INTRODUCTION
Metabolic-associated fatty liver disease (MAFLD), a redefined term for nonalcoholic fatty liver disease (NAFLD) [1], encompasses a spectrum of liver conditions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH). This condition represents a critical global health challenge, frequently associated with common metabolic disorders such as obesity and metabolic syndrome [2–4]. The pathogenesis of MAFLD is multifactorial, with insulin resistance playing a pivotal role in initiating hepatic steatosis [5,6]. The excess influx of free fatty acids (FFAs) into hepatocytes, combined with heightened de novo lipogenesis and impaired lipid export, leads to the hallmark lipid accumulation characteristic of MAFLD [7,8]. Chronic inflammation and cellular stress are key drivers of MAFLD progression from simple steatosis to NASH, leading to further complications [9].
Excess FFA influx under high-energy conditions inhibits AMP-activated protein kinase (AMPK), a central energy sensor that normally suppresses de novo lipogenesis [10]. The AMPK-activating properties of quercetin have been extensively documented across various cell lines. Lee et al. [11] demonstrated that quercetin activates AMPK in cancer cells, resulting in the suppression of cyclooxygenase-2 and the induction of apoptosis. Similarly, Dhanya et al. [12] reported that quercetin activates AMPK in skeletal muscle cells by increasing the AMP/ATP ratio, thereby enhancing glucose uptake through the AMPK-p38 MAPK signaling pathway. Quercetin shares a mechanism similar to that of the well-known drug metformin, underscoring its potential as a promising compound for managing type 2 diabetes [11,12]. Inactive AMPK fails to phosphorylate and inhibit acetyl-CoA carboxylase (ACC), allowing ACC to catalyze malonyl-CoA production. Elevated malonyl-CoA activates fatty acid synthase (FAS), driving unchecked lipid synthesis while simultaneously suppressing mitochondrial β-oxidation via carnitine palmitoyltransferase 1 inhibition. Concurrently, the PI3K/AKT pathway exacerbates lipid accumulation by enhancing glucose uptake and glycolytic flux, thereby providing acetyl-CoA substrates for FAS-mediated lipogenesis. This dual dysregulation of AMPK inhibition and PI3K/AKT hyperactivation creates a metabolic imbalance that amplifies hepatic lipid deposition, disrupts glucose homeostasis, and accelerates insulin resistance [13,14]. Targeting these pathways, along with reducing inflammation and cellular stress, has emerged as a promising therapeutic approach for MAFLD and related metabolic disorders.
Traditional medicinal plants offer a rich reservoir of potential therapeutic agents for the management of MAFLD and are associated with lower evidence of side effects than conventional pharmaceuticals. Several plant-derived compounds have demonstrated efficacy in mitigating MAFLD symptoms by modulating lipid metabolism pathways [15]. Thai kratom (Mitragyna speciosa), traditionally used for its stimulant and analgesic properties, has recently gained attention for its potential metabolic benefits [16]. Preliminary evidence suggests the anti-lipogenic effect of M. speciosa [17–19]; however, the underlying mechanisms remain unexplored.
This study aimed to elucidate the effects of Thai kratom extracts (red and green vein varieties) and their major alkaloid mitragynine and quercetin on lipid metabolism, insulin signaling, and inflammation in an in vitro HepG2 cell model of MAFLD. Our findings demonstrate that kratom extract and mitragynine attenuate FFA-induced lipid accumulation, potentially through AMPK activation, and downregulation of lipogenic enzymes. Additionally, kratom extract enhanced glycogen synthesis by increasing AKT and GSK3 phosphorylation, reducing the availability of precursors for de novo lipogenesis, and subsequently leading to decreased fat accumulation in HepG2 cells. Furthermore, kratom extract exhibited anti-inflammatory effects by decreasing p38 MAPK phosphorylation and downregulating key inflammatory mediators.
These results provide novel insights into the molecular mechanisms underlying the potential therapeutic effects of Thai kratom on MAFLD and its related metabolic complications. Further research, including in vivo studies, is needed to validate the clinical applications of kratom and its active constituents.
MATERIALS AND METHODS
Thai Kratom extracts
Red and green kratom leaves were obtained from a community participation project in Nam Pu Sub-district, Ban Na San district, Surat Thani province, Thailand, as documented in a previous study [18]. This study documents the traditional use of these kratom varieties. The ground and dried powder of the leaves was extracted using a previously described method [18] with slight modifications: 100 g of each kratom powder (red and green) was soaked in 1 l of 95% ethanol for 24 hours. This step was repeated twice. After removing ethanol by rotary evaporation, crude ethanolic extracts of red and green kratom were obtained. LC-MS/MS analysis was performed to identify the major compounds in the extracts. Analysis of the ground and dried powders of red Thai kratom (RTK) and green Thai kratom (GTK) revealed significant differences in their major compounds. A previous publication from our research group found that GTK exhibited mitragynine levels (63%) that were 2 times higher than those found in RTK (37%), with quercetin content ranging from 10.2 to 17.4 mg/g (EtOH extract) [18].
HepG2 cell culture
The human hepatocellular carcinoma cell line HepG2 (HB-8065) was purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM high-glucose medium (Thermo Fisher, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The cells were maintained at 37°C in a humidified incubator with 5% CO2.
MTS assay
To establish a non-cytotoxic dose range for subsequent experiments, the viability of HepG2 cells following treatment with red or green Thai kratom (M. speciosa) extract and pure mitragynine was assessed using the MTS assay (Promega, Madison, WI, USA). HepG2 cells were seeded at a density of 1 × 104 cells/well in 96-well plates and allowed to adhere overnight. Cells were then treated with varying concentrations of red or green kratom extract (0–200 µg/ml) or mitragynine (0–400 µM) for 24 or 48 hours. The mitragynine dose range was selected based on previous studies reporting IC50 values in HepG2 cells of 42–92.85 µM, with non-cytotoxicity in in HL-7702 cells at concentrations >200 µM [20,21]. Kong et al. [21]. To capture the full dose-response, mitragynine was tested at 0, 6.25, 12.5, 25, 50, 100, 200, and 400 µM. Since mitragynine constitutes ~40% of Thai kratom extracts [22], the highest kratom extract dose (200 µg/ml) corresponds to an approximate mitragynine-equivalent concentration of 80 µg/ml (~200 µM), which exceeds the mitragynine IC50. After 24 and 48 hours of incubation at 37°C and 5% CO2, cell viability was determined using the MTS assay. Twenty microliters of MTS solution were added to each well, followed by incubation for an additional 1 hour at 37°C and 5% CO2. The absorbance of the formazan product was measured at 490 nm using a microplate reader (BioTek, Winooski, VT, USA). Cell viability in the treatment groups was normalized to that of the untreated control group (0 µM), which was designated as 100% viability.
Assessment of the impact of Kratom extracts on FFA-induced lipid accumulation in HepG2
To investigate the effect of the test compounds on lipid accumulation, HepG2 cells (4 × 105 cells/well) were plated onto coverslips 24 hours before co-treatment with 500 µM free fatty acids (FFA, mixture of oleic and palmitic acids, 3:1 ratio) and varying concentrations of green and red kratom crude ethanol extracts (0–200 µg/ml), mitragynine (0–200 µM), and quercetin (50 µM) for 24 hours. This co-treatment aimed to induce a fatty liver phenotype while simultaneously evaluating the effects of the test compounds on lipid accumulation. Lipid accumulation was assessed using Oil Red O (ORO) staining. HepG2 cells were fixed with 4% formaldehyde for 10 minutes at room temperature and rinsed with 60% isopropanol. The cells were then stained with ORO solution for 1 hour. The coverslips were then rinsed with 60% isopropanol, washed with PBS three times, and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Lipid accumulation was quantified from the captured images using the ImageJ software (version 2.14.0/1.54f, National Institutes of Health, Bethesda, MD, USA).
Immunoblotting analysis
Western blot analysis was performed as previously described [23], with minor modifications. Briefly, equal amounts of protein from each sample were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked and incubated with primary antibodies against several target proteins shown in Supplementary Table S1. Following primary antibody incubation, the membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour. Immunoreactive proteins were detected using an enhanced chemiluminescence substrate (Pierce, IL, USA) and visualized using a chemiluminescent imaging system. Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). β-actin was used as an internal loading control for normalization.
RNA isolation and RT-qPCR
Total RNA was extracted from samples using the TRIzol reagent as previously described [23]. cDNA was synthesized from 1 µg of RNA using an iScript cDNA Synthesis Kit (Bio-Rad, CA, USA). To assess the transcript abundance, real-time PCR with SYBR Green was performed on an ABI Prism 7,500 Sequence Detection System (Applied Biosystems, CA, USA). Target gene expression was normalized to 18S rRNA, and relative expression was quantified using the Pfaffl method [24]. Primer sequences are listed in Supplementary Table S2.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM) from three independent replicate experiments (n = 3) for all assays. Statistical analyses were performed using GraphPad Prism version 10 (GraphPad Software, CA, USA). Differences between treatment groups were compared using a one-way analysis of variance (ANOVA), followed by an appropriate post-hoc test. Student’s t-test was used to compare the two groups. Statistical significance was defined as a p-value < 0.05.
RESULTS
Effects of red and green Kratom extracts and mitragynine on HepG2 cell viability
To assess the potential cytotoxicity of the red and green kratom extracts, HepG2 cells were treated with varying concentrations (0–200 µg/ml) of each extract for 24 and 48 hours. Cell viability was determined using the MTS assay. Both extracts exhibited dose-dependent cytotoxicity, significantly reducing HepG2 cell viability at 100 and 200 µg/ml after 48 hours of treatment (Fig. 1A and 1B). To minimize cytotoxic effects in subsequent experiments, lower concentrations and a 24-hour incubation period were used. Mitragynine, the major alkaloid in Thai kratom extracts, was also evaluated for its effect on cell viability. Concentrations below 50 µM did not significantly affect HepG2 cell viability. However, mitragynine exhibited dose-dependent cytotoxicity at higher concentrations after both 24- and 48-hour incubations, with calculated IC50 values of 125.5 µM and 115.2 µM, respectively (Fig. 1). The maximum concentration combined with a 24-hour incubation period was determined to mitigate cytotoxicity in subsequent experiments. 0–200 µg/ml of the extract showed no significant toxicity. Given the IC50 of mitragynine of 125 µM, 50 µM was deemed safe for subsequent experiments.
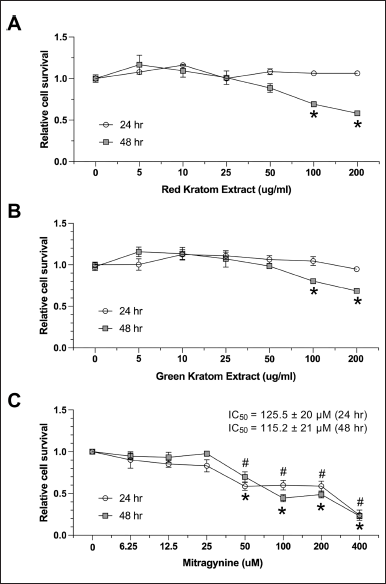 | Figure 1. Cytotoxicity of red and green kratom extracts and mitragynine on HepG2 cells. HepG2 cells were treated with increasing concentrations (0–200 μ g/ml) of red (A) and green (B) kratom extract for 24 and 48 hours. Cell viability was measured using the MTS assay and expressed as a percentage relative to untreated control cells. Additionally, HepG2 cells were treated with increasing concentrations (0–400 μ M) of mitragynine for 24 or 48 hours, and cell viability was measured similarly. IC50 values were calculated to determine cytotoxic effects. Data are presented as mean ± SEM of three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Turkey’s post-hoc test: #p < 0.05 and *p < 0.05 compared to the untreated control at 24 and 48 hours, respectively. [Click here to view] |
Thai Kratom extracts and constituents attenuate FFA-induced lipid accumulation
Previous studies have suggested the potential anti-lipidemic effects of Thai kratom consumption [18,19]. To investigate the effects of Thai kratom on hepatic lipid accumulation, an FFA-induced fatty liver HepG2 cell line was used. HepG2 cells were treated with 0, 50, and 100 µg/ml of red and green kratom for 24 hours, and lipid accumulation was determined by ORO staining. Quantification of lipid accumulation revealed that FFA treatment significantly increased intracellular lipid content compared to the control, resulting in a 27.54 ± 4.83-fold increase. However, red and green kratoms at 50 and 100 µg/ml, respectively, significantly and dose-dependently reduced FFA-induced lipid accumulation compared with FFA treatment alone. Additionally, 50 µM quercetin, a well-known compound that reduces hepatic fat accumulation [25], and 100 µM mitragynine significantly decreased intracellular fat accumulation compared to the FFA-treated group (Fig. 2A and 2B). These findings indicate that Thai kratom extracts and their constituents, particularly mitragynine and quercetin, have the potential to mitigate FFA-induced lipid accumulation in HepG2 cells.
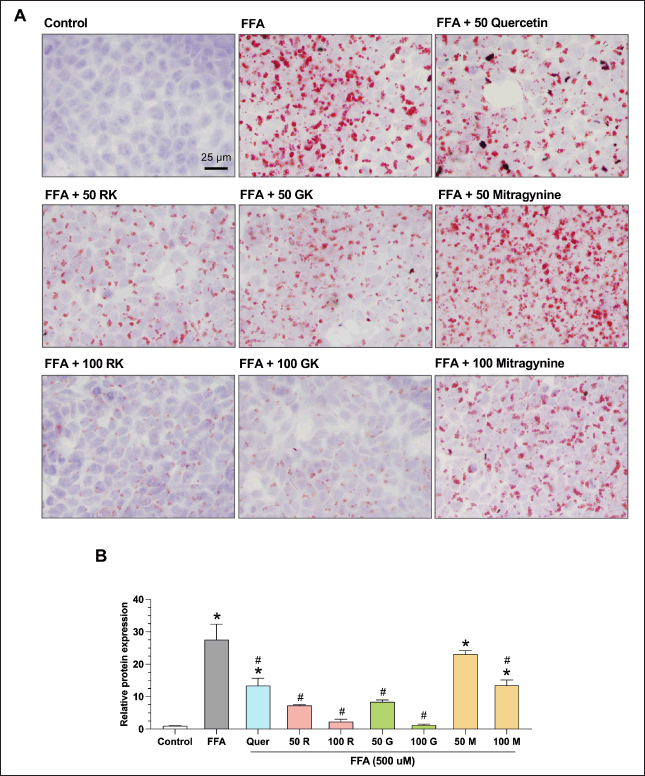 | Figure 2. Effects of kratom extract and mitragynine on intracellular lipid accumulation in HepG2 cells. (A) Representative Oil Red O-stained images of HepG2 cells treated with palmitic and oleic acid (FFA) to induce lipid accumulation. Cells were then co-treated with quercetin (Q), red kratom extract (RK), green kratom extract (GK), or mitragynine (M) at the indicated concentrations. Lipid droplets were stained using Oil Red O. Scale bar = 25 μM. (B) Quantification of the lipid accumulation. The relative intensity of Oil Red O staining was analyzed using ImageJ software. Data are presented as mean ± SEM of three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Turkey’s post-hoc test: *p < 0.05, compared to the control group, #p < 0.05 compared to the FFA-treated group. [Click here to view] |
Effects of Thai Kratom extracts and mitragynine on lipid metabolism
As shown in Figure 3A–D, co-treatment with FFA and red or green kratom extracts led to a dose-dependent reduction in the protein expression of both ACC and FAS. In contrast, mitragynine (50–200 µM) and quercetin (50 µM) did not significantly affect ACC and FAS protein levels. Hepatic de novo lipogenesis is often dysregulated in MAFLD [26] and is known to be suppressed by AMPK, a key regulator of lipid metabolism. AMPK achieves this by inhibiting key enzymes the ACC and FAS [27,28]. We observed that FFA treatment significantly decreased AMPK phosphorylation (0.79 ± 0.07-fold), while both red and green Thai kratom extracts increased the p-AMPK/AMPK ratio. Red kratom extract at 100 and 200 µg/ml increased the ratio by 1.18 ± 0.14-fold and 1.35 ± 0.19-fold, respectively, while green kratom extract induced a 1.44 ± 0.08-fold and 1.71 ± 0.28-fold increase (Fig. 3). AMPK activation was associated with a 50% reduction in ACC and FAS protein levels, indicating dose-dependent suppression of lipogenic enzymes (Fig. 3B and C). Notably, treatment with the pure compounds mitragynine at lower doses (50 and 100 µM) and quercetin did not significantly alter the p-AMPK/AMPK ratio compared to that in the FFA-treated group. However, a higher dose of mitragynine (200 µM) significantly decreased the p-AMPK/AMPK ratio compared to that in the FFA-treated group. These findings suggest that red and green Thai kratom extracts may have a stronger potential to attenuate de novo lipogenesis and mitigate lipid accumulation in HepG2 cells than their constituents mitragynine and quercetin. This effect may be mediated, at least in part, by AMPK activation.
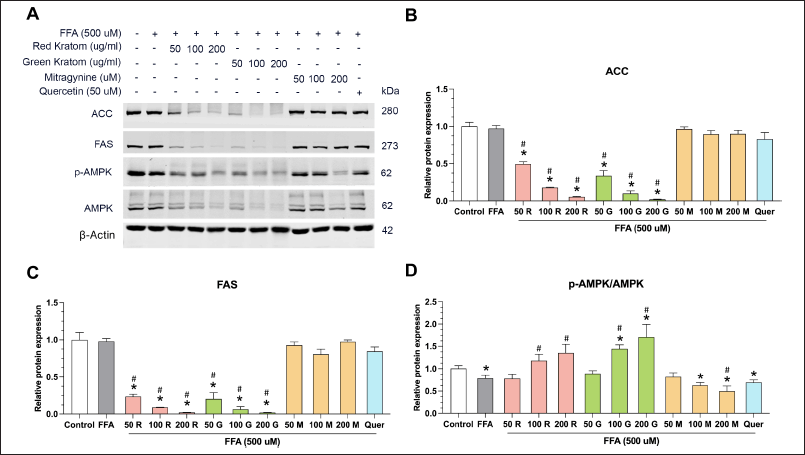 | Figure 3. Effects of kratom extract and mitragynine on lipid metabolism in HepG2 cells. (A) Representative Western blots showing the expression of ACC, FAS, p-AMPK, AMPK, and β-actin (loading control) in HepG2 cells treated with FFA alone or in combination with red kratom extract (R), green kratom extract (G), mitragynine (M), or quercetin (Quer) at the indicated concentrations for 24 hours. Quantification of ACC (B) and FAS (C) protein expression levels relative to β-actin. (D) Quantification of the p-AMPK/AMPK ratio. Data are presented as mean SEM of three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Turkey’s post-hoc test: *p < 0.05 compared to control, #p < 0.05 compared to the FFA-treated group. [Click here to view] |
Effects of Thai Kratom extracts on glucose homeostasis and insulin signaling
Our study investigated the effects of Red and Green Thai Kratom extracts, their primary alkaloid mitragynine, and quercetin (known to enhance insulin signaling and glucose uptake [29]) on insulin signaling and glucose metabolism in HepG2 cells, as shown in Figure 4A–E. We observed a dose-dependent decrease in the p-IRS-1/IRS-1 ratio following mitragynine treatment and a complete disappearance of IRS-1 bands upon kratom extract treatment. This suggests a potential inhibitory effect on IRS-1, possibly through increased degradation or decreased expression [30]. Interestingly, despite this potential inhibition at the IRS-1 level, high doses of kratom extract enhanced downstream insulin signaling by increasing AKT phosphorylation. This suggests that the activation of AKT through alternative pathways is independent of IRS-1. Furthermore, both kratom extracts and mitragynine counteracted the negative effects of FFA treatment on glycogen synthesis, as evidenced by the increased p-GSK3a/GSK3a ratios. However, kratom extract decreased glycogen synthase (GS) levels, whereas mitragynine increased GS levels, suggesting that only mitragynine enhanced GS activity and increased glycogen storage in the liver. This discrepancy suggests that other components within kratom extract may influence GS expression, potentially through complex interactions with signaling pathways or regulatory mechanisms. Notably, quercetin exerted its effects without altering p-AKT/AKT levels, highlighting its distinct mechanisms of action compared to kratom extracts.
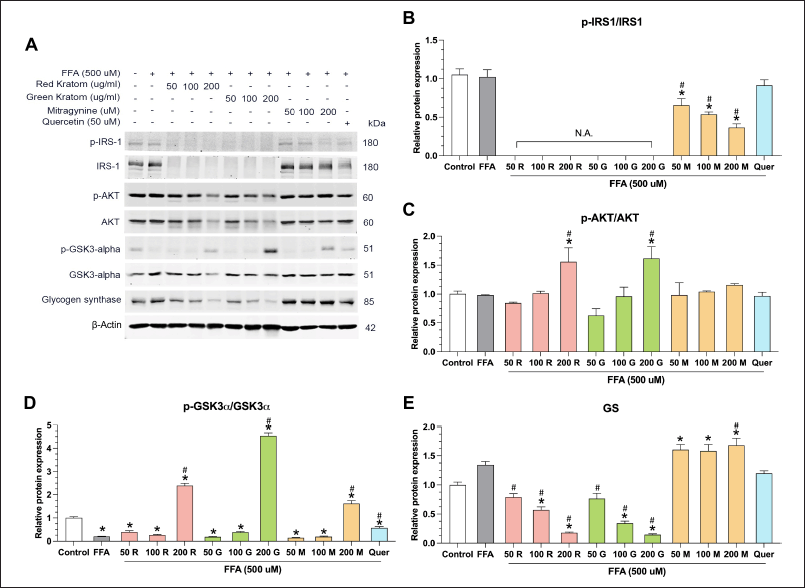 | Figure 4. Effects of thai kratom extracts, mitragynine, and quercetin on insulin signaling and glycogen synthesis in HepG2 cells. (A) representative western blots showing the protein expression of phosphorylated (p-) and total forms of (B) IRS-1, (C) AKT, (D) GSK3a, and (E) Glycogen synthase (GS). HepG2 cells were treated with FFA alone or in combination with Red Thai Kratom (RTK), Green Thai Kratom (GTK), mitragynine, or quercetin at the indicated concentration. β-actin serves as a loading control. “N.A.” indicates that the signal was not detectable. Data are presented as mean ± SEM of three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Turkey’s post-hoc test: *p < 0.05 compared to the control group and #p < 0.05 compared to the FFA-treated group. [Click here to view] |
Effects of Kratom extracts and mitragynine on cellular stress and inflammation
Exposure of HepG2 cells to FFA led to a marked elevation in the phosphorylation of p38 MAPK, a pivotal signaling molecule implicated in cellular stress and inflammatory responses. [31]. Interestingly, treatment with red and green kratom extracts mitigated this effect, decreasing p38 MAPK phosphorylation and suggesting potential anti-inflammatory properties (Fig. 5A and 5B). In contrast, mitragynine did not significantly alter p-p38 MAPK levels, whereas quercetin, which has been previously reported to remarkably increase p38 MAPK phosphorylation [32], further augmented p38 MAPK phosphorylation compared to FFA-treated cells.
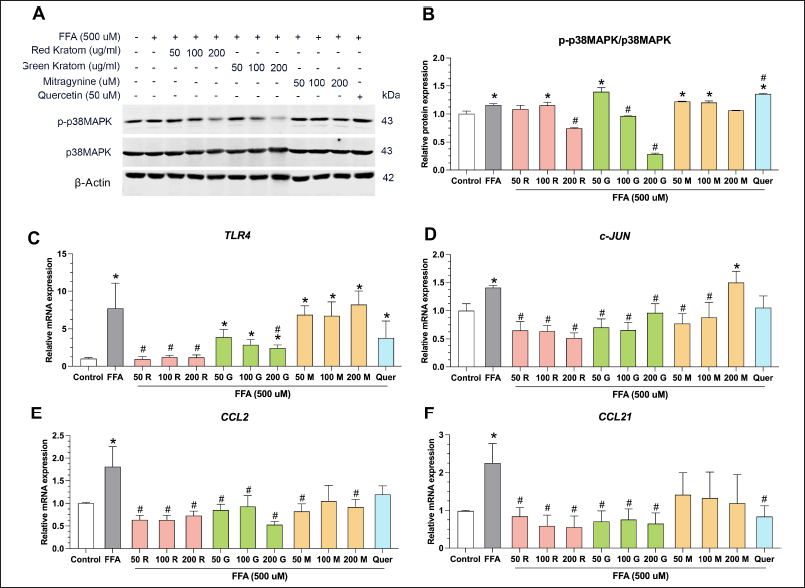 | Figure 5. Effects of Thai kratom extracts and mitragynine on stress and inflammation in HepG2 Cells. (A) representative western blots showing the levels of phosphorylated (p-) and total p38 MAPK in HepG2 cells treated with FFA alone or in combination with Red Thai Kratom (RTK), Green Thai Kratom (GTK), mitragynine, or quercetin at the indicated concentrations. β-actin serves as a loading control. Quantification of stress and inflammatory markers (B) p-p38 MAPK/p38 MAPK ratio. Relative mRNA expression levels of (C) TLR4, (D) c-JUN, (E) CCL2, and (F) CCL21. Data are presented as mean ± SEM of three independent experiments (n = 3). Statistical significance was determined using one-way ANOVA followed by Turkey’s post-hoc test: *p < 0.05, compared with the control group, and #p < 0.05, compared with the FFA-treated group. [Click here to view] |
Hepatocellular function is intricately regulated by a network of signaling pathways, including TLR4, c-Jun, CCL2, and CCL21 [33]. TLR4 activation triggers signaling cascades that culminate in the induction of c-Jun, a transcription factor with diverse roles in cellular processes and inflammation [34]. The interplay between these pathways drives the expression of inflammatory chemokines, such as CCL2 and CCL21 [35]. Our study demonstrated that Thai kratom extract and mitragynine can modulate these pathways in HepG2 cells. Both Thai kratom extracts significantly downregulated the mRNA expression of TLR4, c-Jun, CCL2, and CCL21, indicating their potential anti-inflammatory effects (Fig. 5C–F). In contrast, mitragynine did not significantly alter TLR4 and CCL21 mRNA expression compared to that in FFA-treated cells.
Collectively, these findings suggest that Thai kratom and its constituents, particularly its extracts, may possess protective properties against inflammation and glycogen accumulation in hepatic cells. Further research is warranted to elucidate the precise molecular mechanisms underlying these observations and to explore the potential therapeutic implications of kratom in inflammatory liver diseases.
DISCUSSION
Our study provides evidence supporting the therapeutic benefits of Thai kratom extract and its major alkaloid mitragynine in the context of MAFLD prevention. Treatment with both red and green kratom extracts significantly reduced FFA-induced lipid accumulation in HepG2 cells, indicating their ability to mitigate hepatic steatosis, a hallmark of MAFLD. This observation aligns with a previous study highlighting the anti-lipidemic effects of M. speciosa [19]. Notably, the direct comparison revealed that kratom extracts exerted a greater reduction in lipid accumulation than mitragynine alone, suggesting that additional bioactive compounds in the extracts may act synergistically to enhance this effect. These results suggest that Thai kratom, particularly its extracts, could serve as a promising natural therapeutic strategy for managing hepatic lipid accumulation, a key pathological feature of MAFLD.
The lipid-lowering effects of kratom extracts appear to be mediated, at least in part, by AMPK activation, a key regulator of cellular energy homeostasis and lipid metabolism [36–38]. AMPK activation suppresses de novo lipogenesis by inhibiting key enzymes such as ACC and FAS [39–41]. Our findings demonstrated that palmitic/oleic acid FFA decreased the relative protein levels of p-AMPK/AMPK, which is consistent with in vivo models [42]. However, kratom extracts significantly enhanced AMPK phosphorylation while concurrently downregulating ACC and FAS protein expression, suggesting a potential mechanism underlying its lipid-lowering effects (Fig. 3). Notably, although mitragynine also contributed to AMPK activation, the increase in the p-AMPK/AMPK ratio was more pronounced in kratom extract-treated cells compared to those treated with mitragynine. This finding reinforces the hypothesis that the diverse bioactive compounds in kratom extracts may exert stronger metabolic effects through synergistic interaction. In particular, non-mitragynine components such as quercetin, flavonoids, and other alkaloids may further enhance AMPK signaling, contributing to the superior efficacy of the extract in modulating lipid metabolism [43]. In the previous reported, quercetin also activates AMPK, a critical regulator of glucose uptake and metabolism. This mechanism is directly related to our investigation of the effects of kratom on the AMPK-mediated pathways. The ability of quercetin to enhance glucose uptake and insulin sensitivity in hepatic cells makes it an appropriate comparative standard for evaluating the metabolic effects of kratom [11,12,44–46]. Our findings are consistent with those of several studies demonstrating the potential of plant alkaloids to improve dyslipidemia. For example, i) berberine, an isoquinoline alkaloid found in various medicinal plants, has been shown to reduce cholesterol and triglyceride levels by regulating lipid metabolism pathways [47], and ii) nuciferine, derived from medicinal plants, reduces fat mass in vivo and improves dyslipidemia [48]. Both berberine and nuciferine also reduce de novo lipogenesis by suppressing ACC and FAS expression and have been demonstrated to enhance their overall therapeutic effects by improving MAFLD [49]. However, kratom differs mechanistically from berberine and nuciferine in its ability to modulate both the AMPK and insulin signaling pathways simultaneously, as demonstrated by its dual effect on AKT activation and glycogen synthesis. This distinction suggests that the therapeutic potential of kratom extends beyond AMPK activation alone and may influence broader metabolic pathways. Interestingly, mitragynine alone did not exhibit the same degree of AMPK activation as the whole extract, reinforcing the hypothesis that its efficacy may arise from its complex phytochemical composition, including other alkaloids and flavonoids, such as quercetin [18]. Further research should focus on isolating individual compounds to determine their contribution to the metabolic effects of kratom and their potential synergistic interactions.
An unexpected observation in our study was the lack of ACC and FAS induction by FFA treatment, despite a decrease in the p-AMPK/AMPK ratio. This could be due to compensatory mechanisms triggered by FFA-induced lipid accumulation, counteracting the decrease in AMPK activity, or the involvement of other signaling pathways, such as the mTORC1/SREBP-1c pathway, in regulating ACC and FAS expression [50]. Further investigation is needed to unravel the complex interplay between FFA-induced signaling and lipogenic enzyme regulation in HepG2 cells.
In addition to its impact on lipid metabolism, we observed complex modulation of insulin signaling by kratom extract and mitragynine. Previous studies have shown that FFA treatment increases IRS1 phosphorylation [51]. However, in our study, both kratom extract and mitragynine exhibited antagonistic effects on phosphorylation in the FFA-treated group. Although these treatments led to a decrease in IRS-1 protein levels, potentially inhibiting early insulin signaling, they also increased AKT phosphorylation, which is a key downstream effector of insulin signaling. AKT activation was observed primarily with kratom extract, with a non-significant trend towards increased p-AKT/AKT, which was also observed with mitragynine, but not with quercetin. This observation suggests that kratom extracts may activate AKT and improve insulin sensitivity in HepG2 cells through alternative pathways that bypass IRS-1 [52–54]. Recent evidence indicates that AMPK can activate AKT independently of IRS-1 by directly phosphorylating mTORC2, which in turn phosphorylates AKT at Ser473 [55]. The AMPK-mTORC2-AKT axis may explain our observations. The study by Lee et al. [11] illustrated that quercetin activates AMPK and presents compelling evidence that Compound C, by specifically inhibiting AMPK activity, effectively counteracts this quercetin-induced activation [11]. Additionally, Gao et al. [46] showed that inhibition of AMPK by Compound C prevented mTORC2-mediated AKT activation in HepG2 cells, further supporting the dependency of this pathway on AMPK activity. These studies highlight the complex interplay between AMPK, mTORC2, and AKT in the regulation of cellular responses to energetic stress [46]. Future studies incorporating selective AMPK inhibitors, such as Compound C, will be essential to validate the proposed AMPK-mTORC2-AKT mechanism in the context of kratom extract treatment. Such experiments would provide direct evidence for the involvement of AMPK in mediating AKT activation via mTORC2 and clarify its role in modulating insulin-signaling pathways. Additionally, other potential mechanisms may contribute to this effect, including i) the activation of other receptor tyrosine kinases that can also signal through PI3K to AKT, such as the insulin-like growth factor 1 receptor or epidermal growth factor receptor [56], and ii) crosstalk with G protein-coupled receptors that can phosphorylate and activate AKT independent of IRS-1 [57].
The disappearance of p-IRS-1 and IRS1 protein expression upon kratom extract treatment is also noteworthy. This can be attributed to several mechanisms, including increased protein degradation or decreased protein synthesis. Previous studies have shown that certain natural compounds can promote IRS-1 degradation via the ubiquitin–proteasome pathway [58]. Alternatively, kratom may interfere with IRS-1 gene expression or mRNA stability, leading to reduced protein levels. Further research is necessary to elucidate the precise mechanisms underlying this phenomenon.
An increase in the p-AKT/AKT ratio activates the AKT pathway, including downstream targets such as GSK3α and glycogen synthase, which promote glycogen synthesis [59]. This is consistent with the established role of the AKT pathway in enhancing glucose uptake and glycogen synthesis [60]. Activation of AKT also leads to several downstream effects that could contribute to reduced lipid accumulation, such as i) stimulation of glucose uptake by cells, thereby decreasing glucose availability for conversion into fatty acids [61], and ii) promoting glycogen storage as a non-lipid form of glucose [62]. These findings are consistent with those of previous studies on GSK3α-knockout mice, which exhibited increased hepatic glycogen deposition [63]. Additionally, in vitro studies using GSK3 inhibitors have demonstrated reduced glucose production in hepatic cells by lowering the expression of gluconeogenesis-related genes (PEPCK and G6Pase) independent of glycogen synthesis. This suggests that the mechanisms by which kratom extract and mitragynine activate the AKT pathway may involve the inhibition of GSK3 activity [64].
Chronic inflammation is a hallmark of MAFLD progression [65]. Our study underscores the anti-inflammatory potential of kratom extract. Treatment with these extracts led to a decrease in p38 MAPK phosphorylation, a key mediator of cellular stress and inflammation [31]. Moreover, both kratom extract and quercetin downregulated the expression of the inflammatory mediators TLR4, c-JUN, CCL2, and CCL21, suggesting their ability to suppress inflammatory responses in hepatic cells. These findings align with those of previous reports on the anti-inflammatory properties of kratom and its constituents [66]. The ability to mitigate inflammation is crucial for preventing the progression of MAFLD to NASH, highlighting another potential therapeutic avenue for kratom extracts. This observation is consistent with the established anti-inflammatory properties of quercetin, a component of Thai kratom [67,68].
The findings of this study may provide a mechanistic basis for the observed effects of kratom extract and mitragynine on HepG2 cells. This first report suggests that both kratom and mitragynine can reduce fat accumulation in the HepG2 cell line by i) converting glucose to glycogen via the AKT-GSK3 signaling pathway, thereby suppressing de novo fatty acid synthesis from glucose precursors [69]; ii) decreasing de novo fatty acid synthesis by decreasing ACC and FAS through AMPK activation, which correlates with several herbal compounds [70,71]; and iii) anti-inflammatory activity to prevent NASH progression, consistent with several alkaloid compounds [72,73]. Notably, compared to quercetin, a well-established potential anti-obesity agent, the effects of kratom extract and mitragynine on the AKT pathway appear to be more pronounced [74]. This observation underscores the possibility that kratom extracts may have stronger therapeutic potential by more effectively modulating this signaling pathway. In addition to the MAFLD model, we also observed a 1% ethanol-induced reduction in fat accumulation in cells treated with red and green kratoms (50 and 100 µg/ml) to reduce fat induction (Supplementary Fig. S1). Although this study offers valuable insights derived from cell culture, further in vivo investigations using animal models are necessary to validate these anti-obesity effects and thoroughly assess the therapeutic potential of Thai kratom against MAFLD. Furthermore, the potential therapeutic application of Kratom in MAFLD necessitates addressing safety concerns, particularly given its classification by the US FDA as potentially harmful. The FDA has issued warnings about the safety of kratom, highlighting concerns about its association with multiorgan toxicity, including cardiovascular effects. These effects are primarily linked to mitragynine and 7-hydroxy mitragynine, which exhibit opioid-like properties [75]. Although our study focused on the metabolic benefits of kratom, its safety profile, particularly its cardiovascular effects, remains a critical consideration. A comprehensive review by Leong Bin Abdullah and Singh [75] documented that the most common adverse cardiovascular effects of kratom include tachycardia and hypertension, with possible dose-dependent effects on cardiac rhythm. In vitro studies have indicated that mitragynine can prolong QTc intervals, whereas case reports have described ventricular arrhythmias and cardiopulmonary arrest, although often in cases involving polysubstance use. At physiologically relevant doses, kratom extracts may have different safety profiles than those from isolated high-concentration alkaloids. These findings suggest that standardizing kratom dosage and optimizing extraction methods to maintain beneficial components while minimizing potential cardiotoxic effects may be crucial for therapeutic development. To mitigate these risks, future strategies should isolate or modify specific bioactive compounds, such as mitragynine, to reduce toxicity while retaining therapeutic efficacy. These modifications can involve structural optimization or combination with synergistic compounds to enhance safety profiles. Rigorous preclinical and clinical testing is essential to validate the safety and efficacy of kratom-derived substances. Comprehensive safety assessments, including dose-response studies and long-term evaluations, are critical for establishing Kratom’s viability as a therapeutic option for metabolic disorders.
This study had some limitations. The use of HepG2 cells, an immortalized hepatocellular carcinoma cell line, may not fully replicate the metabolic and signaling dynamics of primary hepatocytes. HepG2 cells exhibit altered lipid metabolism, inflammatory responses, and insulin signaling, which may limit the translational relevance of our findings. Additionally, although in vitro FFA treatment simulates aspects of fatty liver disease, it does not replicate the complexity of in vivo conditions. Finally, the complexity of kratom extracts makes it difficult to pinpoint the specific molecules responsible for the observed effects, warranting further research to isolate and characterize the active compounds.
CONCLUSION
This study presents compelling evidence that Thai kratom extracts, particularly mitragynine, may offer a promising natural approach to managing MAFLD by modulating lipid metabolism, enhancing insulin sensitivity, and reducing inflammation. While further in vivo research is necessary to validate these findings and assess the safety and efficacy of kratom-derived therapies, this study provides the groundwork for the future exploration of kratom as a potential treatment for metabolic disorders.
ACKNOWLEDGMENTS
We would like to extend our sincere gratitude to Zuhair Al-Masri for his meticulous review, invaluable feedback, and thorough proofreading of this manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by Walailak University, Thailand (T.K.), and Walailak University Ph.D. Excellence Scholarships [PE05/2021] (T.K. and J.B.A.).
CONFLICTS OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. CrossRef
2. Afroz A, Alam K, Ali L, Karim A, Alramadan MJ, Habib SH, et al. Type 2 diabetes mellitus in Bangladesh: a prevalence based cost-of-illness study. BMC Health Serv Res. 2019;19:601. CrossRef
3. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. CrossRef
4. Wongrith P, Thirarattanasunthon P, Kaewsawat S. Glycemic control outcome in patients with type 2 diabetes mellitus: chronic care management support of family care team in Thailand. J Diabetes Metab Disord. 2021;20(2):1269–79. CrossRef
5. Bashir A, Duseja A, De A, Mehta M, Tiwari P. Non-alcoholic fatty liver disease development: a multifactorial pathogenic phenomena. Liver Res. 2022;6(2):72–83. CrossRef
6. Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr. 2020;14(6):1875–87. CrossRef
7. Subramanian P, Hampe J, Tacke F, Chavakis T. Fibrogenic pathways in metabolic dysfunction associated fatty liver disease (MAFLD). Int J Mol Sci. 2022;23(13):6996. CrossRef
8. Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2018;76(1):99–128. CrossRef
9. Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64. CrossRef
10. Dusabimana T, Park EJ, Je J, Jeong K, Yun SP, Kim HJ, et al. P2y2r deficiency ameliorates hepatic steatosis by reducing lipogenesis and enhancing fatty acid β-oxidation through AMPK and PGC-1α induction in high-fat diet-fed mice. Int J Mol Sci. 2021;22(11):5528. CrossRef
11. Lee YK, Song YP, Kim YM, Won SL, Ock JP. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41(3):201–7. CrossRef
12. Dhanya R, Arya AD, Nisha P, Jayamurthy P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front Pharmacol. 2017;8:336. CrossRef
13. Wang MY, Zhang SS, An MF, Xia YF, Fan MS, Sun ZR, et al. Neferine ameliorates nonalcoholic steatohepatitis through regulating AMPK pathway. Phytomedicine. 2023;114:154798. CrossRef
14. Kim SH, Yun C, Kwon D, Lee YH, Kwak JH, Jung YS, et al. Effect of isoquercitrin on free fatty acid-induced lipid accumulation in HepG2 cells. Molecules. 2023;28(3):1476. CrossRef
15. Cai Y, Yang Q, Yu Y, Yang F, Bai R, Fan X. Efficacy and underlying mechanisms of berberine against lipid metabolic diseases: a review. Front Pharmacol. 2023;14:1283784. CrossRef
16. Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9(1):55–69. CrossRef
17. Derosa G, Maffioli P. Alkaloids in the nature: pharmacological applications in clinical practice of berberine and mate tea. Curr Top Med Chem. 2014;14(2):200–6. CrossRef
18. Janthongkaw A, Klaophimai S, Khampaya T, Yimthiang S, Yang Y, Ma R, et al. Effect of green and red Thai kratom (Mitragyna speciosa) on pancreatic digestive enzymes (alpha-glucosidase and lipase) and acetyl-carboxylase 1 activity: a possible therapeutic target for obesity prevention. PLoS One. 2023;18(6):e0291738. CrossRef
19. La-up A, Saengow U, Aramrattana A. High serum high-density lipoprotein and low serum triglycerides in kratom users: a study of kratom users in Thailand. Heliyon. 2021;7(6):e06931. CrossRef
20. Karunakaran T, Ganasan J, Rusmadi NN, Santhanam R, Mordi MN. In-vitro hepatotoxic activity of mitragynine and paynantheine isolated from the leaves of Mitragyna speciosa Korth. (Kratom). Nat Prod Res. 2024;20.;1–5. CrossRef
21. Kong WM, Chik Z, Mohamed Z, Alshawsh MA. Physicochemical characterization of Mitragyna speciosa alkaloid extract and mitragynine using in vitro high throughput assays. Comb Chem High Throughput Screen. 2017;20(9):796–803. CrossRef
22. Sempio C, Campos-Palomino J, Klawitter J, Zhao W, Huestis MA, Christians U, et al. Quantification of 11 kratom alkaloids including mitragynine and its main metabolites in human plasma using LC-MS/MS. Anal Bioanal Chem. 2024;416(3):761–9. CrossRef
23. Khamphaya T, Chukijrungroat N, Saengsirisuwan V, Mitchell-Richards KA, Robert ME, Mennone A, et al. Nonalcoholic fatty liver disease impairs expression of the type II inositol 1,4,5-trisphosphate receptor. Hepatology. 2018;67(2):560–74. CrossRef
24. Pfaffl MW, Hageleit M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol Lett. 2001;23(4):275–82. CrossRef
25. Gnoni A, Di Chiara Stanca B, Giannotti L, Gnoni GV, Siculella L, Damiano F. Quercetin reduces lipid accumulation in a cell model of NAFLD by inhibiting de novo fatty acid synthesis through the acetyl-CoA carboxylase 1/AMPK/PP2A axis. Int J Mol Sci. 2022;23(3):1044. CrossRef
26. Zhao J, Liu L, Cao YY, Gao X, Targher G, Byrne CD, et al. MAFLD as part of systemic metabolic dysregulation. Hepatol Int. 2024;18(2):834–47. CrossRef
27. Chen H, Nie T, Zhang P, Ma J, Shan A. Hesperidin attenuates hepatic lipid accumulation in mice fed high-fat diet and oleic acid induced HepG2 via AMPK activation. Life Sci. 2022;296:120428. CrossRef
28. Li Z, Li J, Miao X, Cui W, Miao L, Cai L. A minireview: role of AMP-activated protein kinase (AMPK) signaling in obesity-related renal injury. Life Sci. 2021;265:118828. CrossRef
29. Ho GTT, Kase ET, Wangensteen H, Barsett H. Effect of phenolic compounds from elderflowers on glucose- and fatty acid uptake in human myotubes and HepG2-cells. Molecules. 2017;22(1):90. CrossRef
30. Lee AV, Gooch JL, Oesterreich S, Guler RL, Yee D. Insulin-like growth factor I-induced degradation of insulin receptor substrate 1 is mediated by the 26S proteasome and blocked by phosphatidylinositol 3′-kinase inhibition. Mol Cell Biol. 2000;20(5):1489–96. CrossRef
31. Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92(2):689–737. CrossRef
32. Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact. 2012;195(2):154–64. CrossRef
33. Gusev E, Sarapultsev A. Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int J Mol Sci. 2023;24(9):7910. CrossRef
34. Chang S, Li X, Zheng Y, Shi H, Zhang D, Jing B, et al. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-?B signaling pathway. Phytother Res. 2022;36(4):1678–91. CrossRef
35. Song N, Cui K, Zeng L, Li M, Fan Y, Shi P, et al. Advance in the role of chemokines/chemokine receptors in carcinogenesis: focus on pancreatic cancer. Eur J Pharmacol. 2024;967:176357. CrossRef
36. Hardie DG. Keeping the home fires burning: AMP-activated protein kinase. J R Soc Interface. 2018;15(138):20170774. CrossRef
37. O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol. 2013;366(2):135–51. CrossRef
38. Herzig S, Shaw RJ. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2017;19(2):121–35. CrossRef
39. Zhao P, Saltiel AR. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J Biol Chem. 2020;295(34):12279–89. CrossRef
40. Ren L, Sun D, Zhou X, Yang Y, Huang X, Li Y, et al. Chronic treatment with the modified Longdan Xiegan Tang attenuates olanzapine-induced fatty liver in rats by regulating hepatic de novo lipogenesis and fatty acid beta-oxidation-associated gene expression mediated by SREBP-1c, PPAR-alpha and AMPK-alpha. J Ethnopharmacol. 2019;232:176–87. CrossRef
41. Fariha A, Hami I, Tonmoy MIQ, Akter S, Al Reza H, Bahadur NM, et al. Cell cycle associated miRNAs as target and therapeutics in lung cancer treatment. Heliyon. 2022;8(10):e11081. CrossRef
42. Zhang M, Dong K, Du Q, Xu J, Bai X, Chen L, et al. Chemically synthesized osteocalcin alleviates NAFLD via the AMPK-FOXO1/BCL6-CD36 pathway. J Transl Med. 2024;22(1):1–20. CrossRef
43. Olivares-Vicente M, Sánchez-Marzo N, Encinar JA, de La Luz Cádiz-Gurrea M, Lozano-Sánchez J, Segura-Carretero A, et al. The potential synergistic modulation of AMPK by Lippia citriodora compounds as a target in metabolic disorders. Nutrients. 2019;11(12):2961. CrossRef
44. Jiang H, Yamashita Y, Nakamura A, Croft K, Ashida H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci Rep. 2019;9(1):1–15. CrossRef
45. Wang M, Wang B, Wang S, Lu H, Wu H, Ding M, et al. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers. Front Cell Dev Biol. 2021;9:616219. CrossRef
46. Gao M, Kong Q, Hua H, Yin Y, Wang J, Luo T, et al. AMPK-mediated up-regulation of mTORC2 and MCL-1 compromises the anti-cancer effects of aspirin. Oncotarget. 2016;7(13):16349–61. CrossRef
47. Och A, Och M, Nowak R, Podgórska D, Podgórski R. Berberine, a herbal metabolite in the metabolic syndrome: the risk factors, course, and consequences of the disease. Molecules. 2022;27(4):1351. CrossRef
48. Xu Z, Sheng Y, Zeng G, Zeng Z, Li B, Jiang L, et al. Metabonomic study on the plasma of high-fat diet-induced dyslipidemia rats treated with Ge Gen Qin Lian decoction by ultrahigh-performance liquid chromatography-mass spectrometry. Evid Based Complement Alternat Med. 2021;2021:6692456. CrossRef
49. Xu H, Lyu X, Guo X, Yang H, Duan L, Zhu H, et al. Distinct AMPK-mediated FAS/HSL pathway is implicated in the alleviating effect of nuciferine on obesity and hepatic steatosis in HFD-fed mice. Nutrients. 2022;14(9):1898. CrossRef
50. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin resistant mice. Cell Metab. 2011;13(4):376–88. CrossRef
51. Schmitz-Peiffer C, Whitehead JP. IRS-1 regulation in health and disease. IUBMB Life. 2003;55(7):367–74. CrossRef
52. Zhang Z, Liu H, Liu J. Akt activation: a potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract. 2019;156:107092. CrossRef
53. Takai M, Nakagawa T, Tanabe A, Terai Y, Ohmichi M, Asahi M. Crosstalk between PI3K and Ras pathways via protein phosphatase 2A in human ovarian clear cell carcinoma. Cancer Biol Ther. 2015;16(2):325–35. CrossRef
54. Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–8. CrossRef
55. Kazyken D, Magnuson B, Bodur C, Acosta-Jaquez HA, Zhang D, Tong X, et al. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci Signal. 2019;12(585):eaav3249. CrossRef
56. Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47(1):R1–10. CrossRef
57. Waters C, Pyne S, Pyne NJ. The role of G-protein coupled receptors and associated proteins in receptor tyrosine kinase signal transduction. Semin Cell Dev Biol. 2004;15(3):309–23. CrossRef
58. Ma RH, Ni ZJ, Thakur K, Zhang F, Zhang YY, Zhang JG, et al. Natural compounds play therapeutic roles in various human pathologies via regulating endoplasmic reticulum pathway. Med Drug Discov. 2020;8:100065. CrossRef
59. Mao YP, Song YM, Pan SW, Li N, Wang WX, Feng BB, et al. Effect of codonopsis radix and polygonati rhizoma on the regulation of the IRS1/PI3K/AKT signaling pathway in type 2 diabetic mice. Front Endocrinol (Lausanne). 2022;13:1068555. CrossRef
60. ?widerska E, Strycharz J, Wróblewski A, Szemraj J, Drzewoski J, ?liwi?ska A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. Blood Glucose Levels. 2018. CrossRef
61. Chao HW, Chao SW, Lin H, Ku HC, Cheng CF. Homeostasis of glucose and lipid in non-alcoholic fatty liver disease. Int J Mol Sci. 2019;20(2):298. CrossRef
62. Lu B, Bridges D, Yang Y, Fisher K, Cheng A, Chang L, et al. Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes. 2014;63(9):2935–48. CrossRef
63. Wang L, Li J, Di LJ. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med Res Rev. 2022;42(2):946–82. CrossRef
64. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. CrossRef
65. Córdova-Gallardo J, Keaveny AP, Qi X, Méndez-Sánchez N. Metabolic associated fatty liver disease and acute-on-chronic liver failure: common themes for common problems. Eur J Gastroenterol Hepatol. 2021;33(1S):e84–93. CrossRef
66. Bachu AK, Singal P, Griffin B, Harbaugh L, Prasad S, Jain L, et al. Kratom use and mental health: a systematic literature review and case example. J Addict Dis. 2024;42(4):301–12. CrossRef
67. Hytti M, Piippo N, Salminen A, Honkakoski P, Kaarniranta K, Kauppinen A. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp Eye Res. 2015;132:208–15. CrossRef
68. Cheng SC, Wu YH, Huang WC, Pang JHS, Huang TH, Cheng CY. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine. 2019;116:48–60. CrossRef
69. Papadopoli D, Pollak M, Topisirovic I. The role of GSK3 in metabolic pathway perturbations in cancer. Biochim Biophys Acta Mol Cell Res. 2021;1868(5):119059. CrossRef
70. Hsu CC, Peng D, Cai Z, Lin HK. AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. 2022;85:52–68. CrossRef
71. Foretz M, Even PC, Viollet B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int J Mol Sci. 2018;19(9):2826. CrossRef
72. Argyrou C, Moris D, Vernadakis S. Hepatocellular carcinoma development in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Is it going to be the “plague” of the 21st century? A literature review focusing on pathogenesis, prevention and treatment. J BUON. 2017;22(1):6–20.
73. Shao G, Liu Y, Lu L, Zhang G, Zhou W, Wu T, et al. The pathogenesis of HCC driven by NASH and the preventive and therapeutic effects of natural products. Front Pharmacol. 2022;13:944088. CrossRef
74. Limcharoen T, Pouyfung P, Ngamdokmai N, Prasopthum A, Ahmad AR, Wisdawati W, et al. Inhibition of α-glucosidase and pancreatic lipase properties of Mitragyna speciosa (Korth.) Havil. (Kratom) leaves. Nutrients. 2022;14(19):3909. CrossRef
75. Leong Bin Abdullah MFI, Singh D. The adverse cardiovascular effects and cardiotoxicity of kratom (Mitragyna speciosa Korth.): a comprehensive review. Front Pharmacol. 2021;12:726003. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4590_pdf.pdf]