INTRODUCTION
The rapid population growth in developing countries is straining economic progress and human development, highlighting the need for new contraceptives [1]. Even though there are numerous commercially available synthetic contraceptives available today to manage fertility, most of them have severe adverse effects, which include weight gain, hormonal changes, hypertension, and cancer [2]. Over 80% of people in developing nations rely on affordable traditional plant-based medicines due to the high cost of pharmaceuticals [3, 4]. Ayurvedic and traditional medicine systems have been explored for antifertility effects, and herbal medicine has gained global acceptance [2]. Searching for more advanced and effective herbal medications that are entirely reversible, less expensive, and have minimal to no adverse effects is one of the most challenging tasks [5].
Streblus asper Lour., a small tree from the Moraceae family, native to tropical regions including India [6], is traditionally used to treat various ailments such as piles, tuberculosis, leprosy, and fever. Its bark treats fever, diarrhea, and dysentery; its roots address sinusitis and ulcers; and its latex is used for elephantiasis and glandular swellings [7]. Recent studies highlighted its neuroprotective properties [8]. The plant’s twigs are also used to clean teeth, earning it the name “toothbrush tree” [9, 10]. It is thought that the presence of different chemical elements, such as flavonoids, phenolic acids, and alkaloids, is the reason for the varied pharmacological activity of this plant [11].
The contraceptive activity of S. asper has been previously documented in male mice [12]; however, no published reports are available on its contraceptive effects in females. Also, hormonal contraceptives available in the market are widely used by females. However, male hormonal contraceptives are still undergoing clinical trials. Moreover, S. asper has been traditionally noted for its use in treating various disorders, including antifertility and abortifacient activities. The Tripura tribes of India used the stem of this plant along with other herbs to induce abortion [13]. Additionally, it has been reported to have long-term contraceptive effects with minimal side effects [14], and its fresh stem is known to facilitate abortion [15].
The methanolic leaf extract of S. asper (MESA) contains various bioactive compounds [11] that may regulate reproductive physiology in female mice, potentially influencing the estrous cycle, implantation, and pregnancy. So, it is necessary to validate the contraceptive activity of S. asper in females. If the plant has contraceptive activity in females, it can be used as a candidate for potent herbal contraceptive. Consequently, the current study aims to determine the effect of methanolic leaf extract of S. asper on the estrous cycle, implantation, and pregnancy of female mice.
MATERIALS AND METHODS
Plant materials
In April 2022, the leaves of the fully grown plant were acquired from Deomornoi, District Darrang, Assam, India. The plant material received authentication from the Botanical Survey of India (B.S.I.), Shillong, Meghalaya, with the voucher specimen number ID/1383/S. asper.
Preparation of extracts
The S. asper leaves were collected, cleaned well, and sliced into little pieces. Then, leaves were allowed to air dry in the shade, ground with a mechanical grinder until finely ground, and put in an airtight container. By using a Soxhlet extractor with extractor capacity of 400 ml, powdered leaves (30 g for each extraction) were subjected to extraction with different solvents (300 ml of each solvent) for 20 hours in the ratio of 1:10 (1v of plant material:10v of solvent), namely methanol, ethanol, distilled water, and petroleum ether. After the extraction process, the solvent was removed using a rotary evaporator, resulting in 3.24 g of dried methanolic extract, 2.24 g of dried aqueous extract, 1.31 g of dried ethanolic extract, and 0.078 g of dried petroleum ether extract. After that, the percentage yield was calculated for each solvent. The yield of the leaf extract was then reconstituted with distilled water to get the necessary concentration for every pharmacological test. As the animal study was carried out using methanolic extract due to a higher yield of the leaf extract, methanolic extraction was carried out 5 times to get the required amount of extract for the entire study. On the other hand, aqueous, ethanolic, and petroleum ether extracts of the leaf were prepared only one time to do the phytochemical screening of S. asper leaves.
Phytochemical screening
The phytochemical profiles of crude methanolic, ethanolic, aqueous, and petroleum ether extracts of S. asper leaves were determined using standard methods. The presence of alkaloids, flavonoids, saponins, steroids, tannins, phenol, reducing sugars, carbohydrate, ketone, and anthraquinone glycosides was assessed following the protocols described by Auwal et al. [16]. The screening for glycosides and terpenoids was conducted according to the method outlined by Parekh et al. [17] and Wadood et al. [18], respectively.
Percentage yield
The percentage yield of the different extracts was determined as the percentage of the extract’s weight to the original weight of the dried sample used [19].
The methanolic extract possesses the highest phytoconstituent and percentage yield compared to other solvents, so it was used for further investigation in this study.
Liquid chromatography-mass spectroscopy (LC-MS) analysis
The LC-MS analysis of the leaves of S. asper was performed by an LC-MS/MS (Agilent 6410Triple Quad MS-MS). The detection was performed through direct injection mode with an electrospray ionization probe at the positive mode. The capillary temperature was kept at 300°C, while the sample flow rate was 0.4 ml/min. The mass range was selected from 50 to 1500 m/z. The gas flow was maintained at 6 ml/min, and the maximum pressure was set at 400 bar. As a mobile phase, the ratio of 0.5% formic acid in water and acetonitrile was 95.5 for the High-Performance Liquid Chromatography fraction of S. asper. The MS parameters for each compound were optimized to ensure the most favorable ionization and ion transfer conditions. They attained the optimum signal of both the precursor and fragment ions by infusing the analytes and manually turning the parameters. The source parameter was identical for all of the analytes.
Experimental animals
A healthy female colony breed groups of five albino mice (n = 5) each were kept in conventional laboratory settings with a temperature of 25°C ± 2°C and a 12/12 hours light/dark cycle. The mice were weighed between 25 and 28 g. They were fed a regular pellet diet and given unlimited access to water. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Gauhati University, Guwahati, India (Approval No.: IAEC/Per/2024-24/02-6).
The sample size (n = 5 per group) was determined following previously published studies of a similar nature [20] and animal ethics committee guidelines [Organization for Economic Co-operation and Development (OECD) guideline] that recommend using the minimal number of animals necessary to achieve statistically valid results [21]. This approach balances scientific objectives and animal welfare considerations.
Determination of acute toxicity
Albino mice were used to test the acute toxicity of methanolic leaf extract of S. asper. Prior to the experiment, the animals were fasted for the entire night, and the proper dose was used in accordance with OECD 425 guidelines, 2022 (OECD, 2022) [21]. The extract was given orally to the mice up to the maximum dose of 4,000 mg/kg/day, and animals were observed for any sign of toxicity. All animals were observed for toxicities such as diarrhea, decreased appetite, lacrimation, convulsion, salivation, lethargy, paralysis, and mortality for different time intervals, i.e., 1, 3, 24 hours, and consecutively for 14 days.
Experimental design
Animals were divided into eight groups, with five mice in each group.
Group I: The control group received standard feed and water ad. libitum
Group II: Received 200 mg/kg body weight of plant extract (for 8 days)
Group III: Received 200 mg/kg body weight of plant extract (for 24 days)
Group IV: Received 400 mg/kg body weight of plant extract (for 8 days)
Group V: Received 400 mg/kg body weight of plant extract (for 24 days)
Group VI: Received 17β estradiol (E2) at a dose of 1 μg/kg body weight suspended in olive oil subcutaneously (for 8 days)
Group VII: Received E2 at a dose of 1 μg/kg body weight suspended in olive oil subcutaneously (for 24 days)
Group VIII: The vehicle control (VC) group received olive oil subcutaneously
Pharmacological Screening
Estrous cycle study
The stained preparations of the animals’ vaginal smears were used to examine the estrous cycle every day [22, 23]. Every morning, the vaginal smears of the animals in all groups were examined to look for variations in the proestrus, estrus, metestrus, and diestrus phases duration and compared with the estrous cycle of control mice. Smear analysis was conducted using microscopes with 10 and 40× objective magnifications [24].
Antifertility activity
Anti-implantation and abortifacient activity
To assess the anti-implantation activity of the extract, five groups of mice were used having five animals in each group: Group I (control) received standard feed and water; Group II received 200 mg/kg of plant extract; Group III received 400 mg/kg; Group IV received 1 µg/kg of E2 subcutaneously; and Group V (VC) received olive oil subcutaneously. Female mice in the estrus phase were mated with fertile males (2:1). The female mice that showed vaginal plugs were separated, and that day was designated as the first day of pregnancy. The extracts were given orally to the mice from the first to the seventh day of gestation. Light ether anesthetic laparotomy was performed in sterile conditions on the 10th day, and the number of implantation sites was ascertained by examining the uteri [25].
The plant extract was tested in female albino mice for its abortifacient activity according to the method described by Khanna and Chaudhury [26].
Effect on pregnancy and litter size
To evaluate the extract’s effect on pregnancy parameters, all animal groups were treated for 24 days. In the last 5 days, females were mated with fertile males, and those with vaginal plugs were isolated and allowed to carry the pregnancy to term. Mice were weighed daily to monitor weight gain [27]. Litter size, gestation period, body length, and weight of litters were determined using the procedure described by Hastings-Tolsma et al. [28].
Statistical analysis
Statistical analysis was performed using MS Office 2017 and SPSS 21. The t test and one-way analysis of variance (ANOVA) were employed to evaluate the group differences statistically. Results were expressed as mean ± SE of the mean and differences between means were considered significant at p < 0.05.
RESULTS
Phytochemical screening
The results obtained in the present study showed that extracts obtained by using different solvents from S. asper plant leaves were enriched in phytochemicals such as saponins, flavonoids, alkaloids, steroids, tannins, glycosides, reducing sugar, terpenoid and phenol. Alkaloids, reducing sugars, saponins, and phenols were absent in the water extract. The ethanolic extract lacked alkaloids and glycosides, while the petroleum ether extract lacked reducing sugars, saponins, phenols, glycosides, flavonoids, and tannins. During the study, it was observed that petroleum ether extract contains minimum phytoconstituents, and methanolic extract contains the highest number of phytoconstituents (Table 1).
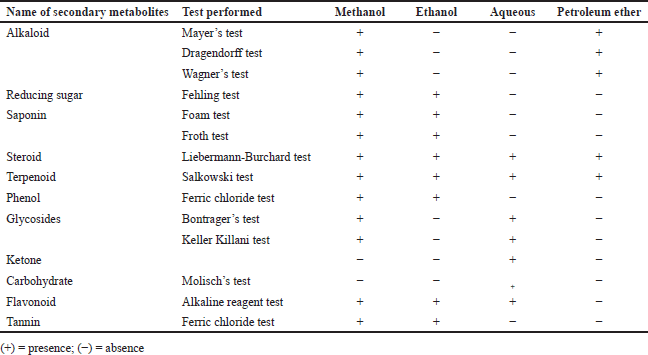 | Table 1. Phytochemical analysis of different extracts of Streblus asper leaves [Click here to view] |
Percentage yield
Percentage yield will give an idea about the extractability of the plant studied under different conditions. Among the different solvents examined in the study, methanolic extract showed the highest percentage yield (10.68%), followed by aqueous extract (7.49%), ethanol extract (4.39 %), and finally, petroleum ether extract (0.26%).
LC-MS analysis
LC/MS chromatogram of methanolic leaf extract of S. asper is presented in Figure 1 and identified compounds with their molecular mass, retention time, m/z ratio, and abundance were presented in Table 2. The mass spectral analysis has identified six compounds with various pharmacological activities.
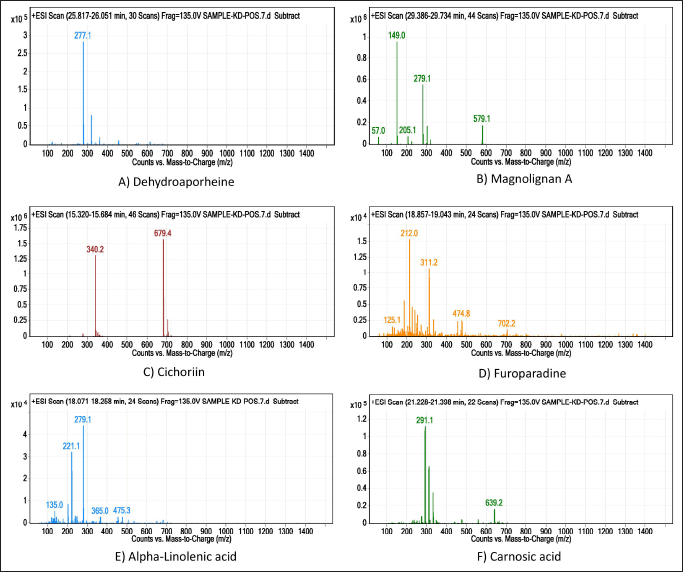 | Figure 1. LC-MS chromatogram of probable phytocompounds identified from methanol extract of S. asper leaves. [Click here to view] |
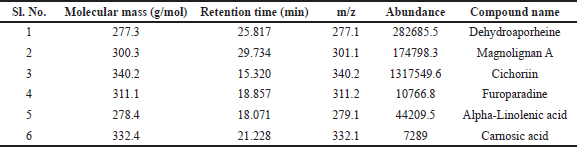 | Table 2. List of probable bioactive compounds quantified from methanolic extract of Streblus asper leaves by LC-MS. [Click here to view] |
Acute oral toxicity study
The MESA exhibited no toxicity in mice up to 4,000 mg/kg body weight. Consequently, 4,000 mg/kg was considered as the maximum tolerated dose. After administering 4,000 mg/kg orally, no adverse effects such as motor activity alterations, diarrhea, lacrimation, convulsions, or coma were observed, and no fatalities occurred during the 14-day observation period. Following OECD guideline 425, the experimental animals were treated with 400 mg/kg (1/10th) as the high dose and 200 mg/kg (1/20th) as the low dose.
Effects of MESA on the estrous cycle of mice
The impact of S. asper methanolic leaf extract on the estrous cycle of mice has been shown in Table 3. Mice administered with 200 or 400 mg/kg body weight of the extract for 8 days showed no visible variations in the duration of the various estrous cycle stages compared to the control group. However, the estrus phase was shorter, and the diestrus phase was longer than the E2-treated group. Over 24 days, all extract-treated and E2-treated groups exhibited longer diestrus and estrus phases and shorter proestrus and metestrus phases than the control group of animals. Various stages of the estrous cycle are depicted in Figure 2A–D.
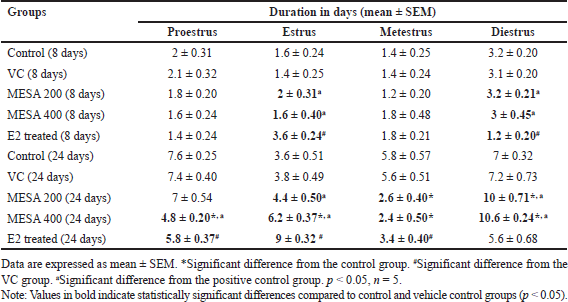 | Table 3. Effects of MESA on the estrous cycle of mice. Data are expressed as mean ± SEM. [Click here to view] |
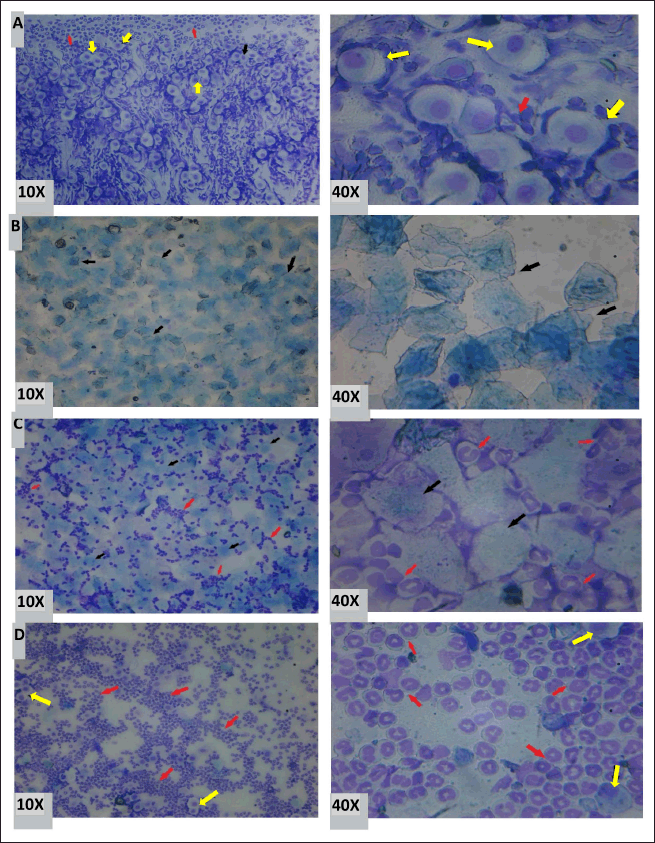 | Figure 2. Different stages of the estrous cycle. A- Proestrus phase, B- Estrus, C- Metestrus, D- Diestrus. (→nucleated epithelial cells;→ Cornified cells;→ Leucocytes) [Click here to view] |
Anti-implantation and abortifacient activity
The current study revealed a significant decrease (p < 0.05) in implantation sites in MESA-treated groups compared to the control group. The 200 and 400 mg/kg doses of MESA showed 40% and 60% anti-implantation efficacy, respectively, with the highest inhibition at 400 mg/kg. The E2-treated group showed 100% inhibition (Fig. 3, Table 4). The extract demonstrated a dose-dependent abortifacient effect, with 47.36% and 91.67% abortion rates at 200 and 400 mg/kg, respectively. As the dose increased, the number of resorptions increased significantly (p < 0.05) (Table 5). There was no vaginal bleeding observed. The 400 mg/kg dose of MESA was substantially more effective (p < 0.05) than the control group. Findings are illustrated in Figure 3 and presented in Tables 4 and 5.
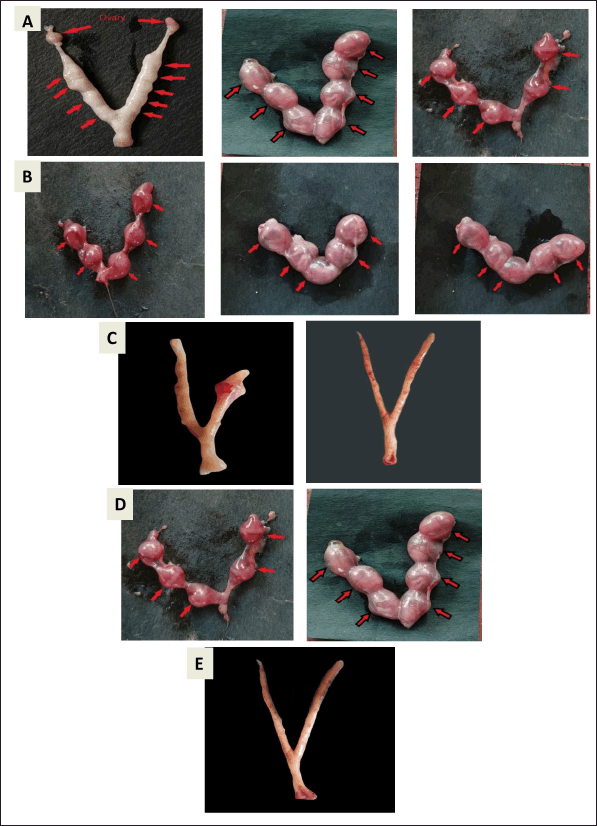 | Figure 3. Effect of MESA on the number of implantations on mice. (A) Control group animal showing 9,7 and 5 implants, (B) 200 mg/kg extract treated group showing five implants, (C) 400 mg/kg extract treated group showing 0 implants, (D) VC group (olive oil) animal showing 5 and 7 implants, (E) E2 treated group (1 μg/kg/bw) animal showing 0 implants. [Click here to view] |
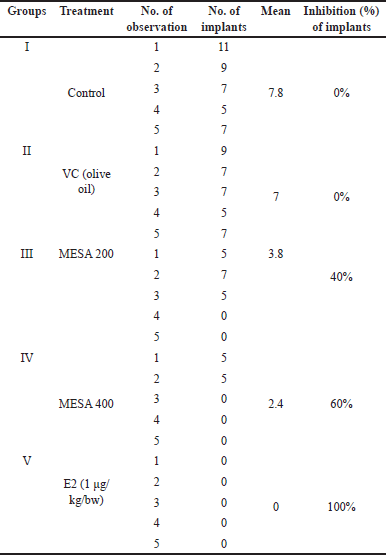 | Table 4. Effect of MESA on implantation in mice. [Click here to view] |
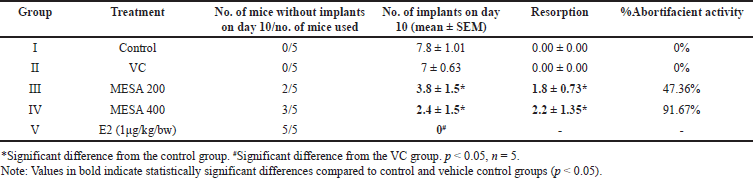 | Table 5. Effect of MESA on resorption index of female mice. [Click here to view] |
Effect on pregnancy and litter size
The study found that oral administration of methanolic leaf extract of S. asper reduced litter size in a dose-dependent manner compared to controls. Mice in the control and VC groups had the largest litter sizes that are 8.4 ± 0.4 and 8.2 ± 0.37, respectively (Table 6). Compared to controls, the MESA-treated groups showed a significant (p < 0.05) decrease in the litter body weight and total body length on the first day of birth. These effects were more pronounced at the 400 mg/kg dose. However, there was no significant difference in gestation duration between the treated and control groups.
 | Table 6. Litter size, body length and weight of litters, and gestation period. Data are expressed as mean ± SEM. [Click here to view] |
DISCUSSION
Despite the benefits of natural remedies, research on the efficacy of traditional medicines is limited [29]. S. asper has been noted for its use in treating various disorders, including its antifertility and abortifacient properties. The Tripura tribes of India utilize its stem, along with other plants, for abortion [13]. It is also reported to prevent pregnancy with long-term effects and minimal side effects [14], and its fresh stem can induce abortion [15].
The study is carried out to evaluate the effects of MESA on the estrous cycle, implantation, and pregnancy in female albino mice. Findings revealed that MESA contains alkaloids, steroids, flavonoids, glycosides, tannins, saponins, and carbohydrates, while petroleum ether extract had the fewest phytoconstituents. The methanolic extract also had a higher percentage yield compared to petroleum ether. Ujagar et al. [30] also reported higher yields from methanolic extracts due to better solubility of phytochemicals in polar solvents.
According to previous literature, flavonoids, saponins, and non-steroidal estrogenic compounds, including flavones, flavonones, isoflavonoids, alkaloids, and phenolics are known for their antifertility properties [31]. Soni et al. [32] reported that flavonoids, which are present in the stem of Musa paradisiaca, might be the source of anti-implantation and abortifacient activity of female rats.
LC/MS analysis revealed the presence of six phytochemical compounds having different pharmacological activities (Table 2, Fig. 1). Among them, cichoriin is a glycoside and coumarin, which possess antidiabetic activity [33] and mitigates oxidative stress [34], carnosic acid is an abietane diterpene which is an antioxidant [35], magnolia A is a kind of biphenyl compound, and biphenyl is an endocrine-disrupting chemical, which may have effects on fertility [36]. Alpha-linolenic acid is a polyunsaturated fatty acid that may reduce pregnancy chances in females [37].
The acute toxicity study revealed that oral administration of the extract at doses up to 4,000 mg/kg did not produce any noticeable adverse effects, such as altered motor activity, diarrhea, excessive tear production, convulsions, or coma, nor were there any fatalities throughout the 14-day observation period. Similarly, research by Kumar et al. [38] on brine shrimps indicated that the methanolic extract of S. asper was non-toxic in both acute and sub-acute toxicity evaluations. However, in subchronic safety studies, the methanolic extract exhibited mild toxicity, whereas the petroleum ether extract was considered non-toxic. Furthermore, Pandey et al. [39] reported that acute toxicity testing of the petroleum ether extract from the stem bark of S. asper confirmed its non-toxic nature. However, further studies on sub-acute and chronic toxicity in mice are necessary to establish a comprehensive safety profile.
The estrous cycle in female mammals, including rats and mice, involves recurring changes in reproductive hormones and is characterized by specific cell types in vaginal smears. In rats and mice, this cycle repeats every 4–5 days [40]. In the current study, treated groups showed increased estrus and diestrus durations and decreased proestrus and metestrus durations compared to control groups. These disturbances, including the prolonged diestrus phase, may be due to the phytoconstituents in the extract [41]. The estrous cycle is regulated by ovarian hormones (progesterone and estrogen) and pituitary gonadotropins [42, 43], and imbalances in these hormones can cause estrous cycle irregularities [44]. Similar studies have noted that such imbalances can lead to prolonged estrus and diestrus phases and reduced the reproductive cycles [45].
Studies have shown that various plant extracts, such as those from Dalbergia saxatilis, Mimosa pudica, Garcinia kola, and Momordica charantia, can lengthen the diestrus phase and disrupt the estrous cycle, potentially leading to reduced fertility and altered reproductive functions [46–49]. Because of the significantly increased length of the diestrus phase, ovulation frequency will decrease. In the absence of pregnancy, the extended diestrus is marked by progesterone-producing activities of the corpus luteum [50]. The current findings with S. asper are consistent with these observations, suggesting that the extract may cause similar disruptions in the estrous cycle, likely due to its anti-inflammatory properties. Saponins in S. asper, known for their anti-inflammatory effects, might inhibit enzymes such as COX-2, essential for ovulation and follicular rupture [51–53].
Implantation is the process where an embryo establishes contact with the mother’s endometrium to initiate pregnancy. In rodents, implantation generally takes place on the fourth or fifth day of gestation, and disruptions during this time might result in losses during the embryo implantation [54]. The uterine endometrium, influenced by estrogen and progesterone, undergoes changes to become receptive to implantation [55]. Disruptions in these hormonal levels can lead to failed implantation and lead to infertility [56, 57]. Chemicals affecting these hormones can make the endometrium non-receptive to the embryo, thereby enhancing antifertility effects [58]. Disruption of the estrous cycle can impair endometrial function, leading to implantation failure [59].
The current study found that MESA contains alkaloids, steroids, flavonoids, glycosides, tannins, saponins, which can potentially prevent pregnancy [60], and also, there are many kinds of literature available on the effect of different plant extracts on implantation and pregnancy of rodents [27,61–64]. Other plants with reported anti-implantation effects include Striga orobanchioides, Calotropis procera, and Lawsonia inermis. [65–67].
The study observed a dose-dependent abortifacient effect with S. asper leaf extract, showing 47.36% and 91.67% abortifacient activity at 200 and 400 mg/kg doses, respectively, indicating that higher doses lead to greater resorption [68] (Table 5). This suggests the extract’s potential abortifacient properties, potentially linked to its estrogenic or anti-estrogenic effects [69]. Flavonoids and other phytochemicals in the extract may contribute to these effects, with flavonoids known for their antifertility activity [70–74]. It could also be due to uncontrollably strong uterine contractions, leading to abortion depending on the estrogen levels in the tissues that could be due to uterotonic effects of the combination of enzymes [75]. Previous studies have shown similar effects with flavonoids from other plants, such as Striga lutea and Butea monosperma [26, 76], steroids from I. trifoliata [77], and alkaloids from Graptophyllum pictum [78]. The contraceptive effects of S. asper may be attributed to its alkaloids, steroids, flavonoids, and saponins.
The study showed that treatment with 200 and 400 mg/kg of S. asper extract significantly reduced litter size and pup body weight, with the 400 mg/kg dose being the most effective (p < 0.05). No litters were observed in the estradiol-treated group due to lack of implantation. Despite significant effects on litter size and pup growth, the gestation duration was unaffected (Table 6). Increased resorption rates indicated disrupted embryo development in post-implantation [25]. The maintenance of pregnancy necessitates a careful balance between estrogen and progesterone, just like in the implantation of embryos in uterine walls, and any disruption in these hormone levels may result in abortion [69, 79]. Similar studies, such as those on Hymenocardia acida and Lepidium meyenii, have also reported dose-dependent impacts on litter size and implantation [80, 81]. Overall, MESA exhibited significant anti-implantation and abortifacient effects.
The present findings aligned with previous research done by Vemula et al. [12] on the effects of S. asper leaf extract in male mice, which demonstrated the antifertility potential of S. asper aqueous and methanolic leaf extracts in male mice. Their study reported a significant reduction in sperm count and motility following extract administration, indicating a possible impairment in spermatogenesis, supporting the hypothesis that S. asper possessed contraceptive properties. These observed effects may be attributed to hormonal disruptions, particularly in luteinizing hormone and follicle-stimulating hormone, both of which are essential regulators of reproductive cycle. An imbalance in these hormones might lead to disruptions in the estrous cycle and impaired folliculogenesis in female mice, which might be a possible mechanism of action observed in the present study.
CONCLUSION
The current study’s findings provide strong evidence for the anti-implantation activity of S. asper leaves. The methanolic extract of these leaves disrupted the estrous cycle, prevented implantation in female mice, and induced abortion, which could potentially lead to infertility. These results validate the traditional use of S. asper leaves as a contraceptive agent. Consequently, this research suggests that the plant could be a reliable and safe alternative for contraception. Further research is required to identify the bioactive compounds responsible for the extract’s anti-implantation and abortifacient effects. These findings underscore the importance of caution when using traditional medicinal plants for reproductive health, highlighting the necessity of investigating reversibility, hormonal effects, and clinical relevance. Ultimately, this study adds to the expanding knowledge of the medicinal applications of plants in fertility management.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
A fellowship awarded to Kakali Deka from the CSIR Government of India is acknowledged (File No: 09/0059(15447)/2022-EMR-I.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details are provided in the ‘Materials and Methods’ section.
DATA AVAILABILITY
All data generated in this study have been incorporated in this article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PLANT IDENTIFICATION AND AUTHENTICATION
The leaves of Streblus asper were collected from the Deomornoi area of Darrang district, Assam (India). The Botanical Survey of India, Shillong Meghalaya, authenticated and identified the plant, and an authentication number was given as BSI/ERC/Tech/2023-24/1383.
REFERENCES
1. Ghosh K, Bhattacharya TK. Preliminary study on the anti-implantation activity of compounds from the extracts of seeds of Thespesia populinea. Indian J Pharmacol. 2004;36(950):288–91.
2. Lopez M, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016;2016(8):CD008815. CrossRef
3. Shah GM, Khan MA, Ahmed M, Zafar M, Khan AA. Observations on antifertility and abortifacient herbal drugs. Afr J Biotechnol. 2009;8(9):1959–64.
4. Kaur A, Sharma R, Kumar R, Kharb R. Rising trends towards herbal contraceptives. J Nat Prod Plant Resour. 2011;1(4):5–12.
5. Lewis ME. Should we be concerned about herbal remedies? J Ethnopharmacol. 2001;75(2–3):141–64. CrossRef
6. Sahana A, Visagaperumal D, Vineeth C. An overview of Streblus asper Lour. Int J Res Publ Rev. 2021;2(8):120–30.
7. Rinkal C, Radhika R, Vasanti S, Richie B. A critical review on phytopharmacology, spectral and computational analysis of phytoconstituents from Streblus asper Lour. Phytomedicine Plus. 2022;2(1):1–23. CrossRef
8. Anchalee P, Atsadang T, Matthew P, Alison TU, Tewin T. Acidbase fractions separated from Streblus asper leaf ethanolic extract exhibited antibacterial, antioxidant, antiacetylcholine esterase, and neuroprotective activities. BMC Complement Altern Med. 2018;18:1–13. CrossRef
9. Kumar M, Prakash S, Kumari N, Pundir A, Punia S, Saurabh V, et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants. 2021;10(7):1061. CrossRef
10. Kadir MF, Bin Sayeed MS, Setu NI, Mostafa A, Mia MM. Ethnopharmacological survey of medicinal plants used by traditional health practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. J Ethnopharmacol. 2014;155(1):495–508. CrossRef
11. Shahed-Al-Mahmud M, Shawon MJA, Islam T, Rahman MM, Rahman MR. In vivo anti-diarrheal activity of methanolic extract of Streblus asper leaves stimulating the Na+/K+-ATPase in Swiss albino rats. Indian J Clin Biochem. 2020;35(1):72–9. CrossRef
12. Vemula R, Reddy GVR, Kumar RV, Reddy MK. Antifertility activity of medicinal plant extracts on albino rats. World J Pharm Res. 2016;5:1333–45.
13. Das B, Talukdar AD, Choudhury MD. A few traditional medicinal plants used as antifertility agents by ethnic people of Tripura, India. Int J Pharm Pharm Sci. 2014;6(3):47–53.
14. Singh P. Pharmacognostic study of root of Streblus asper Lour. Planta Medica. 1963;11(2);191–7. CrossRef
15. Sivamaruthi BS, Prasanth MI, Kesika P, Tencomnao T, Chaiyasut C. Functional properties of Streblus asper Lour.: a review. Food Sci Technol. 2022;42:1–10. CrossRef
16. Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn Mimosa). Vet Res Forum. 2014;5(2):95–100.
17. Parekh J, Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr J Biomed Res. 2007;10:175–81. CrossRef
18. Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2:144. CrossRef
19. Adam OAO, Abadi RSM, Ayoub SMH. The effect of extraction method and solvents on yield and antioxidant activity of certain sudanese medicinal plant extracts. J Phytopharmacol. 2019;8(5):248–52. CrossRef
20. Ojah A, Borthakur MK, Kalita JC. Antifertility efficacy of methanolic root extract of Persicaria hydropiper on ovarian and uterine histomorphometry of female albino mice. J Appl Pharm Sci. 2022;12(12):95–104. CrossRef
21. Test no. 425: acute oral toxicity: 2022. Up-and-down procedure, OECD guidelines for the testing of chemicals, section 4, OECD Publishing, Paris.
22. McLean AC, Valenziela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012;67:1–6. CrossRef
23. Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 2015;43:776–93. CrossRef
24. Ganguly M, Devi N, Mahanta R, Borthakur MK. Effect of Mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifetility activity in albino mice. Contraception. 2008;76(6):482–5. CrossRef
25. Jain S, Choudhary GP, Jain DK. Pharmacological evaluation and antifertility activity of Jatropha gossypifolia in rats. BioMed Res Int. 2013:2013;5. CrossRef
26. Khanna U, Chaudhary RR. Antifertility screening of plants – Part I: investigation of Butea monosperma (Lam) Kutze. Indian J Med Res. 1968;56(1968):1575–9.
27. Pierre W, Esther N, Nguelefack NEPA, Benoit T, Albert K. Reproductive effects of Ficus asperifolia (Moraceae) in female rats. Afr Health Sci. 2009;9(1):49–53.
28. Hastings-Tolsma M, Stoffel RT, Quintana AS, Kane RR, Turner J, Wang X. Effect of Rubus idaeus L. Consumption during pregnancy on maternal mice and their offspring. J Med Food. 2022;25(2):183–91. CrossRef
29. Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;29(5):559. CrossRef
30. Ujagar AB, Rathod MG, Aasore SR, Pathak AP. Phytochemical screening and antibacterial activity of petroleum ether, methanol and acetone extracts Of Catharanthus roseus leaves and flowers. Res Develop Pharm Sci. 2021;1:133–41.
31. Khushalani H, Pratima T, Kamalinder K. Antifertility activity of dried flowers of Woodfordia fruticosa kurz. Indian J Pharm Sci. 2006;68(4):528–9. CrossRef
32. Soni P, Siddiqui AA, Dwivedi J, Soni V. Antiovulatory and estrogenic activity of stem of Musa paradisiaca in female albino rats. J Applied Pharmaceutical Science. 2013;3(8):102–6.
33. Khalil HE, Abdelwahab MF, Ibrahim HM, AlYahya KA, Mohamed AA, Radwan AS, et al. Mechanistic insights into the ameliorative effect of cichoriin on diabetic rats-assisted with an in silico approach. Molecules. 2022;27(21):7192. CrossRef
34. Khalil HE, Abdelwahab MF, Ibrahim HM, AlYahya KA, Altaweel AA, Alasoom AJ, et al. Cichoriin, a biocoumarin, mitigates oxidative stress and associated adverse dysfunctions on high-fat diet-induced obesity in rats. Life (Basel). 2022;12(11):1731. CrossRef
35. Tousson E, Bayomy MF, Ahmed AA. Rosemary extract modulates fertility potential, DNA fragmentation, injury, KI67 and P53 alterations induced by etoposide in rat testes. Biomed Pharmacother. 2018;98:769-74. CrossRef
36. Vessa B, Perlman B, McGovern PG, Morelli SS. Endocrine disruptors and female fertility: a review of pesticide and plasticizer effects. F S Rep. 2022;3(2):86–90. CrossRef
37. Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril. 2011;96(4):880–3. CrossRef
38. Kumar RBS, Puratchikodi A, Prasanna A, Dolai N, Majumder P, Mazumder UK, Haldar PK. Pre clinical studies of Streblus asper Lour in terms of behavioural safety and toxicity. Orient Pharm Exp Med. 2011;11:243–9. CrossRef
39. Pandey MM, Rastogi S. Streblus asper: a phytochemical, ethnopharmacological and pharmacological research update. J Pharmacogn Phytochem. 2022;11(3):7–18. CrossRef
40. Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract. 2020;6:5. CrossRef
41. Muchtaromah B, Romaidi, Griani TP, Hasfita Y. Potencial antifertilty of Centella asiatica leaf extract. Aust J Basic Appl Sci. 2015;9(7):102–5.
42. Sujesh M, Harindran J. Effects of Cyclea peltata (hooks and thom.) root extracts on reproductive system of female rat. World J Pharm Pharm Sci. 2019;8(7):1217–26.
43. Geetha MM, Shankar MB, Mehta RS, Saluja AK. Antifertility activity of Artabotrys odoratissimus Roxb. and Couroupita guianensis Aubl. J Nat Remedies. 2005;5(2):121–5.
44. Sarita M. Antifertility activity of Eugenia jambolana seed extract in female albino rat. Biochem Physiol. 2018;7(2):237.
45. Budreau CH, Singh RP. Effects of fenthion and dimethoate on reproduction of mouse. Toxicol Appl Pharmacol. 1973;26:29–38. CrossRef
46. Uchendu CN, Kamalu TN, Asuzu IU. A preliminary evaluation of antifertility activity of a triterpenoid glycoside from Dalbergia saxatilisin female Wistar rats. Pharm Res. 2000;41:521–5. CrossRef
47. Benie T, Duval J, Thieulant ML. Effects of some traditional plant extracts on rat oestrous cycle compared with clomid. Phytother Res. 2003;17:748–55. CrossRef
48. Akpanthan AO, Oremosu A, Noronha AA, Ekanem TB, Okanlawon AO. Effects of Garcinia kola seed extract on ovulation, oestrous cycle and foetal development in cyclic female Sprague-Dawley rats. Nig J Physiol Sci. 2005;20:58–62.
49. Ifeanyi AC, Yama OE, Duru FI, Osinubi AA, Noronha CC, Okanlawon AO. Effect of Momordica charantia on estrous cycle of Sprague-Dawley rats. Pecific J Med Sci. 2011;8(1):37–48.
50. Bakare A. Medicinal uses of some plants of East Africa. Med Plants East Africa J. 2012; 54:45–47.
51. Abere TA, Okoto PE, Agoreyo FO. Antidiarrhoea and toxicological evaluation of the leaf extract of Dissotis rotundifoliaTriana (Melastomataceae). BMC Complement Altern Med. 2010;10:71. CrossRef
52. Gaytan E, Tmadas E, Moralles C, Bellido C, Sanchez-Criado J. Morphorlogical evidence for uncontrolled proteolytic activity during the ovulatory process in indomethacin-treated rats. Reproduction.2002;123:639–49. CrossRef
53. Olufemi MV, Tams GE, Ipindamitan Adebayo A. Effects of ethanol extract of Dissotis rutundifolia on the histology of the ovary, Uterus and Gonadotropins of adult female Whistar rats. Ann Biologic Sci. 2014;2(3):8–22.
54. Beaudoin AR. Embryology and teratology. In: Baker HJ, Lindsey JR, Weisbroth SH, editors. The laboratory rat (Research application). New York, NY: Academic Press; 1980. pp. 75–94.
55. Turner CD, Bagnara, JT. General endocrinology. 5th edn. Tokyo, Japan: W.B. Saunders Company; 1975.
56. Ding YQ, Zhu LJ, Bagchi MK, Bagchi IC. Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology. 1994;135(5):2265–74. CrossRef
57. Hiremath SP, Rudresh K, Badami S, Patil SB, Patil SR. Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol. 1999;67(3):253–8. CrossRef
58. Mishra M, Gautam RK, Mathur R. Evaluation of antifertility potential of Calotropis gigantea Linn. in female albino rats. J Pharm Res Opin. 2011;1(3):92–3.
59. Wolstenholme W, O’Connor M, editors. Ciba foundation study group on egg implantation. London: Churchil; 1966. pp. 4–28.
60. Hiremath SP, Badami P, Hunasagatta SK, Patil SB. Antifertility and hormonal properties of flavones of Stiriga orobanchioides. Eur J Pharmacol. 2000;391(1–2):193–7. CrossRef
61. Al-Said MS, Al-Khamis KI, Islam MW, Parmar NS, Tariq M, Ageel AM. Post-coital antifertility activity of the seeds of Coriandrum sativum in rats. J Ethnopharmacol. 1987;21(2):165–73. CrossRef
62. Vishwanatha T, Satishagouda S, Patil JS, Patil BS. Anti-implantation activity of Terminalia bellirica bark extracts in female albino rats. Indian J Biotechnol. 2009;3(4):257–60.
63. Vasudeva N, Sharma SK. Post-coital antifertility activity of Hibiscus rosa-sinensis Linn. roots. Evid Based Complement Alternat Med. 2008;5(1):91–4. CrossRef
64. Ahmed M, Khan MY, Khan AA. Effects of Ocimum sanctum (Tulsi) on the reproductive system: an updated review. Biomed Res. 2002;13(2/3):63–7.
65. Hiremath SP, Badami S, Swamy HK, Patil SB, Londonkar RL. Antifertility activity of Striga orobanchioides. Biol Pharm Bull. 1994;17(8):1029–31. CrossRef
66. Kamath JV, Rana AC. Preliminary study on antifertility activity of Calotropis procera roots in female rats. Fitoterapia. 2002;73(1):111–5. CrossRef
67. Agunu A, Samagoro C, Nuhu H. Evaluation of antifertility of Lawsonia inermis L. (Lythraceae) roots found in Kaduna State, Nigeria. Planta Medica. 2011;77:PF30. CrossRef
68. Shah SK, Jhade D, Chouksey R. Antifertility activity of ethanolic and aqueous extracts of aloe vera mill on female wistar rats: rising approaches of herbal contraception. J Pharm Sci Res. 2016;8(9):952–7.
69. Choudhary M, Rani S, Sharma P, Choudhary N, Budhwaar V. Anti-fertility and abortifacient potential of hydroalcoholic leaves extract of Alstonia scholaris in female rats: an ethnomedicine used by Papua women in New Guinea. Bull Fac Pharm Cairo Univ. 2017;55(1):123–7. CrossRef
70. Devarshi P, Patil S, Kanase A. Effect of Plumbago zeylanica root powder induced pre-implantationary loss and abortion on uterine luminal proteins in albino rats. Ind J Exp Biol. 1991;29:521–2.
71. Hiremath SP, Rudresh K, Badami S, Patil SB, Patil RS. Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol. 1991;67:253–8. CrossRef
72. Badami S, Aneesh R, Sankar S, Satishkumar MN, Suresh B, Rajan S. Antifertility activity of Derris brevipes variety Coriacea. J Ethnopharmacol. 2003;84:99–104. CrossRef
73. Vaidya P, Padmashali S, Vagdevi HM, Sathyanarayana ND. Antifertility effect of the plant Balanites roxburghii (Balanitaceae) in female rats. Ind J Pharmal Sci. 2006;3:347–51. CrossRef
74. Namulindwa A, Nkwangu D, Oloro J. Determination of the abortifacient activity of the aqueous extract of Phytolacca dodecandra (L’Her) leaf in Wistar rats. Afric J Pharm Pharmacol. 2015;9(3):43–7. CrossRef
75. Adebiyi A, Adaikan PG, Prasad RNV. Papaya (Carica papaya) consumption is unsafe in pregnancy: facts or fable? Scientific evaluation of a common belief in some parts of Asia using a rat model. Brit J Nut. 2002;88:199–203. CrossRef
76. Hiremath SP, Hanumantha RS. Antifertility efficacy ofthe plant Srtiga lutea (Scrophulariaceae) on rats. Contraception. 1990;42:466–77. CrossRef
77. Sani UM, Sule MI. Antifertility activity of methanol extracts of three different seed varieties of Ricinus communis Linn (Euphorbiaceae). Nig J Pharma Sci. 2007;6:78–83.
78. Stella OOD, Grace EU, Herbert ABC, Samuel AD. Oxytocic and anti-implantation activities of the leaf extract of Graptophyllum pictum (Linn.) Griff. (Acanthaceae). Afr J Biotech. 2009;8:5979–84. CrossRef
79. Sharma P, Manjusha S, Rani S, Malhotra H, Nitesh, Deswal S, et al. Antifertility potential of hydroalcoholic extract of Cordia dichotoma G Forst. Leaves: a folklore medicine used by Meena community in Rajasthan state in India. Asian Pac J Reprod. 2015;4(2):100–5. CrossRef
80. Hyacinth AA, Nwocha UC. Antifertility activity of aqueous ethanolic extract of Hymenocardia acida stem bark in female rats. Iran J Reprod Med. 2011;9(3):217–22.
81. Ruiz-Luna AC, Salazar S, Aspajo NJ, Rubio J, Gasco M, Gonzales GF. Lepidium meyenii (Maca) increases litter size in normal adult female mice. Reprod Biol Endocrinol. 2005;3(16):1–6. CrossRef