by canine dermal fibroblasts and DH82 macrophages, under inflamed conditions [78]. The effects of oleandrin were superior than those of oclacitinib, a medication used to control itching in dogs, associated with allergic dermatitis.
Toxicity
The toxic effects of oleandrin on saline organisms have been reported by Liu et al. [79]. Against the barnacle Balanus albicostatus, the EC50 and LC50 of oleandrin were 15.1 and 16.3 ng/ml. Against the brine shrimp Artemia salina, the LC50 of oleandrin was 28.1 µg/ml. The central steroid nucleus and the sugar moiety at C3 of oleandrin are crucial for its antifouling activity.
CONCLUSION
The anticancer properties of oleandrin, and anticancer drugs of Anvirzel, and PBI-05204 are fairly well-reviewed. In this overview, information on their anticancer properties serves as an update. Of special interest are their other pharmacological properties that present new and interesting findings. Oleandrin and the two anticancer drugs have anticancer effects on a total of 19 types of cancer cells, suggesting that their anticancer activities are very diverse and not cell-type specific. Further research on the anticancer efficacy in combination among themselves and with other anticancer drugs would yield a more in-depth understanding of their anticancer properties. SAR studies of oleandrin especially on the synthesis of derivatives with superior anticancer activities would attract the interest of natural product scientists. Of special interest are the other pharmacological properties of oleandrin, Anvirzel, and PBI-05204. They include neuroprotective, anti-inflammatory, anti-osteolysis, anti-viral, anti-allergy, and cytotoxic effects on saline organisms. Studies have shown that oleandrin, PBI-05204, a derivative of oleandrin, and a fraction of PBI-05204 possess superior neuroprotective properties. Their neuroprotective properties present an exciting field of research especially their ability to penetrate the blood–brain barrier blood. Clinical trials and field studies on the non-cancer pharmacological properties of oleandrin favor further studies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHORS’ DECLARATION
The authors hereby declare that the work in documenting this overview is original and that any liability for claims relating to the content of this article will be borne by them. They have not made use of artificial intelligence (AI) tools when writing and editing the manuscript, including the generation of images.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bandara V, Weinstein SA, White J, Eddleston M. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon. 2010;56(3):273−81. CrossRef
2. Wong SK, Lim YY, Chan EWC. Botany, uses, phytochemistry and pharmacology of selected Apocynaceae species: a review. Pharmacogn Commun. 2013;3:1−11. CrossRef
3. Seher A, Hanif MA, Ayub MA, Jilani MI, Mahomoodally MF. Oleander. In: Hanif MA, Nawaz H, editors. Medicinal plants of South Asia. Amsterdam: Elsevier. 2020;525−39. CrossRef
4. Cao YL, Zhang MH, Lu YF, Li CY, Tang JS, Jiang MM. Cardenolides from the leaves of Nerium oleander. Fitoterapia. 2018;127:293−300. CrossRef
5. Upadhyay RK. Phytochemistry, therapeutic and pharmacological potential of Nerium oleander L. Int J Green Pharm. 2024;18(3):136−46.
6. Bai L, Wang L, Zhao M, Toki A, Hasegawa T, Ogura H, et al. Bioactive pregnanes from Nerium oleander. J Nat Prod. 2007;70(1):14−8. CrossRef
7. Sharma P, Choudhary AS, Parashar P, Sharma MC, Dobhal MP. Chemical constituents of plants from the genus Nerium. Chem Biodivers. 2010;7(5):1198−207. CrossRef cbdv.200900172
8. Chan EWC, Wong SK, Chan HT. Apocynaceae species with antiproliferative and/or antiplasmodial properties: A review of ten genera. J Integr Med. 2016;14(4):269−84. doi: https:// doi.org/10.1016/S2095-4964(16)60261-3
9. Ayouaz S, Arab R, Mouhoubi K, Madani K. Nerium oleander Linn: A review of chemical, pharmacological and traditional uses. J Biomed Res Environ Sci. 2023;4(4):2766−276. doi: https:// doi.org/10.37871/jbres1720
10. Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R. Cardiotonic steroids on the road to anticancer therapy. Biochim Biophys Acta - Rev Cancer. 2007;1776(1):32−57. CrossRef
11. Wen S, Chen Y, Lu Y, Wang Y, Ding L, Jiang M. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia. 2016;112:74−84. CrossRef
12. Zhao M, Bai L, Wang L, Toki A, Hasegawa T, Kikuchi M, et al. Bioactive cardenolides from the stems and twigs of Nerium oleander. J Nat Prod. 2007;70(7):1098−103. CrossRef
13. Bai L, Zhao M, Toki A, Sakai JI, Yang XY, Bai Y, et al. Three new cardenolides from methanol extract of stems and twigs of Nerium oleander. Chem Pharm Bull. 2010;58(8):1088−92. doi: https: //doi.org/10.1248/cpb.58.1088
14. Shams KA, Radwan HM, Tawfik WA, Habib AA, Abdel-Mohsen MM, Abou-Setta LM. Chemical constituents of lipids, proteins and flavonoids of Nerium oleander L. growing in Egypt and their biological activity. Asian J Chem. 2011;23(8):1−6.
15. Ayouaz S, Oliveira-Alves SC, Lefsih K, Serra AT, da Silva AB, Samah M, et al. Phenolic compounds from Nerium oleander leaves: Microwave-assisted extraction, characterization, antiproliferative and cytotoxic activities. Food Funct. 2020;11(7):6319−31. CrossRef
16. Bai Y, Zhao M, Bai L, Hasegawa R, Sakai JI, Hasegawa T, et al. The biological activities of cardenolide triglycosides from stems, twigs, and leaves of Nerium oleander. J Wood Sci. 2011;57:56−65. CrossRef
17. Pandey A, Usmani S, Ahmad M, Khatoon S, Wahab S, Prakash O. Phytochemical and pharmacological attributes of Nerium oleander: a review. Curr Nutr Food Sci. 2024;20(5):570−85. CrossRef
18. Kanwal N, Rasul A, Hussain G, Anwar H, Shah MA, Sarfraz I, et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem Toxicol. 2020;143:111570. CrossRef
19. Francischini CR, Mendonça CR, Barcelos KA, Silva MA, Botelho AF. Antitumor effects of oleandrin in different types of cancers: systematic review. Toxicon. 2022;216:15−27. CrossRef
20. Zhai J, Dong X, Yan F, Guo H, Yang J. Oleandrin: a systematic review of its natural sources, structural properties, detection methods, pharmacokinetics and toxicology. Front Pharmacol. 2022;13:822726. CrossRef
21. Kumar A, De T, Mishra A, Mishra AK. Oleandrin: a cardiac glycoside with potent cytotoxicity. Pharmacogn Rev. 2013;7(14):131−9. CrossRef
22. Tayoub G, Sulaiman H, Alorfi M. Analysis of oleandrin in oleander extract (Nerium oleander) by HPLC. J Nat Prod. 2014;7:73−8.
23. Pedroza HP, Ferreira MG, Carvalho JG, Melo KD, Keller KM, Melo MM, et al. Oleandrin concentrations in Nerium oleander leaves of different flowering colors. Ciencia Rural. 2015;45(5):864−7.
24. Singh Y, Nimoriya R, Rawat P, Mishra DK, Kanojiya S. Quantitative evaluation of cardiac glycosides and their seasonal variation analysis in Nerium oleander using UHPLC-ESI-MS/MS. Phytochem Anal. 2022;33(5):746−53. CrossRef
25. Abdennour S, Lalaouna A, Derouiche MT, Azzouz M, Alvarez JC, Larabi IA. Development and validation of an UHPLC-DAD method for oleandrin content determination in dried leaves of Nerium oleander from Eastern Algeria. Microchem J. 2024;197:109740. CrossRef
26. Karawya MS, Balbaa SI, Khayyal SE. Estimation of cardenolides in Nerium oleander. Planta Med. 1973;23(1):70−3. CrossRef
27. Yang P, Menter DG, Cartwright C, Chan D, Dixon S, Suraokar M, et al. Oleandrin-mediated inhibition of human tumor cell proliferation: Importance of Na+, K+-ATPase α subunits as drug targets. Mol Cancer Ther. 2009;8(8):2319−28. CrossRef
28. Yang P, Cartwright C, Efuet E, Hamilton SR, Wistuba II, Menter D, et al. Cellular location and expression of Na+, K+-ATPase α-subunits affect the anti-proliferative activity of oleandrin. Mol Carcinog. 2014;53(4):253−63. CrossRef
29. Li XX, Wang DQ, Sui CG, Meng FD, Sun SL, Zheng J, et al. Oleandrin induces apoptosis via activating endoplasmic reticulum stress in breast cancer cells. Biomed Pharmacother. 2020;124:109852. CrossRef
30. Pan L, Zhang Y, Zhao W, Zhou X, Wang C, Deng F. The cardiac glycoside oleandrin induces apoptosis in human colon cancer cells via the mitochondrial pathway. Cancer Chemother Pharmacol. 2017;80:91−100. CrossRef
31. Raghavendra PB, Sreenivasan Y, Manna SK. Oleandrin induces apoptosis in human, but not in murine cells: dephosphorylation of Akt, expression of FasL, and alteration of membrane fluidity. Mol Immunol. 2007;44(9):2292−302. CrossRef
32. Reddy D, Kumavath R, Barh D, Azevedo V, Ghosh P. Anticancer and antiviral properties of cardiac glycosides: a review to explore the mechanism of actions. Molecules. 2020;25(16):3596. CrossRef
33. Newman RA, Kondo Y, Yokoyama T, Dixon S, Cartwright C, Chan D, et al. Autophagic cell death of human pancreatic tumor cells mediated by oleandrin, a lipid-soluble cardiac glycoside. Integr Cancer Ther. 2007;6(4):354−64. CrossRef
34. Newman RA, Yang P, Hittelman WN, Lu T, Ho DH, Ni D, et al. Oleandrin-mediated oxidative stress in human melanoma cells. J Exp Ther Oncol. 2006;5(3):167−81.
35. Manna SK, Sreenivasan Y, Sarkar A. Cardiac glycoside inhibits IL-8-induced biological responses by down-regulating IL-8 receptors through altering membrane fluidity. J Cell Physiol. 2006;207(1):195−207. CrossRef
36. Ko YS, Rugira T, Jin H, Park SW, Kim HJ. Oleandrin and its derivative odoroside A, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the STAT-3 signaling pathway. Int J Mol Sci. 2018;19(11):3350. CrossRef
37. Ha DP, Shin WJ, Liu Z, Doche ME, Lau R, Leli NM, et al. Targeting stress induction of GRP78 by cardiac glycoside oleandrin dually suppresses cancer and COVID-19. Cell Biosci. 2024;14(1):115. CrossRef
38. Bao Z, Tian B, Wang X, Feng H, Liang Y, Chen Z, et al. Oleandrin induces DNA damage responses in cancer cells by suppressing the expression of Rad51. Oncotarget. 2016;7(37):59572. CrossRef
39. Wu Q, Liu X, Wang LM, Yang YH, Pan LF, Zhang JJ, et al. Oleandrin enhances radiotherapy sensitivity in lung cancer by inhibiting the ATM/ATR-mediated DNA damage response. Phytother Res. 2024;38(8):4151−67. CrossRef
40. Ma Y, Zhu B, Yong L, Song C, Liu X, Yu H, et al. Regulation of intrinsic and extrinsic apoptotic pathways in osteosarcoma cells following oleandrin treatment. Int J Mol Sci. 2016;17(11):1950. CrossRef
41. Yong L, Ma Y, Liang C, He G, Zhao Z, Yang C, et al. Oleandrin sensitizes human osteosarcoma cells to cisplatin by preventing degradation of the copper transporter 1. Phytother Res. 2019;33(7):1837−50. CrossRef
42. Afaq F, Saleem M, Aziz MH, Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol Appl Pharmacol. 2004;195(3):361−9. CrossRef
43. Güne? CE, Çelik FS, Seçme M, Elmas L, Dodurga Y, Kurar E. Glycoside oleandrin down-regulates toll-like receptor pathway genes and associated miRNAs in human melanoma cells. Gene. 2022;843:146805. CrossRef
44. Sreenivasan Y, Sarkar A, Manna SK. Oleandrin suppresses activation of nuclear transcription factor-κB and activator protein-1 and potentiates apoptosis induced by ceramide. Biochem Pharmacol. 2003;66(11):2223−39. CrossRef
45. Garofalo S, Grimaldi A, Chece G, Porzia A, Morrone S, Mainiero F, et al. The glycoside oleandrin reduces glioma growth with direct and indirect effects on tumor cells. J Neurosci. 2017;37(14):3926−39. CrossRef
46. Fujii T, Katoh M, Ootsubo M, Nguyen OT, Iguchi M, Shimizu T, et al. Cardiac glycosides stimulate endocytosis of GLUT1 via intracellular Na+, K+-ATPase α3-isoform in human cancer cells. J Cell Physiol. 2022;237(7):2980−91. CrossRef
47. Celik FS, Gunes CE, Kurar E. Cardiac glycoside oleandrin suppresses EMT ability in endometrial carcinoma cells. Int J Mol Cell Med. 2023;12(3):220−8. CrossRef
48. Newman RA, Cisneros A, Felix E, Vijjeswarapu M, Lin Y, Yang P, et al. Composition and preliminary pharmacology studies with Anvirzel: an extract of Nerium oleander. J Herb Pharmacother. 2001;1(3):1−6. CrossRef
49. Dunn DE, He DN, Yang P, Johansen M, Newman RA, Lo DC. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J Neurochem. 2011;119(4):805−14. CrossRef
50. Pathak S, Multani AS, Narayan S, Kumar V, Newman RA. Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anti-Cancer Drugs. 2000;11(6):455−63.
51. Smith JA, Madden T, Vijjeswarapu M, Newman RA. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol. 2001;62(4):469−72. CrossRef 10.1016/S0006-2952(01)00690-6
52. Apostolou P, Toloudi M, Chatziioannou M, Papasotiriou I. Determination of efficacy of Anvirzel™ in 37 established cancer cell lines. Int Pharm Indust. 2011;3(3):68−72.
53. Apostolou P, Toloudi M, Chatziioannou M, Papasotiriou I. Studying the effect of Anvirzel, Carboplatin and Docetaxel combination in NSCLC cell lines. J Clin Stud. 2011;3(5):32−4. CrossRef
54. Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Knocke DR, Nester J, et al. Anvirzel in combination with cisplatin in breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines. BMC Pharmacol Toxicol. 2013;14:1−6. CrossRef
55. Terzioglu-Usak S, Nalli A, Elibol B, Ozek E, Hatiboglu MA. Anvirzel regulates cell death through inhibiting GSK-3 activity in human U87 glioma cells. Neurol Res. 2020;42(1):68−75. CrossRef
56. Pan Y, Rhea P, Tan L, Cartwright C, Lee HJ, Ravoori MK, et al. PBI-05204, a supercritical CO2 extract of Nerium oleander, inhibits growth of human pancreatic cancer via targeting the PI3K/mTOR pathway. Investig New Drugs. 2015;33:271−9. CrossRef
57. Colapietro A, Yang P, Rossetti A, Mancini A, Vitale F, Martellucci S, et al. The botanical drug PBI-05204, a supercritical CO2 extract of Nerium oleander, inhibits growth of human glioblastoma, reduces Akt/mTOR activities, and modulates GSC cell-renewal properties. Front Pharmacol. 2020;11:552428. CrossRef
58. Vaccaro S, Rossetti A, Porrazzo A, Camero S, Cassandri M, Pomella S, et al. The botanical drug PBI-05204, a supercritical CO2 extract of Nerium oleander, sensitizes alveolar and embryonal rhabdomyosarcoma to radiotherapy in vitro and in vivo. Front Pharmacol. 2022;13:1071176. CrossRef
59. Chakraborty S, Wei D, Tran M, Lang FF, Newman RA, Yang P. PBI-05204, a supercritical CO2 extract of Nerium oleander, suppresses glioblastoma stem cells by inhibiting GRP78 and inducing programmed necroptotic cell death. Neoplasia. 2024;54:101008. CrossRef
60. Mekhail T, Kaur H, Ganapathi R, Budd GT, Elson P, Bukowski RM. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Investig New Drugs. 2006;24:423−7. CrossRef
61. Hong DS, Henary H, Falchook GS, Naing A, Fu S, Moulder S, et al. First-in-human study of PBI-05204, an oleander-derived inhibitor of akt, fgf-2, nf-κΒ and p70s6k, in patients with advanced solid tumors. Investigational new drugs. 2014;32:1204−12. CrossRef
62. Roth MT, Cardin DB, Borazanci EH, Steinbach M, Picozzi VJ, Rosemury A, et al. A phase II, single-arm, open-label, bayesian adaptive efficacy and safety study of PBI-05204 in patients with stage IV metastatic pancreatic adenocarcinoma. Oncologist. 2020;25(10):e1446−50. CrossRef
63. Ni D, Madden TL, Johansen M, Felix E, Ho DH, Newman RA. Murine pharmacokinetics and metabolism of oleandrin, a cytotoxic component of Nerium oleander. J Exp Therapeut Oncol. 2002;2(5):278−85. CrossRef
64. Van Kanegan MJ, He DN, Dunn DE, Yang P, Newman RA, West AE, et al. BDNF mediates neuroprotection against oxygen-glucose deprivation by the cardiac glycoside oleandrin. J Neurosci. 2014;34(3):963−8. CrossRef
65. Van Kanegan MJ, Dunn DE, Kaltenbach LS, Shah B, He DN, McCoy DD, et al. Dual activities of the anti-cancer drug candidate PBI-05204 provide neuroprotection in brain slice models for neurodegenerative diseases and stroke. Sci Rep. 2016;6(1):25626. CrossRef
66. Elmaci ?, Alturfan EE, Cengiz S, Ozpinar A, Altinoz MA. Neuroprotective and tumoricidal activities of cardiac glycosides. Could oleandrin be a new weapon against stroke and glioblastoma? Int J Neurosci. 2018;128(9):865−77. CrossRef
67. Ünal ?, Çal??kan-Ak E, Üstünda? ÜV, Ate? PS, Alturfan AA, Altinoz MA, et al. Neuroprotective effects of mitoquinone and oleandrin on Parkinson’s disease model in zebrafish. Int J Neurosci. 2020;130(6):574−82. CrossRef
68. Eid S, Zerbes T, Williams D, Wang X, Sackmann C, Meier S, et al. Identification of a cardiac glycoside exhibiting favorable brain bioavailability and potency for reducing levels of the cellular prion protein. Int J Mol Sci. 2022;23(23):14823. CrossRef
69. Nguyen LD, Wei Z, Silva MC, Barberán-Soler S, Rabinovsky R, Muratore CR, et al. Small molecule inducers of neuroprotective miR-132 identified by HTS-HTS in human iPSC-derived neurons. BioRxiv. 2022; 2022:1−84. CrossRef
70. Manna SK, Sah NK, Newman RA, Cisneros A, Aggarwal BB. Oleandrin suppresses activation of nuclear transcription factor-κB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000;60(14):3838−47.
71. Li Z, Chen K, Yu Q, Li Y, Tong S, Xu R, et al. Suppression of NFATc1 through NF-κB/PI3K signaling pathway by oleandrin to inhibit osteoclastogenesis and bone resorption. Eng Regen. 2024;5(3):342−9. CrossRef
72. Xiang C, Cao J, Hu R, Li K, Meng T, Xia Y, et al. Oleandrin inhibits osteoclast differentiation by targeting the LRP4/MAPK/NF-κB signaling pathway to treat osteoporosis. Int Immunopharmacol. 2025;148:114073. CrossRef
73. Newman RA, Sastry KJ, Arav-Boger R, Cai H, Matos R, Harrod R. Antiviral effects of oleandrin. J Exp Pharmacol. 2020;12:503−15. CrossRef
74. Singh S, Shenoy S, Nehete PN, Yang P, Nehete B, Fontenot D, et al. Nerium oleander derived cardiac glycoside oleandrin is a novel inhibitor of HIV infectivity. Fitoterapia. 2013;84:32−9. CrossRef
75. Hutchison T, Yapindi L, Malu A, Newman RA, Sastry KJ, Harrod R. The botanical glycoside oleandrin inhibits human T-cell leukemia virus type-1 infectivity and env-dependent virological synapse formation. J Antivir Antiretrovir. 2019;11(3):1−24. CrossRef
76. Plante KS, Dwivedi V, Plante JA, Fernandez D, Mirchandani D, Bopp N, et al. Antiviral activity of oleandrin and a defined extract of Nerium oleander against SARS-CoV-2. Biomed Pharmacother. 2021;138:111457. CrossRef
77. Jensen GS, Yu L, Iloba I, Cruickshank D, Matos JR, Newman RA. Differential activities of the botanical extract PBI-05204 and oleandrin on innate immune functions under viral challenge versus inflammatory culture conditions. Molecules. 2023;28(12):4799. CrossRef
78. Fossum TW, Jensen GS, Newman RA, Matos JR. Botanical oleander extract and oleandrin have superior effects on innate immune functions pertaining to dermal allergic reactions in canine cells when compared to oclacitinib. Am J Vet Res. 2024;85(12):1−7. CrossRef
79. Liu H, Chen SY, Guo JY, Su P, Qiu YK, Ke CH, et al. Effective natural antifouling compounds from the plant Nerium oleander and testing. Int Biodeterior Biodegrad. 2018;127:170−7. CrossRef