INTRODUCTION
The carob tree (Ceratonia siliqua L.), a resilient and versatile species of the Fabaceae family, has long been an integral component of Mediterranean ecosystems. Known for its adaptability to arid and semi-arid climates, the carob tree thrives in nutrient-poor soils, providing ecological and economic benefits to the regions where it grows [1]. Historically, this species has been cultivated for its pods, which serve as a valuable source of food, feed, and raw materials for various industries. Its seeds are particularly prized for producing locust bean gum, a thickening agent widely used in the food and cosmetic sectors [2].
Moroccan carob, in particular, holds a distinctive position within this species’ global distribution. As the second-largest producer of carob globally, Morocco yields approximately 22,000 tons annually, with primary cultivation zones in the Atlas Mountains, Beni Mellal, Essaouira, and the Rif Mountains. These regions provide optimal conditions for carob cultivation, reflecting the interplay between environmental factors, genetic diversity, and traditional agricultural practices [3,4]. The unique morphological, phytochemical, and nutritional attributes of carob have garnered increasing research interest among researchers in phytochemistry, ethnopharmacology, and related disciplines. In Morocco, carob pods and seeds are widely used in ethnomedicine to treat gastrointestinal ailments, diabetes, and respiratory conditions, among other health issues. Despite this rich cultural and therapeutic heritage, Moroccan carob remains underrepresented in scientific literature compared to its counterparts from other Mediterranean countries [4,5].
In recent years, there has been a growing recognition of the therapeutic potential of C. siliqua as a natural source of bioactive compounds with significant health benefits. Phytochemical analyses reveal a rich composition of polyphenols, flavonoids, tannins, and dietary fibers [5], which collectively contribute to its antioxidant, anti-inflammatory, antidiabetic, and antimicrobial properties [4,6,7]. These pharmacological effects have positioned carob as a promising candidate for functional foods, nutraceuticals, and therapeutic applications. Despite growing interest, most studies on C. siliqua have centered on non-Moroccan varieties, leaving the distinctive phytochemical and pharmacological traits of Moroccan carob largely underexplored [8].
This review seeks to bridge this gap by presenting a comprehensive synthesis of the phytochemical composition, nutritional value, and pharmacological properties of Moroccan carob. Particular attention is given to its ethnomedicinal uses, morphological diversity, and potential industrial applications. By consolidating recent advancements in carob research, this work aims to highlight the multidimensional benefits of Moroccan carob and to identify avenues for future investigations that could enhance its medicinal, and economic value.
MATERIALS AND METHODS
Bibliographic search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency, reproducibility, and a structured synthesis of the literature on C. siliqua. The PRISMA framework established the study selection criteria, screening process, data extraction methods, and synthesis strategy, enabling a comprehensive and unbiased assessment of the phytochemical, pharmacological, nutritional, and ethnomedicinal properties of Moroccan and Mediterranean carob varieties.
A systematic and exhaustive bibliographic search was performed across major academic databases, including PubMed/Medline, Scopus, ScienceDirect, Cochrane Library, Web of Science, SciELO, SpringerLink, Capes Periodicals, virtual health library (VHL), Regional Portal, and Google Scholar. These databases were selected based on their broad disciplinary coverage, their indexing of high-impact, peer-reviewed journals, and their relevance to biomedical, pharmaceutical, ethnobotanical, and nutritional sciences. The literature search covered studies published between January 2010 and January 2025 (Fig. 1).
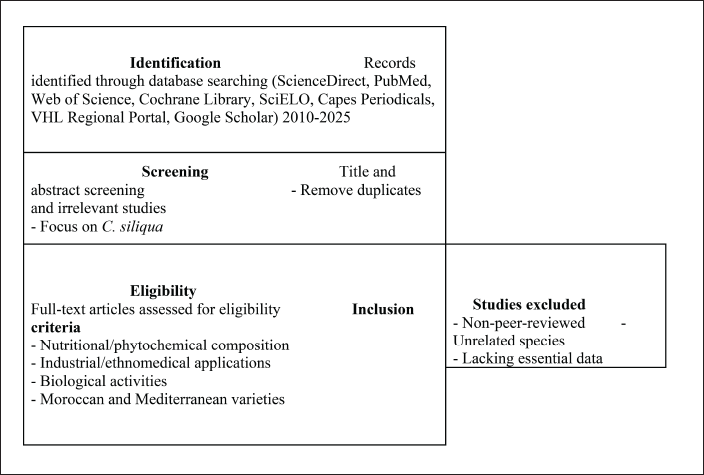 | Figure 1. Systematic selection process of studies on C. siliqua L. (carob). [Click here to view] |
To ensure an inclusive and rigorous search process, a combination of relevant keywords and Boolean operators was applied, including “Carob”, “Ceratonia siliqua”, “phytochemical composition”, “antioxidant activity”, “polyphenols”, “nutritional value”, “pharmacological properties”, “ethnomedicine”, and “morphological variation”. Boolean operators such as AND, OR, and NOT were employed to refine search results and minimize irrelevant studies. Google Scholar was used as a supplementary resource to identify grey literature, conference proceedings, and other non-indexed but relevant studies, reducing publication bias and integrating diverse perspectives, particularly on Moroccan carob varieties.
Study selection process
A systematic selection process was applied to identify, assess, and include only high-quality and relevant studies. The selection procedure followed a four-phase approach illustrated in a PRISMA flow diagram (Fig. 1). Initially, a total of 625 articles were identified through database searches. After the removal of 102 duplicate records, 523 articles remained for title and abstract screening. At this stage, 287 studies were excluded due to a lack of relevance to C. siliqua or failure to meet the inclusion criteria. The full texts of 236 articles were retrieved and assessed for eligibility, leading to the exclusion of 113 studies due to missing quantitative phytochemical data, lack of pharmacological validation, or failure to provide comparative analyses of Moroccan and Mediterranean carob varieties. Ultimately, 123 studies met the inclusion criteria and were incorporated into the qualitative and quantitative synthesis.
Inclusion and exclusion criteria
The inclusion criteria ensured that only well-designed and relevant studies on C. siliqua were considered. Studies were selected based on their focus on phytochemical composition, bioactive compounds, nutritional properties, pharmacological potential, and ethnomedicinal applications. Research on Moroccan populations was prioritized, with comparative analyses involving other Mediterranean varieties included to provide broader contextual insights into intraspecific variations. Experimental and observational studies, including in vitro, in vivo, and clinical research, were eligible for inclusion. Systematic reviews and meta-analyses were also considered if they contributed to a well-rounded synthesis of the existing literature.
To ensure consistency, reliability, and relevance, only studies offering detailed analyses of the phytochemical composition of C. silique, particularly polyphenols, flavonoids, tannins, and carbohydrates were retained for this review.
Pharmacological studies evaluating the antioxidant, antimicrobial, anti-inflammatory, antidiabetic, and anticancer properties of carob were prioritized. Morphological studies assessing intraspecific diversity, particularly those comparing Moroccan varieties with other Mediterranean populations, were included. Ethnomedicinal research detailing the traditional applications of Moroccan carob in food, pharmacology, and traditional medicine was integrated to provide a comprehensive overview of its therapeutic uses and cultural value. Only peer-reviewed articles published in English were selected.
Studies focusing exclusively on C. siliqua populations outside the Mediterranean region, without any comparative analysis involving Moroccan or other Mediterranean varieties, were excluded. Research lacking experimental validation or presenting unsubstantiated claims regarding the benefits of carob was also omitted. Articles unrelated to C. siliqua including those addressing other plant species or agricultural practices without direct relevance to the species’ phytochemical, nutritional, pharmacological, or industrial attributes were not considered. Studies that did not explore these specific aspects were excluded unless they offered essential contextual information relevant to the review’s objectives. Duplicate publications and studies with overlapping datasets were removed to avoid redundancy. Additionally, studies for which the full text was unavailable or accessible only through restricted platforms were excluded, unless the abstracts contained sufficient detail to justify inclusion.
A two-stage screening process was implemented to ensure the systematic identification of relevant studies. The first stage involved an initial review of titles and abstracts to eliminate duplicate records and studies lacking relevance. At this stage, articles that did not specifically address C. siliqua or failed to present essential data on its phytochemical composition, nutritional value, or pharmacological properties were excluded. This initial filtering allowed for the retention of studies most pertinent to the objectives of the review. In the second stage, the full texts of the remaining articles were retrieved and evaluated in detail according to predefined inclusion and exclusion criteria. Studies were included if they presented quantitative data on the phytochemical or nutritional composition of carob, examined its ethnomedicinal or pharmacological applications, or provided experimental evidence of biological activities such as antioxidant, antibacterial, anti-inflammatory, and antidiabetic effects. Conversely, studies were excluded if they were not peer-reviewed, lacked quantitative findings, or focused on plant species unrelated to C. siliqua.
Research synthesis and statistical analyses
To provide a comprehensive overview of research trends, a statistical synthesis was conducted on the 223 selected studies. The distribution of study types indicated that 65% of the included research focused on in vitro investigations, 22% on in vivo models, and only 13% on clinical trials, highlighting a critical gap in translational research. Among phytochemical investigations, 78% of studies reported polyphenol content, with significant variability depending on extraction methods and geographical origin. Antioxidant activity was the most frequently studied pharmacological property, assessed in 63% of reviewed articles, followed by antidiabetic effects (41%), anti-inflammatory properties (35%), and antimicrobial activity (28%). Ethnomedicinal applications were documented in 24% of studies, yet only 9% attempted to establish direct mechanistic links between bioactive compounds and traditional therapeutic uses.
RESULTS AND DISCUSSION
Ethnobotanical aspects
Ceratonia siliqua, a resilient member of the Fabaceae family, has held a central position in the traditional cultures, economies, and agricultural systems of Mediterranean societies, particularly in North Africa, including Morocco. Its historical relevance extends over millennia, with ancient civilizations such as the Phoenicians, Greeks, and Romans documenting its cultivation and applications. Across the Mediterranean, carob has been consistently recognized for its nutritional and therapeutic attributes, contributing to its enduring status as a key element within the region’s ethnobotanical and cultural heritage [9].
In Morocco, carob holds a central place in cultural practices, cuisine, and folk medicine. Carob pods, seeds, and derivatives are processed into various forms, such as flour and natural sweeteners, contributing to traditional sweets and beverages. Carob syrup, known for its high nutritional value, is a staple energy source in rural areas. Medicinally, carob has been used to treat gastrointestinal disorders like diarrhea and indigestion, due to its tannin content, as well as respiratory conditions, including coughs and throat irritations. These remedies remain prevalent in rural Moroccan communities [10].
Beyond its medicinal and nutritional roles, the carob tree holds economic importance in the broader Mediterranean region. Carob seeds are a source of locust bean gum, a highly sought-after thickening agent used extensively in the food and cosmetic industries. Its nutrient-rich pods are also utilized as livestock fodder, underscoring its versatility and value [11–13].
The growing recognition of carob as an invaluable cultural and scientific resource has spurred efforts to document and preserve traditional knowledge associated with its uses. Particularly in Morocco, this aligns with initiatives in sustainable agriculture and biodiversity conservation, where C. siliqua symbolizes the enduring interplay between human societies and their natural environment [14,15].
Structural traits of Moroccan C. siliqua
Morphological description
The carob tree, a robust member of the Fabaceae family, thrives in nutrient-poor and rocky soils, showcasing remarkable adaptability to challenging environments [16]. Typically reaching heights of 5–10 m, with optimal conditions producing specimens up to 15 m, the carob tree features a stout, gnarled trunk covered with rough, dark bark that develops deep furrows over time. Its broad, spreading crown forms a wide canopy, providing ample shade, while its long lifespan often exceeding a century reinforces its suitability for Mediterranean landscapes [17].
The tree’s compound, pinnate leaves are alternately arranged along the branches, with 4–8 obovate to elliptical leaflets (occasionally up to 12). These glossy leaflets, measuring 2–8 cm in length and 1–4.5 cm in width, are dark green on the upper surface and dull grey–green underneath. This dual coloration, along with a thick, waxy cuticle, minimizes water loss, enabling survival in arid climates. As an evergreen species, C. siliqua provides year-round foliage for shade and fodder [1].
The carob tree exhibits sexual dimorphism, being primarily dioecious, though some individuals produce both male and female flowers. These small, petal-less flowers grow in clusters along short racemes on old wood (cauliflory). Male flowers contain five stamens, while female flowers have a single ovary with a short style and stigma. Insect pollination predominates, though wind pollination can occur under specific conditions [18]. Flowering typically occurs from late summer to early autumn, attracting pollinators during periods of scarce floral resources [1–19].
The carob pod, the tree’s leguminous fruit, measures 10–30 cm in length and 1.5–3.5 cm in width. Initially green, the pods darken to brown or black as they mature, with a fibrous structure encasing a sweet pulp and 10–15 hard, shiny seeds. Notably, the seeds are consistent in size and weight, historically serving as a standard for measuring precious stones [17]. Pods take 11–12 months to mature, and trees begin bearing fruit after 6–8 years, reaching full productivity by 15 years. Mature pods can remain on the tree for extended periods, facilitating prolonged harvests [1].
Carob seeds (Fig. 2), encased in a hard endosperm, are rich in galactomannans polysaccharides widely utilized in the food and pharmaceutical industries as stabilizers and thickeners, commonly known as locust bean gum [20,21].
 | Figure 2. Morphological characteristics of the C. siliqua tree, pods, seeds, and seed structure. [Click here to view] |
Morphological characteristics of Moroccan C. siliqua pods
Moroccan carob trees exhibit significant morphological diversity (Table 1), shaped by both environmental and genetic factors. Trees located in well-preserved and favorable environmental conditions, such as those in Ait Oum El Bakht, tend to reach the greatest heights. However, site-specific factors contribute to growth variability observed across different studies [4,22].
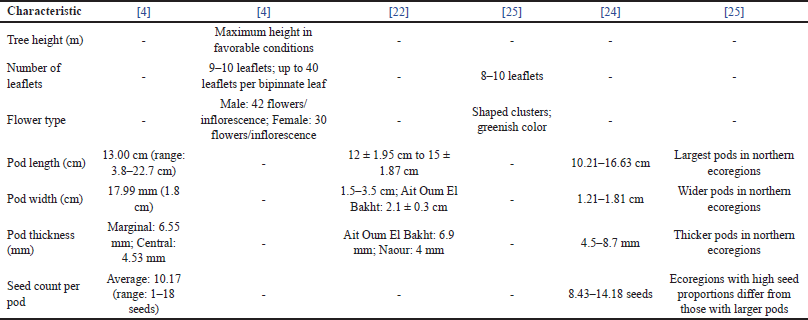 | Table 1. Morphological characteristics of Moroccan C. siliqua pods. [Click here to view] |
Leaflet numbers vary markedly among populations. The unproductive (Dkar) variant often has 9–10 leaflets per leaf, occasionally reaching up to 40 in a bipinnate arrangement. Similarly, El Kahkahi reported leaflet counts ranging from 8 to 10, reflecting genetic diversity and environmental influences [4,22,23]. Flower morphology also varies by gender. Male trees produce more flowers per inflorescence (42) than females (30), with flowers clustered, greenish, and featuring free sepals traits vital for understanding pollination dynamics [4,22,23].
Pod morphology demonstrates substantial variability. Pod lengths range from 10.21 cm to 16.63 cm [24] and 12 ± 1.95 cm to 15 ± 1.87 cm [22], with extremes of 3.8 cm–22.7 cm [4]. Pod width varies from 1.21 cm to 1.81 cm [24] and up to 3.4 cm [22], highlighting the influence of climatic and geographic factors [22–24]. Thicknesses range from 0.4 cm to 0.69 cm [22] to 4.5 mm to 8.7 mm indicating soil fertility and climate as critical determinants [24].
The seed count per pod also reflects ecological variability. Counts range from an average of 10.17 seeds to 8.43–14.18 seeds, with Sidina noting that regions with larger pods often have fewer seeds [4–21,4,22–25].
Geographic differences in pod morphology highlight the ecological plasticity of C. siliqua under diverse environmental conditions. Pods from northern Moroccan ecoregions, characterized by higher altitudes and latitudes, are notably larger and thicker, demonstrating the influence of local edaphic and climatic factors [25].
Morphological characteristics of Moroccan C. siliqua seeds
Comparative analyses of C. siliqua seed characteristics from Algeria and Morocco reveal significant variations in key traits, including seed length, width, weight, and germination rate (Table 2). These differences highlight the influence of geographic and environmental factors, suggesting regional adaptation and phenotypic diversity.
 | Table 2. Comparative seed morphological and germination traits of C. siliqua across different studies in Morocco and Algeria. [Click here to view] |
Seed length, a fundamental morphological trait, exhibits notable variability across regions. In Algeria, studies by Boublenza et al. [26] and Smaili et al. [27] reported length variations specific to local populations, with Yatim et al. [28,29,30–37] indicating measurements ranging from 6.06 mm to 8.07 mm. Moroccan carob displays broader ranges, with Kassout et al. [4] reporting lengths between 8.17 mm and 10.19 mm. Smaili et al. [27] also highlighted regional disparities within Algeria, with seed lengths varying from 7.35 mm in Blida to 9.98 mm in Skikda, suggesting localized ecological or genetic adaptations.
Seed width shows similar variability. Moroccan studies by Yatim et al. [28] reported widths ranging from 3.91 mm to 6.33 mm, while Kassout et al. [4] recorded a slightly broader range of 6.17 mm to 7.66 mm. In Algeria, Smaili et al. [27] observed the widest seeds in Blida, averaging 4.63 mm, emphasizing the role of local climate and soil conditions in shaping seed morphology (Table 2).
Seed weight, often correlated with nutrient content and pod structure, also varies across regions. In Morocco, Yatim et al. [28, 30–37] reported weights ranging from 0.787 g to 3.079 g per pod, while Kassout et al. [4] documented weights between 0.75 g and 2.58 g. In Algeria, Smaili et al. [27] reported average weights of 2.77 g in Blida and 2.37 g in Chelef, underscoring the combined influence of environmental factors and genetic variability [27].
Germination rates, a critical indicator of seed viability, were reported by Yatim et al. [28] for Moroccan populations, ranging from 16.3% to 34.4% under controlled conditions (sterile distilled water at 25°C) [28]. However, germination data were not available in Algerian studies by Kassout et al. [4] and Smaili et al. [27], highlighting a gap in research and the need for further exploration of seed viability across diverse environmental conditions [38,39–41].
Carob production areas
The carob tree thrives in arid and semi-arid climates, making it a valuable agricultural species globally. While the Mediterranean Basin remains the primary production zone, carob cultivation has expanded to regions with similar climates, including parts of the Americas, Asia, and Australia [42].
In the Mediterranean, carob production is concentrated in countries along the northern, southern, and eastern shores, with Morocco, Spain, Italy, Greece, Turkey, Tunisia, and Portugal dominating global output (Fig. 3). The Mediterranean climate characterized by hot, dry summers and mild, wet winters provides ideal conditions for carob growth [43].
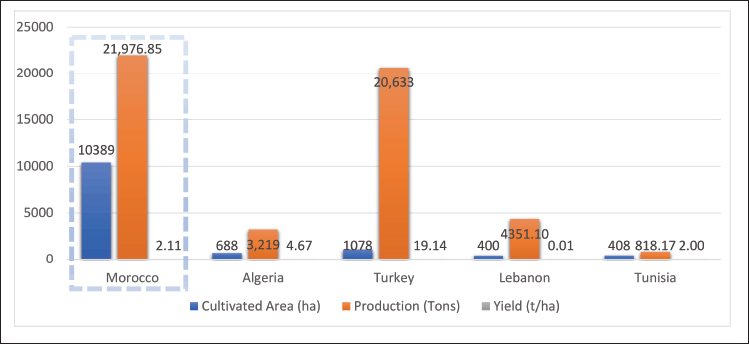 | Figure 3. Carob production metrics by country [3] . [Click here to view] |
Spain leads global production, accounting for approximately 40% of total output. It is the second-largest producer of carob seeds (locust bean) after Morocco, with Valencia, Catalonia, and the Balearic Islands recognized for their extensive orchards [44–46].
In North Africa, Morocco, Algeria, and Tunisia are significant contributors. Morocco, the region’s largest producer, primarily cultivates carob in the Rif and Atlas Mountains, integrating it into traditional agroforestry systems. Algeria and Tunisia also emphasize cultivation in semi-arid areas, utilizing the ability of C. siliqua to thrive in poor soils and under minimal water conditions to support local economies and promote land rehabilitation.
In Asia Minor, Turkey stands out as a major producer, particularly in the southwestern regions of Antalya, Mugla, and Mersin. Turkish carob, valued for its high sugar content, is widely used in traditional sweets and food products [47]. In the Middle East, Lebanon incorporates carob into agroforestry systems on marginal lands, leveraging its drought resistance and minimal irrigation needs for sustainable agricultural output and environmental conservation [2].
Beyond the Mediterranean, carob cultivation has reached North and South America. In the United States, carob is grown in California and Arizona, gaining popularity as a health-conscious alternative to cocoa and sweeteners. South American countries like Argentina and Chile have adopted carob cultivation in regions such as Mendoza and San Juan, where its drought resilience aligns with sustainable farming practices [44].
The productivity of carob cultivation varies across regions (Fig. 3). Morocco leads in cultivated area with 10,389 hectares producing 22 million tons (2.11 tons per hectare). Algeria, though cultivating only 688 hectares, achieves a higher yield of 4.67 tons per hectare, likely due to efficient farming practices or optimized varieties. Turkey records one of the highest yields at 19.14 tons per hectare, producing 20,633 tons from 1,078 hectares, reflecting advanced agricultural methods and favorable conditions. In contrast, Lebanon and Tunisia exhibit lower yields, with Lebanon producing just 0.01 tons per hectare, indicative of suboptimal growing conditions or less intensive cultivation practices (Fig. 3).
Ethnomedicine of C. siliqua
Widely valued in ethnomedical practices, the carob has long been esteemed for its therapeutic applications across Mediterranean, North African, and Middle Eastern cultures. Traditional healers have employed their pods, seeds, and bark to treat a variety of ailments, underscoring its dual function as both a nutritional source and a medicinal agent well before the advent of modern pharmacology [48].
Carob is primarily valued in traditional medicine for treating digestive ailments. In Mediterranean folk practices, carob pods are boiled to create remedies for diarrhea and gastrointestinal disturbances, an effect attributed to their high tannin content, which reduces intestinal secretions and combats pathogenic bacteria. In North Africa, carob extracts are used to manage dysentery and colitis, underscoring their effectiveness in promoting digestive health [49].
In Moroccan ethnomedicine, carob-based infusions and decoctions are used to manage blood sugar levels in individuals with diabetes (Table 3). The low glycemic index of carob, coupled with its ability to modulate glucose absorption, makes it a natural treatment for hyperglycemia. Studies confirm that its high fiber content and polyphenols help lower postprandial blood glucose levels, supporting its role in diabetic diets [14].
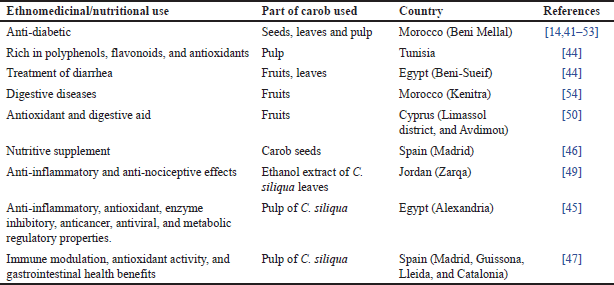 | Table 3. Ethnomedicinal and nutritional utilization of C. siliqua across different regions. [Click here to view] |
Ceratonia siliqua fiber-rich pods enhance satiety and regulate appetite, aiding in weight loss and metabolic health. Herbal remedies derived from carob are traditionally prescribed in rural Mediterranean communities for these purposes. Recent studies validate these applications, showing that carob polyphenols and dietary fibers inhibit fat accumulation and support metabolism [50].
Carob seeds and bark are used in North African communities as poultices for wound care and skin infections, leveraging their anti-inflammatory and antimicrobial properties. Modern studies have identified bioactive compounds like gallic acid, which exhibit strong antioxidant and anti-inflammatory effects, corroborating its traditional use for treating skin conditions [51].
In Algeria, carob is widely used to treat diarrhea, while Moroccan healers recommend carob-based remedies for their soothing effects on the digestive tract and hypoglycemic properties. In Lebanon and other Middle Eastern countries, carob syrup (dibs el kharrub) is traditionally used to relieve respiratory ailments, such as coughs and bronchial irritation, due to its expectorant and soothing qualities [49–52]. In Portugal, carob pods have been incorporated into bread-making for their high fiber content, which supports gut health. Similarly, in Greece, carob is used as a natural sweetener, providing a sugar alternative that regulates energy levels without causing blood sugar spikes [53].
Ethnomedicinal and nutritional uses of C. siliqua
Ethnomedicinal practices involving C. siliqua are widely recognized throughout the Mediterranean and North Africa, with notable prevalence in Morocco. In the Beni Mellal region, seeds, leaves, and fruits are traditionally used to manage diabetes, reflecting long-standing local knowledge. Comparable practices have been observed in Tunisia, where carob pulp is valued for its high content of polyphenols, flavonoids, and antioxidants, which contribute to its nutritional and therapeutic potential [14,41,42,54].
In Egypt (Table 3), carob is traditionally employed to treat diarrhea, particularly in Beni-Sueif, where fruits and leaves are used for this purpose. In Alexandria, carob pulp extract is recognized for its broad pharmacological properties, including anti-inflammatory, antioxidant, anticancer, antiviral, and metabolic regulatory effects [44,45].
The nutritional benefits of C. siliqua are widely acknowledged in Bulgaria, particularly in Plovdiv, where its pods and seeds rich in proteins, lipids, phenolic compounds, and antioxidants offer functional advantages when incorporated into food products [48]. In Spain, carob seeds are valued as nutritional supplements, while the pulp is utilized for its immune-modulating, antioxidant, and gastrointestinal health benefits, particularly in Madrid [46,47].
Pharmacological investigations highlight the promising therapeutic potential of carob. In Jordan, ethanol extracts of carob leaves collected from the Zarqa region have shown notable anti-inflammatory and anti-nociceptive effects [49]. Similarly, in Cyprus, carob fruits are highly regarded for their antioxidant properties and digestive benefits, particularly in Limassol and Avdimou [50]. These findings underscore the extensive use of C. siliqua across diverse Mediterranean and neighboring regions for its medicinal and nutritional attributes (Table 3).
Ethnomedicine and therapeutic potential
The traditional use of C. siliqua in Moroccan and Mediterranean ethnomedicine is grounded in generations of practical knowledge and cultural experience. However, its pharmacological properties have only recently started to receive attention in modern research. Many of the therapeutic uses described in traditional practices appear to align with the plant’s diverse phytochemical profile especially its rich content of polyphenols, flavonoids, tannins, and bioactive carbohydrates. Exploring these connections helps shed light on the biological mechanisms through which these compounds may produce the health benefits long observed in traditional settings.
Carob has been widely used in Moroccan folk medicine to manage gastrointestinal disorders, including diarrhea and indigestion (Table 3). This traditional application can be attributed to its high tannin content, which exhibits astringent properties that reduce intestinal motility and fluid loss, thereby alleviating diarrhea. Additionally, tannins have demonstrated antimicrobial activity, particularly against Escherichia coli and Salmonella, two major bacterial agents of gastrointestinal infections. The presence of galactomannans and dietary fibers further supports digestive health by promoting beneficial gut microbiota, which aligns with the traditional use of C. siliqua as a digestive aid.
In traditional Moroccan and Mediterranean medicine, carob-based remedies have long been used to help manage diabetes (Table 3). Recent research has identified flavonoids such as quercetin, myricetin, and gallic acid in carob as having antihyperglycemic effects. These compounds exert their effects by inhibiting α-glucosidase and α-amylase enzymes involved in carbohydrate digestion which helps reduce post-meal blood sugar spikes. In addition, polyphenols found in carob have been shown to improve insulin sensitivity and influence glucose transporter activity. Together, these findings offer a possible explanation for the traditional use of carob in supporting blood sugar regulation.
Widely used in traditional medicine for treating wounds and respiratory ailments, the anti-inflammatory potential of carob is closely associated with its high levels of flavonoids and phenolic acids. Bioactive compounds such as catechins and epicatechins have been shown to suppress pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, thereby reducing systemic inflammation. Additionally, the strong antioxidant activity of carob polyphenols helps support tissue repair by counteracting oxidative stress, a key contributor to chronic inflammatory conditions.
In Moroccan traditional medicine, carob extracts have long been used to treat respiratory infections and oral health problems, based on their known antimicrobial effects (Table 3). Studies have shown that compounds such as tannins, flavonoids, and alkaloids in carob possess both bacteriostatic and bactericidal activity against a range of pathogens, including Staphylococcus aureus and Pseudomonas aeruginosa. These findings help explain the traditional use of carob in managing throat infections, gum disease, and respiratory conditions.
Carob is also valued in traditional healing practices for its heart-protective benefits. Its high fiber and polyphenol content contribute substantially to cardiovascular health by helping to lower cholesterol and improve vascular function. Soluble fibers in carob can bind to bile acids, reducing cholesterol absorption and enhancing lipid metabolism. Meanwhile, flavonoids and tannins have been shown to support healthy blood pressure by promoting nitric oxide production and improving blood vessel dilation.
Phytochemistry
Primary and secondary metabolites of C. siliqua pulp
The pulp, constituting the main consumable part of the carob pod, contains a high concentration of bioactive constituents that have been linked to beneficial effects on human health and nutrition (Table 4). Accounting for approximately 90% of the pod’s mass, the pulp is a primary source of its overall nutritional and pharmacological value. Its chemical profile is dominated by carbohydrates, dietary fibers, polyphenols, minerals, and other essential nutrients, with variations influenced by cultivar, geographic origin, and harvest conditions [55].
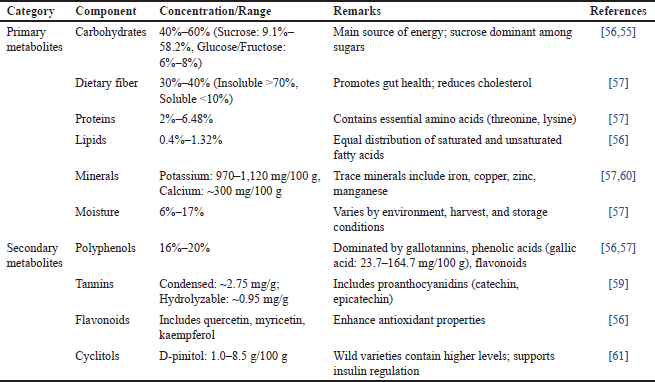 | Table 4. Nutritional and phytochemical profile of carob pulp: primary and secondary metabolites. [Click here to view] |
Carbohydrates are the predominant component, comprising from 40% to 60% of the pulp. Sucrose is the most abundant sugar (from 9.11% to 58.2%), followed by glucose and fructose (6%–8%) (Table 4). The pulp also contains high levels of dietary fiber (around 3% of dry weight) but relatively low amounts of protein (from 2% to 6.5%) and lipids (0.4%–1.3%), with a balanced distribution of saturated and unsaturated fatty acids. Carob pulp provides an energy value of approximately 17 kJ/g of dry weight [55].
The pulp of Moroccan carob is particularly valued for its high fiber content especially cellulose and hemicellulose, which together account for approximately 18% contributing to its strong nutritional profile. Its naturally low fat and protein levels further support its suitability for low-calorie dietary applications. Differences in carbohydrate composition among various carob types are often influenced by factors such as regional origin, harvest timing, and storage conditions [56].
Polyphenols, key secondary metabolites, are abundant in carob pulp, imparting strong antioxidant properties. Polyphenol levels range from 16% to 20%, with tannins comprising the majority. The pulp is rich in gallotannins, phenolic acids (e.g., gallic acid), and flavonoids such as quercetin, myricetin, and kaempferol, with their concentrations influenced by extraction techniques, geographic location, and storage methods [55]. Gallic acid is particularly prominent, with levels ranging from 23.7 mg/100 g to 164.7 mg/100 g, establishing carob as one of the richest natural sources of this compound [57].
Flavonoids in carob, primarily flavonols, contribute significantly to its antioxidant effects. The most common are quercetin, myricetin, and kaempferol derivatives, along with other flavonoids like apigenin, luteolin, and naringenin, which enhance the bioactivity of the pulp [58]. Tannins, particularly condensed tannins (proanthocyanidins), provide an astringent taste and offer health-promoting properties, including anti-inflammatory and anticancer effects. Condensed tannins are found at approximately 2.75 mg/g, while hydrolyzable tannins reach about 0.95 mg/g [59].
Dietary fiber constitutes 30%–40% of the dry weight, with insoluble fibers like cellulose, hemicellulose, and lignin comprising more than 70%. This fiber content supports cholesterol reduction and gut health through improved bowel regularity. Soluble fibers account for up to 10% of total fiber [57].
Carob pulp also contains amino acids, including essential ones like threonine, methionine, valine, isoleucine, leucine, phenylalanine, and lysine. Protein content (around 4.45%) aligns with World Health Organization standards for essential amino acids. Minerals are another critical component, with potassium (970–1,120 mg/100 g dry weight) and calcium (up to 300 mg/100 g) being particularly abundant. Trace minerals such as iron, copper, zinc, and manganese further enhance the nutritional profile, with iron being the most prevalent [57–60].
Moisture content in carob pulp varies between 6% and 13.88%, influenced by environmental conditions, harvest timing, and storage duration. Additionally, carob pulp is a rich source of cyclitols, especially D-pinitol, known for its insulin-regulating and antioxidant effects. D-pinitol levels range from 1.0 to 8.5 g per 100 g of dry matter, with wild varieties typically having higher concentrations than cultivated types [61].
Chemical composition of C. siliqua seed
Carob seeds, or kernels, are notable for their diverse chemical composition, which provides considerable nutritional and functional benefits across the food, pharmaceutical, and nutraceutical industries (Fig. 4 and Table 5). The seeds consist of three main anatomical layers testa, tegmen, and endosperm each contributing distinct biomolecules with varied applications [7].
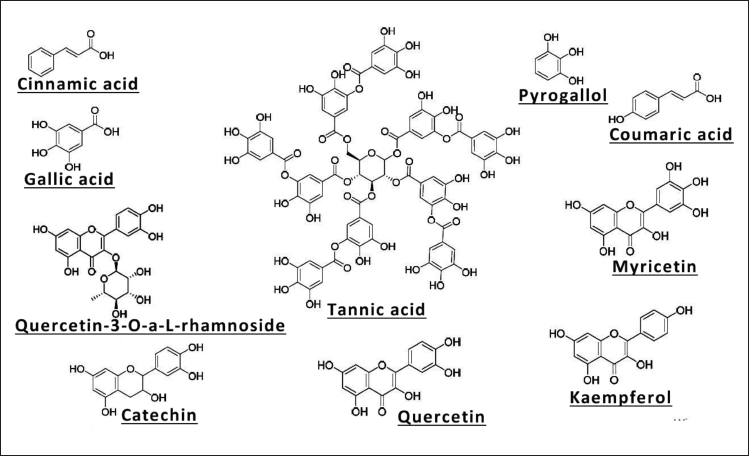 | Figure 4. Phytochemical constituents identified in C. siliqua. [Click here to view] |
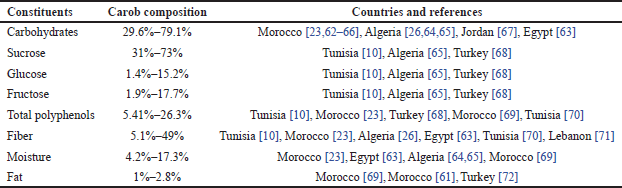 | Table 5. The chemical composition of C. siliqua in various Mediterranean countries. [Click here to view] |
The outer testa (from 30% to 33%) acts as a protective covering, while the softer, lighter tegmen forms the inner coat. The endosperm (42%–46%), the largest portion, contains essential reserves that support embryo development and enhance the seed’s value as a natural thickening and gelling agent in the food industry [7].
A key component of carob seeds is their high galactomannan content, concentrated in the endosperm. These polysaccharides, composed of a mannose backbone with galactose side chains, form locust bean gum (LBG), a high-molecular-weight hydrocolloid. LBG accounts for approximately 85%–90% of the endosperm and is highly valued in food processing for its gelling and stabilizing properties. The typical 3:1 mannose-to-galactose ratio in galactomannans determines their functional behavior, establishing carob seeds as an economically valuable source of hydrocolloids.
Despite comprising only 4%–7% of the seed’s dry weight, carob seed proteins are rich in essential amino acids such as lysine, leucine, and threonine, enhancing their nutritional appeal [17]. These proteins also include enzymes such as peroxidases and polyphenol oxidases, which play roles in metabolic processes and hold potential for biotechnological applications. Additionally, bioactive peptides derived from these proteins exhibit antioxidant and antimicrobial properties, further enhancing the seed’s therapeutic potential [73,74].
Carob seeds contain moderate lipid levels (1%–3%), composed primarily of unsaturated fatty acids such as oleic and linoleic acids. These lipids contribute to cardiovascular health, reinforcing the nutritional value of the seeds within the context of functional foods. Additionally, the lipid fraction includes bioactive compounds such as phytosterols and tocopherols (vitamin E), which possess antioxidant properties and help reduce oxidative stress [61].
Carob seeds are rich in polyphenolic compounds, including phenolic acids, flavonoids, and tannins, which act as potent antioxidants. These compounds neutralize free radicals and reduce oxidative damage, lowering the risk of cardiovascular and other chronic diseases. Consequently, carob seed extracts are widely utilized in nutraceuticals and functional foods for their high antioxidant capacity [75].
Carob seeds contain substantial amounts of calcium, potassium, magnesium, and phosphorus, which support bone health, electrolyte balance, and enzymatic functions. Calcium, in particular, is abundant, aiding in osteoporosis prevention and promoting skeletal health. Trace minerals such as iron and zinc further enhance the nutritional value, addressing dietary micronutrient deficiencies [60].
Carob seeds also contain secondary metabolites, including tannins, phenolic compounds, and alkaloids. Tannins, primarily proanthocyanidins, provide an astringent taste and exhibit strong antioxidant effects. These properties contribute to the seeds’ therapeutic potential in managing oxidative stress and reducing the risk of chronic diseases [59].
Environmental and genetic influences on carob phytochemical profiles
The phytochemical composition and pharmacological properties of C. siliqua exhibit considerable variability across geographical regions, shaped by both environmental conditions and genetic diversity. Previous studies have reported pronounced differences in polyphenol content, flavonoid concentrations, and tannin profiles between Moroccan and other Mediterranean carob populations; however, the underlying factors driving this variability remain insufficiently understood.
Among the environmental influences, climatic conditions, soil characteristics, altitude, and water availability are key determinants of the phytochemical profile of C. siliqua. Carob trees cultivated in semi-arid and arid regions, such as Morocco’s Rif and Atlas Mountains, are frequently exposed to drought stress, intense solar radiation, and nutrient-deficient soil factors known to stimulate the biosynthesis of polyphenols, flavonoids, and tannins. This observation is consistent with the broader principle of stress-induced secondary metabolite production, whereby plants increase the accumulation of antioxidant and protective compounds in response to abiotic stressors. Conversely, carob trees grown in more temperate Mediterranean environments, including parts of Spain, Morocco, Italy, and Greece, tend to exhibit higher carbohydrate content and comparatively lower polyphenolic concentrations, reflecting distinct metabolic adaptations to their respective growing conditions.
Soil composition and mineral availability further influence the variability in phytochemical profiles. In Morocco, soils characterized by calcareous sediments and low organic content impact mineral uptake and, consequently, enzymatic pathways involved in phenolic biosynthesis. Moreover, microbial communities within the rhizosphere contribute to shaping the plant’s metabolic profile; however, research on plant-microbe interactions in carob cultivation remains limited.
Beyond environmental factors, genetic diversity within C. siliqua represents a major contributor to variations in phytochemical composition and associated pharmacological effects. The species exhibits considerable genetic variability across its natural range, with distinct ecotypes and chemotypes characterized by unique secondary metabolite profiles. Several studies have reported differences in gallic acid, catechin, and epicatechin content among Moroccan carob accessions, suggesting that genotypic factors substantially influence polyphenol biosynthesis. Nonetheless, the lack of comprehensive genomic and metabolomic investigations continues to limit our understanding of how genetic variation interacts with environmental conditions to shape the phytochemical landscape of carob. Emerging technologies in next-generation sequencing and advanced metabolomic profiling offer promising tools to identify key biosynthetic genes involved in polyphenol production, thereby informing future breeding programs aimed at enhancing the nutraceutical value of carob.
The pharmacological potential of C. siliqua is closely tied to its phytochemical diversity. However, many studies overlook the influence of environmental and genetic factors when evaluating bioactivity, which may explain the inconsistencies frequently observed in pharmacological assessments. For instance, variations in IC50 values reported for antioxidant assays such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) can often be traced to differences in geographical origin, seasonal factors, or extraction methods. Similar discrepancies are evident in studies evaluating the antimicrobial and antidiabetic activities of carob extracts, suggesting that uncontrolled environmental and genetic variables may contribute to inconsistent outcomes. Standardizing research protocols by incorporating information on geographical provenance, seasonal variability, and genetic background would improve the reliability and comparability of pharmacological findings.
Medicinal properties of C. siliqua
Antioxidant activity
Renowned for its potent antioxidant capacity, the carob tree owes this property to its diverse phytochemical composition, particularly its high concentrations of polyphenols, flavonoids, and tannins. These bioactive compounds contribute to the neutralization of free radicals, the reduction of oxidative stress, and the prevention of chronic diseases associated with oxidative damage, including cancer, cardiovascular disorders, and neurodegenerative disorders.
The primary antioxidant compounds in carob are polyphenols, which are abundantly present in both pods and seeds. Among these, gallic acid, catechins, and epicatechins are particularly effective in neutralizing free radicals and alleviating oxidative stress. Several studies have reported that carob pod extracts contain high levels of total phenolic content and exhibit strong radical scavenging activity (Table 6), as confirmed by 2,2-diphenyl-1-picrylhydrazyl and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assays [76].
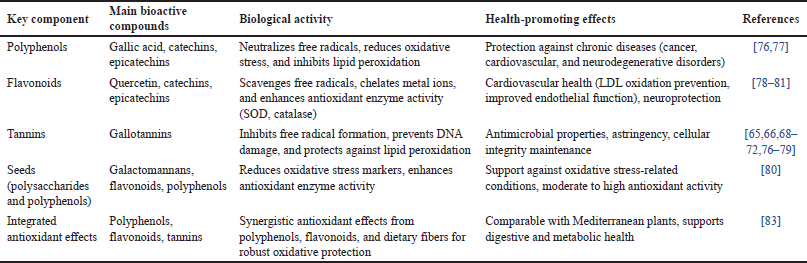 | Table 6. Antioxidant activity, major compounds, and health-promoting properties of C. siliqua. [Click here to view] |
Among the phenolic compounds identified in carob, one of the most prominent is gallic acid, which contributes substantially to its antioxidant activity. This compound acts by scavenging reactive oxygen species (ROS) and inhibiting lipid peroxidation, thereby protecting cellular structures from oxidative damage (Table 6). Its abundance in carob pods and seeds underscores the therapeutic potential of carob for managing oxidative stress-related conditions [77].
Flavonoids in carob (Table 6), including quercetin, catechins, and epicatechins, further enhance its antioxidant defense. These compounds not only scavenge free radicals but also chelate metal ions, preventing the generation of harmful reactive species. They also boost the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, strengthening the body’s oxidative defenses [78].
Tannins, particularly hydrolysable types such as gallotannins, enhance the antioxidant efficacy of C. siliqua by inhibiting a free radical formation and protecting DNA from oxidative damage (Table 6). Tannins also prevent lipid peroxidation, maintaining cellular integrity and reducing inflammation linked to chronic diseases [65,66,68–72,76–79].
Carob seeds, although primarily valued for their galactomannan content, also exhibit significant antioxidant activity due to their polyphenol and flavonoid content. Research shows moderate to high antioxidant activity in carob seed extracts, which can lower oxidative stress markers and enhance antioxidant enzyme activity, depending on the extraction methods used [80].
The antioxidant potential of carob has been widely investigated for its protective effects against conditions associated with oxidative stress, underscoring its value in both prevention and therapy. Oxidative stress serves as a driving factor in the development of cardiovascular diseases by promoting atherosclerosis through the oxidation of low-density lipoproteins (LDLs). Carob-derived flavonoids and polyphenols help counteract this process by inhibiting LDL oxidation, enhancing endothelial function, and exerting antihypertensive effects, thereby contributing to a reduced risk of cardiovascular disorders [81].
Carob, characterized by an antioxidant-rich profile, particularly its high levels of gallic acid and flavonoids, also exhibits anticancer properties. These compounds mitigate oxidative damage to DNA, proteins, and lipids, inhibiting the initiation and growth of cancer cells. Furthermore, they may protect healthy tissues during cancer treatments, enhancing the efficacy of conventional therapies [82].
In the context of neurodegenerative diseases like Alzheimer’s and Parkinson’s, flavonoids derived from C. siliqua have demonstrated neuroprotective effects by reducing ROS levels and supporting neuronal health. This is achieved through the modulation of oxidative stress pathways and reduction of neural inflammation. Compared to other polyphenol-rich Mediterranean plants such as pomegranate, olive, and grape, carob stands out for its unique combination of polyphenols, tannins, and dietary fibers, offering robust antioxidant protection alongside digestive and metabolic health benefits [83].
Antibacterial effects
The antibacterial potential of carob has attracted considerable interest, largely due to its rich phytochemical composition, particularly its abundance of polyphenols, flavonoids, and tannins. These bioactive compounds exhibit broad-spectrum antimicrobial activity, positioning carob as a promising natural option for the management of bacterial infections (Table 8) [19,84]. Extracts derived from carob have shown effectiveness against both Gram-positive and Gram-negative bacteria, lending support to its traditional use in infection treatment and health promotion [19].
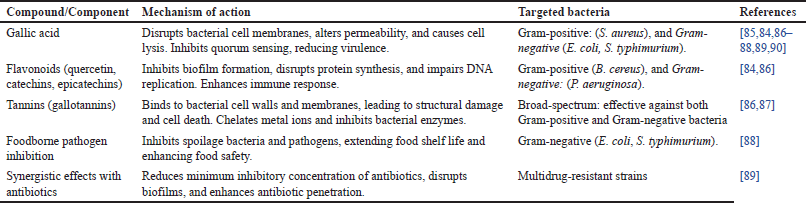 | Table 7. Antibacterial potential of C. siliqua demonstrating mechanisms of action and targeted bacteria. [Click here to view] |
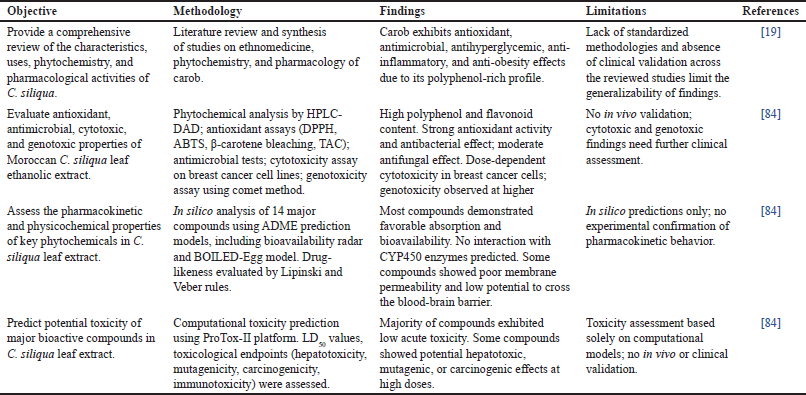 | Table 8. Pharmacological and toxicological profile of C. siliqua. [Click here to view] |
The high polyphenolic content in carob is a key determinant of its antibacterial effects. Phenolic compounds, including gallic acid, flavonoids, and tannins, disrupt bacterial cell membranes, inhibit enzymatic functions, and interfere with bacterial growth cycles.
Among the polyphenols found in carob, gallic acid is recognized for its ability to disrupt bacterial cell membranes, altering their permeability and leading to cell lysis (Table 7). Moreover, gallic acid interferes with bacterial quorum sensing, thereby reducing the expression of virulence factors and limiting bacterial pathogenicity [85].
Flavonoids, including compounds such as quercetin, catechins, and epicatechins inhibit bacterial biofilm formation a key defense mechanism against antibiotics. By preventing biofilm development, flavonoids enhance the immune system’s ability to combat bacterial infections (Table 7). They also inhibit bacterial protein synthesis and DNA replication, further suppressing bacterial proliferation [84,86].
Tannins are particularly abundant in carob, especially gallotannins, which bind to bacterial cell walls, compromise membrane integrity, and induce cell death (Table 7). Tannins also coagulate intracellular proteins, chelate essential metal ions, and inhibit bacterial enzymes. These mechanisms make tannins effective against Gram-positive bacteria like S. aureus and Gram-negative strains such as E. coli and Salmonella typhimurium, demonstrating their broad-spectrum activity [86,87].
Carob extracts show promise in food preservation by inhibiting foodborne pathogens. The tannins in carob reduce the growth of bacteria responsible for food spoilage and contamination, such as E. coli and S. typhimurium, extending the shelf life of food products and enhancing food safety [88].
An emerging area of interest is the ability of carob extracts to enhance the efficacy of conventional antibiotics. Studies suggest that polyphenols and flavonoids present in C. siliqua can act synergistically with antibiotics, improving their effectiveness against multidrug-resistant bacterial strains. This combination reduces the minimum inhibitory concentration of antibiotics required to combat resistant bacteria, offering a potential adjuvant therapy for infections caused by antibiotic-resistant pathogens. By disrupting bacterial biofilms and enhancing antibiotic penetration, carob extracts may restore the potency of antibiotics against resistant infections [89].
Significant activity has been observed against S. aureus and Bacillus cereus, which are responsible for skin infections, food poisoning, and respiratory illnesses (Table 7). The disruption of bacterial membranes and inhibition of enzymatic functions are central to the antimicrobial effectiveness of C. siliqua against these strains [39].
Carob exhibits notable efficacy against E. coli, S. typhimurium, and P. aeruginosa. These pathogens, often linked to gastrointestinal, urinary tract, and hospital-acquired infections, are typically resistant to antibiotics due to their lipid-rich outer membranes. Polyphenols and tannins from C. siliqua effectively disrupt these barriers, enhancing antibacterial activity [90].
Antidiarrheal benefits
Traditionally valued for its antidiarrheal properties, carob has been used to manage diarrhea and promote digestive health, owing to its high content of tannins, polyphenols, and dietary fiber. The tannins present in carob help form a protective layer over the intestinal mucosa, reducing secretions, limiting fluid loss, and binding to toxins released by pathogenic microorganisms. In parallel, polyphenols exert anti-inflammatory and antimicrobial effects by inhibiting the growth of harmful bacteria such as E. coli and Salmonella, both commonly associated with infectious diarrhea [91].
Carob contains soluble fibers, including pectin, absorb water to form a gel that bulks stools, slows intestinal transit, and improves stool consistency. Insoluble fibers, on the other hand, promote the growth of beneficial gut bacteria, which helps reduce the risk of gastrointestinal infections. Studies have shown that carob powder is particularly effective in managing pediatric diarrhea. Clinical trials in children with acute diarrhea report that carob powder reduces symptom duration by up to 50% and improves stool consistency within 24–48 hours [92].
Animal studies further corroborate the therapeutic potential of C. siliqua, demonstrating reduced defecation frequency and improved stool form due to its anti-inflammatory and astringent tannins [19]. Its traditional use in treating diarrhea is deeply rooted in traditional medicine across Mediterranean, Middle Eastern, and North African cultures. Particularly in rural areas with limited access to pharmaceuticals, carob has served as a natural remedy for gastrointestinal issues [93].
Anti-inflammatory potential
Rich in bioactive compounds such as polyphenols, flavonoids, and tannins, carob is widely acknowledged for its anti-inflammatory potential. These constituents help modulate inflammatory processes by suppressing pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, thereby contributing to the prevention of chronic conditions such as arthritis, cardiovascular diseases, and metabolic disorders. Among these bioactives, polyphenols particularly gallic acid are known to attenuate inflammatory responses, while flavonoids such as quercetin and catechin exert antioxidant effects by reducing ROS that further aggravate inflammation [94].
Tannins in carob also contribute significantly to its anti-inflammatory effects by inhibiting the enzymes cyclooxygenase and lipoxygenase, which are responsible for the production of pro-inflammatory mediators. This action reduces pain and swelling. Additionally, carob disrupts the NF-κB signaling pathway, a key regulator of inflammation, thereby decreasing the expression of inflammation-promoting molecules. Animal studies have shown that carob extracts effectively reduce swelling and inflammatory markers, with efficacy comparable to non-steroidal anti-inflammatory drugs but with fewer side effects [95].
Limited human studies suggest that carob may alleviate symptoms associated with inflammatory conditions such as arthritis and inflammatory bowel disease. The fiber content in carob also supports gut health, which may further lower systemic inflammation and improve overall well-being [95].
The anti-inflammatory properties of C. siliqua highlight its potential in managing chronic inflammation-related diseases, including rheumatoid arthritis, cardiovascular conditions, and metabolic disorders. Its polyphenols and fiber work synergistically to reduce oxidative stress, inflammation, and insulin resistance, thereby promoting metabolic and cardiovascular health. Traditionally used in Mediterranean and Middle Eastern cultures for treating inflammation-related ailments, carob remains a valued natural remedy for alleviating symptoms of tissue irritation and supporting the healing process [96].
Anti-hyperglycemic action
Carob has shown considerable potential in the management of hyperglycemia and metabolic disorders such as diabetes, largely due to its high content of fiber, polyphenols, flavonoids, and tannins. Its substantial fiber content, particularly galactomannan, slows glucose absorption by forming a gel-like matrix in the intestines, thereby stabilizing blood sugar levels and preventing postprandial spikes. This delayed digestion of carbohydrates also enhances satiety, contributing to weight management an essential component in the control of type 2 diabetes [97].
Polyphenols and flavonoids in carob, including gallic acid, quercetin, and catechin, enhance insulin sensitivity and protect pancreatic β-cells from oxidative damage, a common feature of diabetes. These compounds inhibit enzymes like alpha-amylase and alpha-glucosidase, thereby reducing postprandial hyperglycemia by slowing carbohydrate breakdown. Tannins further support glycemic control by inhibiting carbohydrate-digesting enzymes, preventing rapid increases in blood sugar levels after meals.
Preclinical studies underscore the anti-hyperglycemic effects of C. siliqua, demonstrating enhanced glucose tolerance and improved insulin sensitivity in diabetic animal models. Emerging human studies reinforce these findings, showing that carob supplements reduce post-meal glucose levels and enhance insulin sensitivity in type 2 diabetes patients. These benefits are partly attributed to the substantial fiber content of C. siliqua, which has a central function in glycemic control and weight management [80].
Anti-obesity effects
Carob is a promising natural agent for obesity management, an essential factor in preventing chronic conditions such as type 2 diabetes, cardiovascular diseases, and metabolic syndrome. Its high content of dietary fibers, polyphenols, and tannins supports weight control by enhancing satiety, regulating lipid metabolism, and reducing fat storage [37].
Carob pods are low in calories and rich in fiber, particularly soluble fibers like galactomannan, which slow digestion, stabilize postprandial insulin levels, and promote hunger control. This fiber increases feelings of fullness, helping to limit overall calorie intake. Additionally, polyphenols such as quercetin and catechins inhibit fat cell formation, reduce fat absorption, and promote fat breakdown. Tannins complement these effects by binding to dietary fats and inhibiting pancreatic lipase, further minimizing fat absorption [98].
Evidence from both preclinical and clinical studies supports the anti-obesity effects attributed to C. siliqua. Animal models have shown reductions in body weight, fat mass, and food intake following carob extract supplementation. Similarly, human clinical trials involving carob fiber supplements have demonstrated significant weight loss and improved lipid profiles, emphasizing its effects on appetite control and lipid regulation [37]. The satiety-inducing effects of carob fiber are well-supported by recent clinical studies [99].
Antihypertensive properties
The potential of carob in managing hypertension a major risk factor for cardiovascular diseases has been attributed to its high content of polyphenols, dietary fiber, and essential minerals. Polyphenolic compounds, particularly flavonoids and tannins, support vascular health by stimulating nitric oxide (NO) production, which promotes vasodilation and helps lower blood pressure. In addition, these bioactives offer antioxidant protection, mitigating oxidative stress that can impair vascular function and contribute to the development of hypertension [100]. Furthermore, polyphenols may inhibit the renin-angiotensin system, specifically the angiotensin-converting enzyme (ACE), which regulates vasoconstriction. By blocking ACE, carob helps maintain lower blood pressure.
The soluble dietary fiber from C. siliqua, particularly galactomannans, supports cardiovascular health by reducing cholesterol levels and positively influencing gut microbiota, both of which are critical in blood pressure regulation. Fiber lowers cholesterol by binding bile acids, improving lipid metabolism, and promoting arterial health. Through gut fermentation, fiber produces short-chain fatty acids, which exhibit vasodilatory and anti-inflammatory properties, further contributing to blood pressure control [98].
The mineral content of carob, particularly potassium and magnesium, is essential for blood pressure regulation. Potassium helps counteract the hypertensive effects of sodium by promoting its excretion, while magnesium contributes to vascular relaxation and NO production, thereby supporting blood pressure reduction [19].
Animal studies highlight the blood pressure-lowering properties of C. siliqua, showing that carob extracts can improve endothelial function and reduce oxidative stress, thereby lowering blood pressure. Preliminary human studies suggest similar benefits, with carob fiber demonstrating a potential in reducing both blood pressure and cholesterol levels. However, further research involving larger populations is necessary to confirm these findings and establish clinical applications [101].
Hepatoprotective role
Recognized for its hepatoprotective potential, C. siliqua has emerged as a promising natural remedy for liver disorders such as hepatic steatosis, fibrosis, and cirrhosis. These beneficial effects are primarily attributed to its strong antioxidant, anti-inflammatory, and anti-fibrotic activities, driven by its high polyphenol content, particularly catechins, quercetin, and gallic acid [101].
Antioxidant compounds present in C. siliqua neutralize free radicals, reducing oxidative stress a critical factor in liver damage associated with conditions like non-alcoholic fatty liver disease and toxin-induced hepatotoxicity. Additionally, carob enhances the activity of endogenous liver antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase, thereby strengthening the liver’s defense mechanisms against reactive oxygen species [37].
Protective effects against hepatotoxins, including heavy metals and acetaminophen, have been associated with carob consumption. Its capacity to chelate heavy metals limits their accumulation in liver tissue, while its antioxidant properties help counteract oxidative damage induced by drugs such as acetaminophen, thereby enhancing liver resilience and supporting detoxification processes [102].
Anti-fibrotic properties make an important contribution to the hepatoprotective potential of carob. By inhibiting the activation of hepatic stellate cells, which are primarily responsible for collagen production during fibrosis, carob helps reduce excessive collagen deposition in liver tissue, thereby limiting fibrosis and preserving liver function. Animal studies further suggest that it promotes liver regeneration by stimulating hepatocyte proliferation, facilitating recovery from liver injury, and maintaining liver function in chronic liver diseases [103].
Recent investigations have examined the phytochemical profile, biological activities, and safety aspects of C. siliqua, providing valuable insights into its potential hepatoprotective properties. Dahmani [19] conducted a comprehensive review of ethnomedicinal applications, phytochemical composition, and pharmacological activities exhibited by C. siliqua. Their synthesis highlighted the broad spectrum of biological effects associated with carob, including antioxidant, antimicrobial, antihyperglycemic, anti-inflammatory, and anti-obesity properties, largely attributed to its polyphenol-rich content. Nonetheless, the absence of standardized methodologies and clinical validation across the reviewed studies limits the broader applicability of these findings.
Complementary experimental research by Amine et al. [84] evaluated the antioxidant, antimicrobial, cytotoxic, and genotoxic potential of Moroccan carob leaf ethanolic extract. The extract demonstrated high polyphenol and flavonoid content, potent antioxidant and antibacterial activity, and a moderate antifungal effect. Moreover, dose-dependent cytotoxicity against breast cancer cell lines and genotoxic effects at higher concentrations were reported. However, the lack of in vivo validation and clinical assessment restricts the translation of these results into therapeutic applications.
Computational analyses conducted in the same study assessed the pharmacokinetic and physicochemical properties of major bioactive compounds in carob leaves. The findings revealed that most compounds possessed favorable absorption and bioavailability profiles, with no predicted interactions with CYP450 enzymes. Nonetheless, certain compounds exhibited low membrane permeability and limited ability to cross the blood–brain barrier. These predictions, however, remain unconfirmed in experimental settings.
Furthermore, computational toxicity predictions indicated that while most bioactive compounds present low acute toxicity, some may exhibit hepatotoxic, mutagenic, or carcinogenic effects at high concentrations. These results, derived exclusively from in silico models, require empirical validation through in vivo and clinical studies.
Pharmacological limitations of C. siliqua
While extensive research has investigated the pharmacological properties of C. siliqua, several methodological limitations continue to hinder the application of these findings in clinical settings. A critical evaluation of the available studies highlights major challenges related to small sample sizes, variability in bioavailability, the predominance of in vitro and animal models, and the absence of standardized experimental protocols.
One of the primary limitations in pharmacological research on carob is the small sample sizes used in most studies. Many investigations rely on limited experimental groups, particularly in in vivo models, which raises concerns about the statistical power and reproducibility of the reported effects. The absence of large-scale, well-controlled clinical trials greatly restricts the ability to draw definitive conclusions regarding the efficacy and safety of carob-derived bioactive compounds in human populations.
Another critical issue is the variability in bioavailability and metabolism of key bioactive compounds. Polyphenols, flavonoids, and tannins, which are largely responsible for the pharmacological properties of carob, exhibit low solubility, rapid metabolism, and limited absorption in the gastrointestinal tract. Despite numerous in vitro studies demonstrating antioxidant, anti-inflammatory, antidiabetic, and antimicrobial effects, the extent to which these compounds exert similar effects in vivo remains unclear due to poor systemic bioavailability. The influence of gut microbiota on polyphenol metabolism further complicates pharmacokinetic predictions, as individual differences in microbiome composition can lead to substantial inter-individual variations in metabolite formation and physiological response.
The over-reliance on in vitro and animal models is another limitation that restricts the clinical relevance of current findings. While these models provide valuable insights into mechanisms of action, they do not fully replicate human physiological conditions. A substantial number of pharmacological claims are derived from cell culture studies conducted at concentrations of bioactive compounds that do not reflect physiological relevance, leading to effects that may not be achievable through dietary intake or therapeutic use. Similarly, rodent models, although widely employed in pharmacology, may not accurately reflect human metabolic and immune responses. The scarcity of randomized controlled trials (RCTs) in human subjects is a major gap that needs to be addressed to substantiate the therapeutic potential of C. siliqua in clinical settings.
Another concern is the lack of standardized methodologies in pharmacological research on carob. Studies differ significantly in extraction techniques, solvent compositions, dosage formulations, and administration routes, leading to discrepancies in reported bioactivity. Ethanol, methanol, and aqueous extracts yield different polyphenolic profiles, which may explain inconsistencies in pharmacological findings. Furthermore, dose-response relationships remain poorly characterized, with some studies reporting beneficial effects at relatively low concentrations, while others require significantly higher doses to observe similar outcomes. This lack of standardization hinders the comparability of results across studies and limits the potential for establishing universally accepted therapeutic applications.
To advance pharmacological research on C. siliqua, future studies should focus on large-scale human clinical trials, comprehensive pharmacokinetic analyses, and the development of bioavailability-enhanced formulations. Nanotechnology-based delivery systems, encapsulation techniques, and formulation with lipid carriers could improve the absorption and stability of carob-derived bioactive compounds. Additionally, integrating omics technologies, such as metabolomics and transcriptomics, could provide deeper insights into the molecular mechanisms underlying its pharmacological effects. Standardizing experimental protocols and harmonizing methodologies across studies would also facilitate a more accurate evaluation of the therapeutic potential of C. siliqua.
Toxicology of C. siliqua
Recognized for its safety profile, this plant has been widely used in both food and traditional medicine without reports of adverse effects. The European Food Safety Authority has classified it as GRAS, with no evidence of toxicity even at high doses. Moreover, clinical studies have confirmed that its consumption is not associated with carcinogenic, teratogenic, or mutagenic risks [104].
Although phytochemicals found in C. siliqua such as polyphenols, tannins, and dietary fiber offer numerous health benefits, excessive intake may cause mild digestive issues. High tannin levels can interfere with nutrient absorption and potentially lead to digestive discomfort, though this occurs only at very high doses. Similarly, the substantial fiber content of C. siliqua may exert a mild laxative effect, occasionally causing bloating or diarrhea when consumed in excessive amounts. Despite these minor concerns, carob is hypoallergenic, however, individuals with legume allergies may be susceptible to cross-reactivity. Processing techniques, particularly roasting, have been shown to lower the allergenic potential of carob products [105].
For sensitive populations, including pregnant and breastfeeding women, infants, and individuals with chronic conditions, carob is considered safe when consumed in moderate amounts. It is commonly used in infant formulas as a thickening agent without adverse effects, although careful monitoring is recommended to avoid potential nutrient absorption issues [103].
Comparative analysis of Moroccan and Mediterranean C. siliqua varieties
Several investigations have reported elevated levels of gallic acid, catechins, and proanthocyanidins in Moroccan carob compared to varieties from other Mediterranean regions. These compositional differences may contribute to enhanced antioxidant and anti-inflammatory effects, which align with the traditional medicinal use of Moroccan carob in addressing gastrointestinal disorders, metabolic imbalances, and inflammatory conditions. Conversely, carob populations from Spain, Italy, and Greece tend to exhibit higher sugar content and improved nutritional attributes, making them particularly suitable for food-based applications. This contrast in bioactive compound profiles and nutritional value reflects the impact of regional environmental factors on the therapeutic potential of C. siliqua.
Pharmacological studies have also reported notable variations in antimicrobial and antidiabetic activities among carob varieties. Moroccan carob extracts have demonstrated pronounced antibacterial effects against Gram-negative bacteria, whereas Mediterranean varieties have been associated with stronger antifungal activity possibly reflecting region-specific adaptations in secondary metabolite production. Additionally, antidiabetic assays indicate that polyphenols from Moroccan carob may exert greater inhibitory effects on α-glucosidase and α-amylase, enzymes central to carbohydrate metabolism, while Mediterranean varieties appear to offer more pronounced lipid-lowering properties. These observations emphasize the importance of conducting comprehensive comparative studies, integrating metabolomic profiling and pharmacokinetic evaluations to clarify the functional distinctions between Moroccan and Mediterranean C. siliqua.
Meta-analysis of pharmacological activities
To provide a clearer overview of the pharmacological potential of C. siliqua, a quantitative review of published studies was carried out to examine patterns in reported bioactivities. Analysis of the compiled data shows that antioxidant activity remains the most frequently investigated property, with 63% of studies reporting strong radical scavenging effects attributed to polyphenol-rich extracts. Antidiabetic activity, primarily evaluated through inhibition of α-glucosidase and α-amylase, was documented in 47% of studies; however, uncertainties persist regarding effective dosage ranges and bioavailability outcomes. Antimicrobial properties were reported in 39% of studies, though there was wide variation in the types of pathogens tested and the extraction solvents employed. Anti-inflammatory effects were assessed in 31% of studies, predominantly in in vitro macrophage models.
This quantitative assessment also draws attention to the limited number of human clinical studies, which accounted for only 13% of the reviewed literature. This gap highlights the need for future research to focus more directly on human trials, particularly those evaluating the bioavailability, pharmacokinetics, and long-term safety of carob-derived bioactive compounds. Furthermore, methodological diversity especially in extraction protocols and bioassay conditions has contributed to variability in reported outcomes. Addressing these methodological differences through standardized research frameworks would facilitate a more reliable assessment of the pharmacological potential of C. siliqua.
Limitations in the study of C. siliqua
Pharmacological investigations reveal methodological limitations, particularly in the evaluation of dose-response relationships, mechanistic pathways, and pharmacokinetic behavior. Many studies highlight strong in vitro antioxidant, antimicrobial, and anti-inflammatory effects, yet the extent to which these findings apply to in vivo models and human health remains underexplored. Moreover, the bioavailability and metabolism of key bioactive compounds, especially polyphenols, are rarely investigated, despite their known instability and limited systemic persistence. Without pharmacokinetic data, it is unclear whether the concentrations required to achieve therapeutic effects can be obtained through dietary intake or pharmaceutical formulations.
The limited number of clinical trials represents another major research gap. While numerous in vitro and in vivo studies support the pharmacological potential of carob, only a small proportion (approximately 13%) of investigations have advanced to clinical evaluation. The lack of RCTs assessing the efficacy of C. siliqua extracts in metabolic, gastrointestinal, and inflammatory conditions restricts their clinical relevance and regulatory acceptance. Existing trials often suffer from methodological weaknesses, including small sample sizes, short intervention periods, and lack of proper control groups, limiting the generalizability and statistical strength of the findings. Large-scale, multi-center clinical trials are needed to validate therapeutic claims and establish standardized dosage recommendations.
Another challenge stems from methodological diversity in extraction techniques and solvent systems, which affects the reproducibility of pharmacological results. Studies employ various solvents (ethanol, methanol, water, and acetone) and extraction conditions, leading to considerable variability in polyphenol yield and bioactivity. Comparative analyses often fail to account for these differences, making it difficult to determine whether observed variations arise from plant characteristics or methodological factors. Establishing standardized extraction protocols and reporting guidelines, aligned with pharmacopoeial standards, would improve the reliability of pharmacological assessments.
Ethnomedicinal data on carob also lack systematic documentation and correlation with pharmacological evidence. Although traditional uses of carob in Moroccan medicine are frequently reported, few studies have linked these practices to specific bioactive compounds or demonstrated underlying mechanisms. The absence of integrative ethnopharmacological approaches limits the ability to develop evidence-based therapeutic applications grounded in traditional knowledge. In addition, the industrial and environmental dimensions of Moroccan C. siliqua remain underexplored. Although carob is recognized as a drought-tolerant and environmentally sustainable crop, few studies have assessed its long-term ecological benefits, carbon sequestration potential, or contribution to sustainable agroforestry systems. Economic aspects related to large-scale carob production, market dynamics, and consumer acceptance are also poorly addressed. Future investigations should integrate ecological, economic, and industrial perspectives to fully assess the role of carob in sustainable agriculture and bioeconomy development.
CONCLUSION
The Moroccan carob emerges as a multifaceted plant with exceptional potential across nutritional and pharmacological domains. Its rich phytochemical profile encompassing polyphenols, flavonoids, tannins, and dietary fibers forms the basis of its diverse health-promoting properties, including antioxidant, anti-inflammatory, antidiabetic, and antimicrobial activities. Beyond its established role in traditional medicine, Moroccan carob offers significant promise for modern applications, such as functional foods, nutraceuticals, and sustainable industrial products.
This review highlights the distinctive characteristics of Moroccan carob, underscoring the influence of regional environmental factors and genetic diversity on its morphological and biochemical properties. Moreover, it emphasizes the importance of integrating traditional knowledge with contemporary scientific approaches to fully exploit its therapeutic and economic potential. Despite its evident value, several gaps in research remain, particularly concerning its clinical validation, optimization of cultivation practices, and scalability for industrial use.
Future studies should prioritize addressing these gaps by focusing on advanced molecular analyses, clinical trials, and sustainable agro-industrial models to enhance the global utilization of Moroccan carob. By bridging traditional practices with cutting-edge scientific innovation, Moroccan carob has the potential to become a cornerstone of sustainable development and a model for promoting biodiversity in the Mediterranean and beyond.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
AUTHORS’ DECLARATION
The authors declare that the content of this article represents original work. They accept full responsibility for any claims or disputes arising from the material presented within this publication.
REFERENCES
1. Thomas PA, Garcia-Martí X, Mukassabi TA, Tous J. International biological flora: Ceratonia siliqua. J Ecol. 2024;112(8):1885−922. CrossRef
2. Martins-Loução MA, Correia PJ, Romano A. Carob: a Mediterranean resource for the future. Plants. 2024;13:1188. CrossRef
3. FAO. FAOSTAT crops and livestock products data. Rome, Italy: FAO; 2021. Available from: https://www.fao.org/faostat/en/#data/QCL
4. Kassout J, Hmimsa Y, Fatehi SE, Kadaoui K, Houssni M, Chakkour S, et al. Aridity gradients shape intraspecific variability of morphological traits in native Ceratonia siliqua L. of Morocco. Plants. 2023;12:3447. CrossRef
5. El Batal H, Hasib A, Dehbi F, Zaki N, Ouatmane A, Boulli A. Assessment of nutritional composition of carob pulp (Ceratonia siliqua L.) collected from various locations in Morocco. J Mater Environ Sci. 2016;7(9):3278−85.
6. Mouniane Y, Chriqui A, El-Khadir I, Hbyaj K, Rochd A, Aitouhanni I, et al. Assessing morphological trait variability in Moroccan carob (Ceratonia siliqua L.) ecotypes for adaptive breeding in response to climate change and sustainable food security. BIO Web Conf. 2024;109:01040. CrossRef
7. Shahrajabian MH, Sun W. Carob (Ceratonia siliqua L.), pharmacological and phytochemical activities of neglected legume of the Mediterranean basin, as functional food. rev recent clin trials. 2024;19(2):127−42. CrossRef
8. Tzatzani TT, Ouzounidou G. Carob as an agrifood chain product of cultural, agricultural and economic importance in the Mediterranean region. J Innov Econ Manag. 2023;42(3):127−47.
9. Asma B, Meriem H, Rafika L. Ethnobotanical knowledge and socio-economic importance of Ceratonia siliqua L. (Fabaceae) in the north of Setif (north-east of Algeria). Acta Ecol Sin. 2023;43(4):712−20. CrossRef
10. Rtibi K, Selmi S, Grami D, Amri M, Eto B, El-Benna J. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: a review. Biomed. Pharmacother. 2017;93:522−8. CrossRef
11. Karababa E, Coskuner Y. Physical properties of carob bean (Ceratonia siliqua L.): an industrial gum yielding crop. Ind Crops Prod. 2013;42:440−6. CrossRef
12. Palaiogianni A, Stylianou M, Sarris D, Agapiou A. Carob: agro-industrial waste and potential uses in the circular economy. In: Mediterranean fruits bio-wastes: Chemistry, functionality and technological applications. Cham, Switzerland: Springer International Publishing; 2022. pp. 765−97.
13. Ajjoun M, Fakchich J, Elachouri M. First insight on ethnobotanical appraisal of plants used traditionally as medicine by berber community (amazigh-speaking), living in Driouch province (north-eastern Morocco). Ethnobot Res Appl. 2021;22:1−71. CrossRef
14. Laaraj S, Hussain A, Mouhaddach A, Noutfia Y, Gorsi FI, Yaqub S, et al. Nutritional benefits and antihyperglycemic potential of carob fruit (Ceratonia siliqua L.): an overview. Ecol Eng Environ Technol. 2024;25:124–32. CrossRef
15. Menconi ME, Abbate R, Grohmann D. Exploring the uses and landscape value of Ceratonia siliqua L.: a systematic review. For Trees Livelihoods. 2024;33(3):1−17. CrossRef
16. Serradj AMK, Salima RAIS, Adnane Y, Mahdad MY, Mediouni R, Bechir Suheil Gaouar S. Morpho-geometric identification of leaflets from male, female, and hermaphrodite populations of the carob tree (Ceratonia siliqua L.) in Algeria. Genet Biodivers J. 2024;8(2):57−75.
17. Laaraj S, Salmaoui S, Addi M, El-Rhouttais C, Tikent A, Elbouzidi A, et al. Carob (Ceratonia siliqua L.) seed constituents: a comprehensive review of composition, chemical profile, and diverse applications. J Food Qual. 2023;2023(1):3438179. CrossRef
18. Gómez-Martínez C, González-Estévez MÁ, deCastro-Arrazola I, Unglaub P, Lázaro A. Landscape conservation and orchard management influence carob tree yield through changes in pollinator communities. PLoS One. 2025;20(2):e0307357. CrossRef
19. Dahmani W, Elaouni N, Abousalim A, Akissi ZLE, Legssyer A, Ziyyat A, et al. Exploring carob (Ceratonia siliqua L.): a comprehensive assessment of its characteristics, ethnomedicinal uses, phytochemical aspects, and pharmacological activities. Plants. 2023;12(18):3303. CrossRef
20. Prajapati VD, Maheriya PM, Roy SD. Locust bean gum-derived hydrogels. In: Plant and algal hydrogels for drug delivery and regenerative medicine. Sawston, Cambridge: Woodhead Publishing; 2021. pp. 217−60. CrossRef
21. Dadach M, Bhatt A, Gairola S, Elnaggar A, El-Keblawy A. Germination responses of carob (Ceratonia siliqua L.) seeds to salinity and water stresses: implications for the restoration of Mediterranean marginal lands. Botany. 2024; 103:1–13. CrossRef
22. Elfazazi K, Jbilou M, Assaidi A, Benbati M, Harrak H. Morphological and biochemical variability of Moroccan carob (Ceratonia siliqua L.) produced in Beni mellal region. Int J Pure Appl Biosci. 2017;5(4):14−21. CrossRef
23. El Kahkahi R, Zouhair R, Diouri M, Ait Chitt M, Errakhi R. Morphological and biochemical characterization of Morocco carob tree (Ceratonia siliqua L.). Int J Biol Med Res. 2015;6(2):4946−52.
24. El Kahkahi R, Zouhair R, Ait Chitt M, Errakhi R. Morocco carob (Ceratonia siliqua L.) populations: morphological variability of pods and kernel. Int J Pure Appl Biosci. 2014;2(4):38−47.
25. Sidina MM, El Hansali M, Wahid N, Ouatmane A, Boulli A, Haddioui A. Fruit and seed diversity of domesticated carob (Ceratonia siliqua L.) in Morocco. Sci Hortic. 2009;123(1):110−6. CrossRef
26. Boublenza I, Ghezlaoui S, Mahdad M, Vasaï F, Chemat F. Algerian carob (Ceratonia siliqua L.) populations: morphological and chemical variability of their fruits and seeds. Sci Hortic. 2019;256:108537. CrossRef
27. Smaili O, Chebouti-Meziou N, Scollo F, La Malfa S, Gentile A, Distefano G. Evaluation of the morphological and physicochemical diversity of carob (Ceratonia siliqua, Fabaceae) germplasm from Algeria. Forests. 2024;15:1423.
28. Yatim M, Kahkahi RE, Sbata IE, Askri TE, ElOirdi S, Lakhlifi T, et al. Effects of pre-sowing treatments and abiotic stress on the germination of Ceratonia siliqua seeds of four Moroccan biomes. Annu Res Rev Biol. 2020;35(12):11−31. CrossRef
29. Toumi S, Acem K, Abdelhamid D, Khedim K, Lagraa I, Loumani Z. Morphobiometric characterisation of carob tree pods cultivated in Algeria and evaluation of physicochemical, nutritional, and sensory properties of their powders. Proc Latv Acad Sci Sect B Nat Exact Appl Sci. 2023;78(2):153−63. CrossRef
30. Hussain A, Arif MR, Ahmed A, Laaraj S, Firdous N, Ali MQ, et al. Evaluation of carob tree (Ceratonia siliqua L.) pods, through three different drying techniques, and ultrasonic-assisted extraction, for presence of bioactives. S Afr J Bot. 2024;173:388−96. CrossRef
31. Mayordomo-Maya S, Hermosilla-Pla J. Heritage evaluation of the carob tree MTAS in the territory of Valencia: analysis and social perception of the ecosystem services and values from cultivating it. Land. 2024;13(7):922. CrossRef
32. Ertas TB. Ormanciligimizda keçiboynuzu (Ceratonia siliqua L.). Theor Appl For. 2023;3(1):18–21. CrossRef
33. Tous J, Esbenshade H. Comparison of Spanish and Australian carob orchards. In: XXIX international horticultural congress on horticulture: sustaining lives, livelihoods and landscapes. Brisbane, Australia. 2014. pp. 171−8. CrossRef
34. Correia PJ, Guerreiro JF, Pestana M, Martins-Loução MA. Management of carob tree orchards in Mediterranean ecosystems: strategies for a carbon economy implementation. Agrofor Syst. 2017;91:295−306. CrossRef
35. Ayad R, Ayad R, Boudjerda S, Bessibes S, Idoui T. The effects of boiling and freezing processing methods on the quality characteristics, bioactive compound content, and antioxidant capacity of Jijel region carob pods, Ceratonia siliqua L. Mor J Chem. 2023;11(4):11−14. CrossRef
36. Torche S, Beroual K, Zaouani M, Boujellaba S. Ethnobotanical study of medicinal plants used for traditional diarrhea treatment in north-east Algeria. Res J Pharm Technol. 2024;17(2):811−9. CrossRef
37. Raškovic A, Martic N, Tomas A, Andrejic-Višnjic B, Bosanac M, Atanaskovic M, et al. Carob extract (Ceratonia siliqua L.): Effects on dyslipidemia and obesity in a high-fat diet-fed rat model. Pharmaceutics. 2023;15(11):2611. CrossRef
38. Djellal S, Dahmoune F, Aoun O, Remini H, Belbahi A, Dairi S, et al. Optimization of ultrasound-assisted extraction of galactomannan from carob seeds (Ceratonia siliqua L.) and evaluation of their functional properties and in vitro anti-inflammatory activity. Sep Sci Technol. 2024;59(1):41−58. CrossRef
39. Moumou M, Mokhtari I, Milenkovic D, Amrani S, Harnafi H. Carob (Ceratonia siliqua L.): a comprehensive review on traditional uses, chemical composition, pharmacological effects and toxicology (2002-2022). J Biol Act Prod Nat. 2023;13(3):179−223. CrossRef
40. Ydjedd S, Bouriche S, López-Nicolás R, Sánchez-Moya T, Frontela-Saseta C, Ros-Berruezo G, et al. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J Agric Food Chem. 2017;65(4):827–35.
41. Mrabti HN, Jaradat N, Kachmar MR, Ed-Dra A, Ouahbi A, Cherrah Y, et al. Integrative herbal treatments of diabetes in Beni Mellal region of Morocco. J Integr Med. 2019;17:93−9. CrossRef
42. Orch H, Zidane L, Douira A. Ethnobotanical study of the plants used in the treatment of digestive diseases by the riverine population of the forest of Izarène. Int J Recent Sci Res. 2017;8:15213−20.
43. AbouZid SF, Mohamed AA. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J Ethnobiol Ethnomed. 2011;7:18. CrossRef
44. El-Zeftawy M, Ghareeb D. Pharmacological bioactivity of Ceratonia siliqua pulp extract: In vitro screening and molecular docking analysis, implication of Keap-1/Nrf2/NF-kB pathway. Sci Rep. 2023;13:12209. CrossRef
45. Benito -Vázquez I, Garrido-Romero M, Hontoria-Caballo G, García-García C, Díez-Municio M, Moreno FJ. Carob (Ceratonia siliqua) flour as source of bioactive compounds: production, characterization and nutraceutical value. Foods. 2024;13(19):3024. CrossRef
46. Pelegrin -Valls J, Álvarez-Rodríguez J, Martín-Alonso MJ, Aquilué B, Serrano-Pérez B. Impact of carob (Ceratonia siliqua L.) pulp inclusion and warm season on gastrointestinal morphological parameters, immune-redox defences, and coccidiosis in concentrate-fed light lambs. Res Vet Sci. 2023;163:104969. CrossRef
47. Fidan H, Stankov S, Petkova N, Petkova Z, Iliev A, Stoyanova M, et al. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J Food Sci Technol. 2020;57:2404−13. CrossRef
48. Alqudah A, Qnais EY, Wedyan MA, Oqal M, Alqudah M, AbuDalo R, et al. Ceratonia siliqua leaves ethanol extracts exert anti-nociceptive and anti-inflammatory effects. Heliyon. 2022;8(8):e10400. CrossRef
49. Goulas V, Hadjisolomou A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion. LWT—Food Sci Technol. 2019;101:269−75. CrossRef
50. Nasrallah K, Khaled S, El Khatib S. Nutritional, biochemical, and health properties of locust beans and its applications in the food industry: a review. J Food Sci Technol. 2024;61:621−30. CrossRef
51. Ibrahim IMA, Aal HZA, Saleh HM. Utilization of hibiscus, tamarind, and carob in production of low-calorie healthy soft drinks. Eur J Nutr Food Saf. 2024;16(10):160−73. CrossRef
52. Testa M, Malandrino O, Santini C, Supino S. Nutraceutical and functional value of carob-based products: the LBG Sicilia Srl case study. In: Case studies on the business of nutraceuticals, functional and super foods. Amsterdam, Netherlands: Woodhead Publishing; 2023. pp. 107−20. CrossRef
53. Loullis A, Pinakoulaki E. Carob as cocoa substitute: a review on composition, health benefits, and food applications. Eur Food Res Technol. 2018;244:959−77. CrossRef
54. Khelouf I, Jabri Karoui I, Abderrabba M. Chemical composition, in vitro antioxidant and antimicrobial activities of carob pulp (Ceratonia siliqua L.) from Tunisia. Chem Pap. 2023;77(10):6125−34. CrossRef
55. Gregoriou G, Neophytou CM, Vasincu A, Gregoriou Y, Hadjipakkou H, Pinakoulaki E. Anti-cancer activity and phenolic content of extracts derived from cypriot carob (Ceratonia siliqua l.) pods using different solvents. Molecules. 2021;26:5017. CrossRef
56. Hasib A, EL Batal H. Optimization of production of carob pulp syrup from different populations of Moroccan carob (Ceratonia siliqua L.). Int J Emerg Technol Adv Eng. 2014;4(3):855−63.
57. Basharat Z, Afzaal M, Saeed F, Islam F, Hussain M, Ikram A, et al. Nutritional and functional profile of carob bean (Ceratonia siliqua): a compre hensive review. Int J Food Prop. 2023;26(1):389−413. CrossRef
58. Djebari S, Wrona M, Nerín C, Djaoudene O, Guemouni S, Boudria A, et al. Phenolic compounds profile of macerates of different edible parts of carob tree (Ceratonia siliqua l.) using UPLC-ESI-Q-TOF-MSE: phytochemical screening and biological activities. Fitoterapia. 2024;172:105696. CrossRef
59. Dammak A, Chtourou F, Luca SV, Skalicka-Wozniak K, Bouaziz M. Insights into the phytochemical composition and antioxidant potential of the tunisian Ceratonia siliqua L. Fitoterapia. 2024;175:105919. CrossRef
60. Ikram A, Khalid W, Zafar W, Ali A, Afzal MF, Aziz A. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci Nutr. 2023;11(7):3641−54. CrossRef
61. Amar YMB, Potortì AG, Albergamo A, Litrenta F, Rando R, Mouad LB, et al. Study of the lipid fraction of Moroccan and Italian carobs (Ceratonia siliqua L.). Eur J Lipid Sci Technol. 2024;126(9):2400036. CrossRef
62. El Batal H, Hasib A, Ouatmane A, Dehbi F, Jaouad A, Boulli A. Sugar composition and yield of syrup production from the pulp of Moroccan carob pods (Ceratonia siliqua L.). Arab J Chem. 2011;9(2):955−9. CrossRef
63. Youssef MKE, El-Manfaloty MM, Ali HM. Assessment of proximate chemical composition, nutritional status, fatty acid composition, and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Health. 2013;3(6):304−8.
64. Ouis N, Hariri A. Phytochemical analysis and antioxidant activity of the flavonoid extracts from pods of Ceratonia siliqua L. J Pharm Pharmaceutics. 2017;4(2):159−65.
65. Mahtout R, Ortiz-Martínez VM, Salar-García MJ, Gracia I, Hernández-Fernández FJ, Pérez de los Ríos A, et al. Algerian carob tree products: a comprehensive valorization analysis and future prospects. Sustainability. 2018;10:90. CrossRef
66. Ettaleb I, Aoujdad J, Ouajdi M, Arba M, El Antry S, Satrani B, et al. Biochemical parameters of carob (Ceratonia siliqua L.) pods and seeds as affected by different geographical sites in Morocco. Food Hum. 2024;3:100391. CrossRef
67. Shawakfeh KQ, Ereifej KI. Pod characteristics of two Ceratonia siliqua L. varieties from Jordan. Ital J Food Sci. 2005;17(2).
68. Gubbuk H, Kafkas E, Guven D, Gunes E. Physical and phytochemical profile of wild and domesticated carob (Ceratonia siliqua L.) genotypes. Span J Agric Res. 2010;8(4):1129−36. CrossRef
69. Khlifa M, Bahloul A, Kitane S. Determination of chemical composition of carob pod (Ceratonia siliqua L.) and its morphological study. J Mater Environ Sci. 2013;4(3):348−53.
70. Rtibi K, Jabri MA, Selmi S, Sebai H, Amri M, Marzouki L, et al. Preventive effect of carob (Ceratonia siliqua L.) in a dextran sulfate sodium-induced ulcerative colitis in rat. RSC Adv. 2016;6:19992−20000. CrossRef
71. Haddarah A, Ismail A, Bassal A, Hamieh T, Ioannou I, Ghoul M. Morphological and chemical variability of Lebanese carob varieties. Eur Sci J. 2013;9(18):353−69.
72. Matthaus B, Özcan MM. Lipid evaluation of cultivated and wild carob (Ceratonia siliqua L.) seed oil growing in Turkey. Sci Hortic. 2011;130:181−4. CrossRef
73. Laaraj S, Choubbane H, Elrherabi A, Tikent A, Farihi A, Laaroussi M. Influence of harvesting stage on phytochemical composition, antioxidant, and antidiabetic activity of immature Ceratonia siliqua L. pulp from Béni Mellal-Khenifra region, Morocco: in silico, in vitro, and in vivo approaches. Curr Issues Mol Biol. 2024;46:10991−1020. CrossRef
74. Boutasknit A, Ait-El-Mokhtar M, Fassih B, Ben-Laouane R, Wahbi S, Meddich A. Effect of arbuscular mycorrhizal fungi and rock phosphate on growth, physiology, and biochemistry of carob under water stress and after rehydration in vermicompost-amended soil. Metabolites. 2024;14:202. CrossRef
75. Amrani A, Bouakline H, Elkabous M, Brahmi M, Karzazi Y, El Bachiri A, et al. Ceratonia siliqua L seeds extract: experimental analysis and simulation study. Mater Today Proc. 2023;72:3705−11. CrossRef
76. Darwish WS, Khadr AES, Kamel MAEN, Abd Eldaim MA, El Sayed IET, Abdel-Bary HM, et al. Phytochemical characterization and evaluation of biological activities of Egyptian carob pods (Ceratonia siliqua L.) aqueous extract: in vitro study. Plants. 2021;10:2626. CrossRef
77. Ünal E, Sulukan E, Senol O, Baran A, Nadaroglu H, Kankaynar M, et al. Antioxidant/protective effects of carob pod (Ceratonia siliqua L.) water extract against deltamethrin-induced oxidative stress/toxicity in zebrafish larvae. Comp Biochem Physiol C Toxicol Pharmacol. 2023;267:109584. CrossRef
78. Aicha M, Maameri Z, Sihem H, Nadia Z, Amira N, Abdelmalik B. Phytochemical investigation of Algerian Ceratonia siliqua L. Leaves extract, evaluation of antioxidants, and analgesic effects. Egypt J Chem. 2023;66(3):519−28. CrossRef
79. De Luca M, Tuberoso CIG, Pons R, García MT, Morán MdC, Martelli G, et al. Ceratonia siliqua L. pod extract: from phytochemical characterization to liposomal formulation and evaluation of behaviour in cells. Antioxidants. 2023;12:1209. CrossRef
80. Siano F, Mamone G, Vasca E, Puppo MC, Picariello G. Pasta fortified with c-glycosides-rich carob (Ceratonia siliqua L.) seed germ flour: inhibitory activity against carbohydrate digesting enzymes. Food Res Int. 2023;170:112962. CrossRef
81. Moumou M, Mokhtari I, Tayebi A, Milenkovic D, Amrani S, Harnafi H. Immature carob pods extract and its fractions prevent lipid metabolism disorders and lipoprotein-rich plasma oxidation in mice: A phytochemical and pharmacological study. J Ethnopharmacol. 2024;322:117557. CrossRef
82. Ioannou GD, Savva IK, Christou A, Stavrou IJ, Kapnissi-Christodoulou CP. Phenolic profile, antioxidant activity, and chemometric classification of carob pulp and products. Molecules. 2023;28:2269. CrossRef
83. Antón-Domínguez BI, López-Moral A, Romero-Salguero FJ, Trapero A, Trapero C, Agustí-Brisach C. Bioprotection of olive trees against Verticillium wilt by pomegranate and carob extracts. Plant Dis. 2024;108(4):1073−82. CrossRef
84. Elbouzidi A, Taibi M, Ouassou H, Ouahhoud S, Ou-Yahia D, Loukili EH, et al. Exploring the multi-faceted potential of carob (Ceratonia siliqua var. Rahma) leaves from Morocco: a comprehensive analysis of polyphenols profile, antimicrobial activity, cytotoxicity against breast cancer cell lines, and genotoxicity. Pharmaceuticals. 2023;16:840. CrossRef
85. Meziani S, Oomah BD, Zaidi F, Simon-Levert A, Bertrand C, Zaidi-Yahiaoui R. Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum. Microb Pathog. 2015;78:95−102. CrossRef
86. Karmous I, Taheur FB, Zuverza-Mena N, Jebahi S, Vaidya S, Tlahig S, et al. Phytosynthesis of zinc oxide nanoparticles using Ceratonia siliqua L. and evidence of antimicrobial activity. Plants. 2022;11:3079. CrossRef
87. Drioiche A, Ailli A, Remok F, Saidi S, Gourich AA, Asbabou A, et al. Analysis of the chemical composition and evaluation of the antioxidant, antimicrobial, anticoagulant, and antidiabetic properties of Pistacia lentiscus from Boulemane as a natural nutraceutical preservative. Biomedicines. 2023;11:2372. CrossRef
88. Khider M, Seliem KAEH, Ebid WMA. Development of functional synbiotic flavored fermented skim milk drinks supplemented with doum (Hyphaene thebaica L.) and carob (Ceratonia siliqua) fruits powder for nutritional, antimicrobial, and high antioxidant activities. Food Nutr Sci. 2022;13(11):878−905. CrossRef
89. Yahiaoui K, Bouchenak O, Boumaza S, Toubal S, Blizak D, Nouani A, et al. Characterization and assessment of the antimicrobial function of total polyphenol extracts from pulps, leaves, and seeds of two Ceratonia siliqua L. varieties. Algerian J Environ Sci Technol. 2021;7(2):1958−63.
90. Al-Azzawi SMM, Al-Azzawi MMNA. Evaluation of the inhibitory and antioxidant activity of Ceratonia siliqua leaf and seed extract. In: IOP Conf Ser Earth Environ Sci. 2023;1213(1):012063. CrossRef
91. Ali SH, Ibrahim AS, Mohamed NI. Inhibitory activity of aqueous extract of pomegranate peel and carob against bacteria isolated from diarrheal patient in Kirkuk Hospital. Plant Arch. 2020;20(1):2467−70.
92. Kahkahi ER, Moustaine M, Zouhair R. Botanical, chemical, and pharmacological characteristics of carob tree (Ceratonia siliqua L.). Med Discov. 2024;3(6):1168.
93. Elachouri M, Chaachouay N, Elbouzidi A, Taibi M, Idrissi A. Ceratonia siliqua L. Fabaceae. In: Bussmann RW, Elachouri M, Kikvidze Z, editors. Ethnobotany of northern Africa and levant. ethnobotany of mountain regions. Cham, Switzerland: Springer; 2024. CrossRef
94. Ahmed AF, Cevher SC, Peker EGG, Balabanli B, Ebegil M. Synergistic effects of thymoquinone and carob powder versus dexamethasone in the model of asthma in pregnant rats: new insights into their therapeutic effects. Gazi Med J. 2024;35(4):393−400. CrossRef
95. Touiss I, Khaili A, Khouya T, Bekkouch O, Harnafi M, Lahmass I, et al. The synergistic effect of the combination of Ocimum basilicum and Ceratonia siliqua on inflammation and oxidative stress. In: E3S Web Conf. 2024;527:01023. CrossRef
96. Faraag RA, Abd Elhamid OM, Abdelmagid AD, Elsenosi YAE, Said AM. Potential protective role of carob ethanolic extract against diclofenac sodium-induced acute hepatorenal toxicity in male rats: biochemical and histopathological studies. Benha Vet Med J. 2024;46(1):58−62. CrossRef
97. Esposito L, Casolani N, Ruggeri M, Spizzirri UG, Aiello F, Chiodo E, et al. Sensory evaluation and consumers’ acceptance of a low glycemic and gluten-free carob-based bakery product. Foods. 2024;13:2815. CrossRef
98. Gioxari A, Amerikanou C, Nestoridi I, Gourgari E, Pratsinis H, Kalogeropoulos N, et al. Carob: a sustainable opportunity for metabolic health. Foods. 2022;11:2154. CrossRef
99. Martic N, Zahorec J, Stilinovic N, Andrejic-Višnjic B, Pavlic B, Kladar N, et al. Hepatoprotective effect of carob pulp flour (Ceratonia siliqua L.) extract obtained by optimized microwave-assisted extraction. Pharmaceutics. 2022;14:657. CrossRef
100. Hamid I, Janbaz KH, Iqbal R, Akhtar MF, Saleem A, Ali S, et al. Therapeutic potential of Ceratonia siliqua extract for the management of asthma and hypertension. Cell Mol Biol. 2021;67(5):6−15. CrossRef
101. Atta AH, Atta SA, Khattab MS. Ceratonia siliqua pods (carob) methanol extract alleviates doxorubicin-induced nephrotoxicity via antioxidant, anti-inflammatory, and anti-apoptotic pathways in rats. Environ Sci Pollut Res. 2023;30:83421−38. CrossRef
102. Zahorec J, Šoronja-Simovic D, Kocic-Tanackov S, Bulut S, Martic N, Bijelic K. Carob pulp flour extract obtained by a microwave-assisted extraction technique: a prospective antioxidant and antimicrobial agent. Separations. 2023;10:465. CrossRef
103. Abd-Elhak N, Sayed H, Hashem M. Fennel, carob, rosemary extracts as anti-inflammatory activity functions on formalin-induced paw edema in albino rats. Food Technol Res J. 2024;3(1):14−32. CrossRef
104. Younes M, Aquilina G, Castle L, Degen G, Engel KH, Gundert-Remy U. Re-evaluation of locust bean gum (E 410) as a food additive in foods for infants below 16 weeks of age and follow-up of its re-evaluation as a food additive for uses in foods for all population groups. EFSA J. 2023;21(2):e07775. CrossRef
105. Ben Ayache S, Behija Saafi E, Emhemmed F, Flamini G, Achour L, Muller CD. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules. 2020;25(14):3120.