INTRODUCTION
Chronic kidney disease (CKD) has emerged as an escalating global public health concern, with its prevalence rising consistently over the past few decades. Hemodialysis, an essential aspect of kidney replacement therapy, is vital for supporting the quality of health and well-being among patients with end-stage renal disease (ESRD) [1]. Despite progress in hemodialysis technology and management, individuals undergoing hemodialysis remain prone to nutritional deficiencies due to metabolic changes, dietary restrictions, and increased nutrient losses during dialysis sessions [2].
Nutritional imbalances in patients undergoing hemodialysis (HD) can result in complications such as anemia, bone disorders, and weakened immune function. Consequently, healthcare providers frequently prescribe a variety of dietary supplements, including multivitamins and multi-minerals, to correct these deficiencies and enhance patient outcomes. However, the rationale and consistency of prescribing practices for these supplements within the HD context continue to raise concerns [3].
Vitamins are essential organic compounds required in small quantities to support critical biochemical functions. Since the body cannot synthesize most vitamins, they must be obtained from dietary sources [4]. Chronic HD patients are at an increased risk of vitamin deficiencies due to multiple factors. Dietary restrictions associated with kidney disease often limit vitamin intake, while dialysis itself removes water-soluble vitamins from the bloodstream. In addition, uremic toxins may impair vitamin metabolism and reduced appetite or poor dietary intake can further contribute to deficiencies. Certain medications and comorbid conditions commonly seen in HD patients may also interfere with vitamin absorption or efficacy, exacerbating the risk of nutritional imbalances [5–7].
The Kidney Disease Outcome Quality Initiative (KDOQI) 2005 advocates prioritizing supplementation to prevent deficiencies, especially when it is safe to do so at prescribed dosages. As a result, dialysis patients are likely to benefit from daily vitamin supplementation that matches the recommended vitamin profile for their needs, with an emphasis on B vitamins and folic acid [8]. According to the Kidney Disease Improving Global Outcomes (KDIGOs) guidelines, vitamin D insufficiency in HD patients should be managed similarly to the general population. However, calcitriol or a vitamin D analog is recommended only for individuals with elevated parathyroid hormone levels to help regulate bone and mineral metabolism [9]. These suggestions are backed by the European Kidney Clinical Excellence guidelines [10]. Hemodialysis patients are frequently prescribed multivitamins due to an increased risk of deficiencies, particularly in water-soluble vitamins. However, our study indicates insufficient evidence to encourage habitual supplementation with these vitamins. A recent epidemiological investigation of dialysis outcomes and practice patterns investigation discovered that increased usage of water-soluble vitamin supplements was related to a decreased death rate [11].
Patients on long-term HD are typically advised to limit their intake of extra fluids, potassium, and phosphates in their diet, while also managing interdialytic weight gain within the recommended guidelines as part of their daily routine. Patients on long-term HD are generally advised to limit extra fluid, sodium, and phosphorus intake and to keep weight gain between dialysis sessions within recommended limits as part of their daily regimen. This suggests their diets may be limited to vegetables, fruits, and dairy products such as milk, yogurt, cheese, and fruit juices. Consequently, these dietary restrictions may lead to inadequate consumption of essential macro- and micronutrients. Furthermore, throughout their lives, individuals on long-term HD may experience anorexia, resulting in an even more diminished intake of macro- and micronutrients [12–14]. Studies repeatedly indicate that patients with long-term HD often exhibit significantly lower plasma levels of various minerals and certain vitamins compared to healthy control participants [15,16].
High-flux, high-efficiency dialysis may eliminate more water-soluble vitamins from the body than initially anticipated during the early stages of HD therapy [17]. Emerging evidence links hyperhomocysteinemia to an increased risk of vascular and cardiac complications [18–20]. Individuals with ESRD and CKD often exhibit elevated homocysteine levels, making them particularly vulnerable to these adverse outcomes [21–23]. Recent studies suggest that supplementation with cyanocobalamin (B12), pyridoxine (B6), and folic acid can effectively lower homocysteine levels in HD patients [24,25]. Given the potential benefits of these water-soluble vitamins, further research is needed to assess their long-term impact on cardiovascular health and overall outcomes in HD patients.
The southern part of Karnataka region in India, marked by its diverse population and distinct healthcare practices, provides a unique backdrop to examine the prescribing patterns of multivitamins, multi-minerals, and dietary supplements among HD patients. While studies have explored supplement usage in CKD populations globally, there is a lack of research explicitly investigating these practices in the South Karnataka region. This multi-centric study aims to bridge the research gap by analyzing the prescribing patterns of multivitamins, multi-minerals, and dietary supplements among HD patients in South Karnataka. By examining patient demographics, the types of supplements prescribed, and the key factors influencing prescription decisions, this study seeks to provide valuable insights into the patterns and rationale behind supplement utilization in this patient population.
Currently, India lacks standardized guidelines for the prescription of multivitamins, multi-minerals, hematinics, and dietary supplements (MMHDSs) in HD patients. Unlike international organizations such as KDIGO and KDOQI, which provide evidence-based recommendations for managing nutritional deficiencies in CKD, Indian healthcare providers rely on individual clinical judgment, leading to significant variability in prescribing practices. This inconsistency increases the risk of both under-supplementation, which may leave deficiencies unaddressed, and over-supplementation, which can lead to toxicity and adverse interactions. The absence of national guidelines underscores the urgent need for India-specific recommendations tailored to regional dietary patterns, economic factors, and patient needs to ensure the safe and effective use of MMHDS in HD care.
This multi-centric study aims to bridge the knowledge gap by investigating the prevalence, types, and factors associated with multivitamin, multi-mineral, and dietary supplement prescriptions among South Karnataka, India, HD patients. The study provides insights that can guide healthcare practitioners in making informed decisions regarding supplement therapies by uncovering the nuances of these prescribing patterns. In addition, a better understanding of these patterns can contribute to developing guidelines and interventions to improve HD patients’ nutritional status and overall well-being.
Pharmacists can play a crucial role in guiding the appropriate use of nutritional supplements in HD patients by providing evidence-based recommendations, monitoring potential interactions, and optimizing therapy to improve patient outcomes.
METHODOLOGY
This multicentric prospective observational study was conducted from August 2021 to August 2022 at HD units in three hospitals in South Karnataka, India: Dr. TMA Pai Hospital Udupi (200 beds), Father Muller Medical College Hospital Mangalore (FMMCH) (1,250 beds), and Kasturba Medical College Hospital Manipal (KMC) (2,032 beds). The study included 384 participants: 22 from Dr. TMA Pai Hospital, 134 from Father Muller Medical College, and 228 from KMC Hospital.
Sample size calculation
Cochran’s formula was used for sample size calculations.
n0 = Sample size
Z = Z-score (1.96 for 95% confidence level)
p = Expected proportion of MMHDS use (0.5 was used for the maximum sample size)
e = Margin of error (0.05).
Sources of data collection
Medical and pharmaceutical data were collected from patient case sheets, medication prescription charts, and demographic interviews with patients and caregivers. Each participant provided written informed consent for a 3-month follow-up to meet the inclusion criteria.
Inclusion criteria
- Outpatient HD patients.
- Age ≥ 18 years.
- Patients who voluntarily consent to participate in the study.
- Ability to speak and understand English or Kannada.
- Mentally alert and cooperative individuals.
Exclusion criteria
- Patients undergoing HD for acute kidney failure.
- Individuals who did not provide informed consent.
- Pregnant or breastfeeding women.
- Patients undergoing post-kidney transplant follow-up.
- Pediatric patients (age < 18 years).
- Individuals with cancer or traumatic injuries.
- Patients requiring occasional continuous HD due to severe medical conditions.
- Individuals with kidney stones requiring laparoscopic intervention.
- Patients receiving peritoneal dialysis instead of HD.
- Critical care unit patients.
- Patients who declined treatment on the study visit day.
Data acquisition and procedures
Data were collected from individuals who met the eligibility criteria, focusing on outpatient HD patients. A custom questionnaire was developed to gather comprehensive patient information, including demographics, medical history, medication charts, pharmaceutical records, laboratory test results, social history, HD treatment details, and primary complaints. Study-specific data were extracted from patient event files, with a justification recorded for each prescribed medication.
Medications were categorized based on the anatomical therapeutic chemical (ATC) classification system, documenting prescribed drugs, associated medical conditions, and prognosis. The data were analyzed using descriptive statistics, with findings presented as categories and proportions to assess prescribing trends.
To maintain patient confidentiality, all names were excluded from case records, and laboratory investigations were based on the most recent test results available in clinical records. The data collection process adhered to strict confidentiality protocols, ensuring ethical compliance. In addition, pharmaceutical brand names were standardized to their generic equivalents using the current index of medical specialties, India, 2022 edition.
The data collection process and questionnaire focused on the challenges faced by hemodialysis patients.
Information was collected from individual treatment records of 384 HD patients who were followed up throughout the study period. Dialysis-related complications were documented, and potential consequences were assessed by reviewing nursing records, patient symptoms, and physicians’ treatment plans.
A custom-designed questionnaire was used to record HD frequency, gender, age group, dialysis duration, and the underlying cause of ESRD. Complications were classified into three categories: technical, interdialytic, and intradialytic issues. Appropriate medical interventions were provided whenever complications arose, ensuring timely patient management.
Evaluating patterns of drug use
Drug data and patient characteristics were analyzed to identify medication usage patterns among individuals with CKD. Each drug was divided into groups according to its therapeutic and pharmacological effects.
Statistical analysis
Information was gathered using Microsoft Excel’s spreadsheet platform. Frequency and percentage were used to describe the data. The mean and SD demonstrate quantitative aspects of patients’ clinical profiles.
RESULTS
The study’s findings highlight the diverse patterns of supplement utilization, patient demographics, and the most commonly prescribed supplements in HD care. The analysis revealed significant variability in prescribing practices among healthcare providers, with most HD patients receiving some form of nutritional supplementation. Prescriptions included a broad range of supplements, such as multivitamins, individual dietary supplements, B-complex vitamins, vitamin D, and essential minerals such as iron, calcium, and zinc. The frequent use of combination supplements reflects an effort to address the complex nutritional needs of HD patients and compensate for dialysis-related nutrient losses.
Multivitamins emerged as the most prescribed supplements, given their comprehensive formulations designed to cater to the intricate nutrient demands of HD patients. Vitamin D supplementation was prevalent, likely driven by the well-documented incidence of serum vitamin D insufficiency among individuals with persistent renal failure. Iron supplementation was frequently prescribed, particularly among patients exhibiting signs of anemia. Including individual vitamins and minerals in the prescription regimen highlighted a tailored approach to supplement selection based on specific patient requirements.
The duration of HD treatment showed a significant correlation with the frequency of supplement prescriptions. Patients undergoing long-term HD were more likely to receive supplementation, likely due to cumulative nutrient losses over time. In addition, the presence of comorbidities influenced supplement utilization, with patients managing multiple health conditions receiving specific supplements tailored to their needs.
Table 1 presents the prescribing patterns of multivitamins, multi-minerals, and nutritional supplements among CKD patients undergoing maintenance HD at FMMCH, KMC, and Dr. TMA Pai Hospital (Udupi).
 | Table 1. Usage patterns of multivitamins, multi-minerals, and nutritional supplements among CKD patients undergoing maintenance HD at FMMCH, KMC, and Dr. TMA Pai Hospital in Udupi. [Click here to view] |
In this study, 384 HD patients received a total of 1,399 medications across 12 different classes of vitamins, minerals, and nutritional supplements. The five most commonly prescribed classes were vitamins, minerals, and antianemics (38.31%), vitamin B complex (20.3%), calcium supplements (13.63%), vitamins and minerals (10.65%), and agents affecting bone metabolism (6.07%). Other prescribed categories included vitamin C (3.35%), protein and calorie-rich supplements for renal nutrition (2.64%), multivitamins and multi-minerals (1.71%), vitamin D analogs (1.21%), supplements and adjuvant therapy (1%), enteral/nutritional products (0.78%), and minerals (0.28%).
Figure 1 illustrates the overall consumption of multivitamins, multi-minerals, and dietary supplements among CKD patients undergoing HD at FMMCH Mangalore, KMC Manipal, and Dr. TMA Pai Hospital.
 | Figure 1. Overall consumption of multivitamins, multi-minerals, and dietary supplements among CKD patients undergoing HD at FMMCH Mangalore, KMC Manipal, and Dr. TMA Pai Hospital. [Click here to view] |
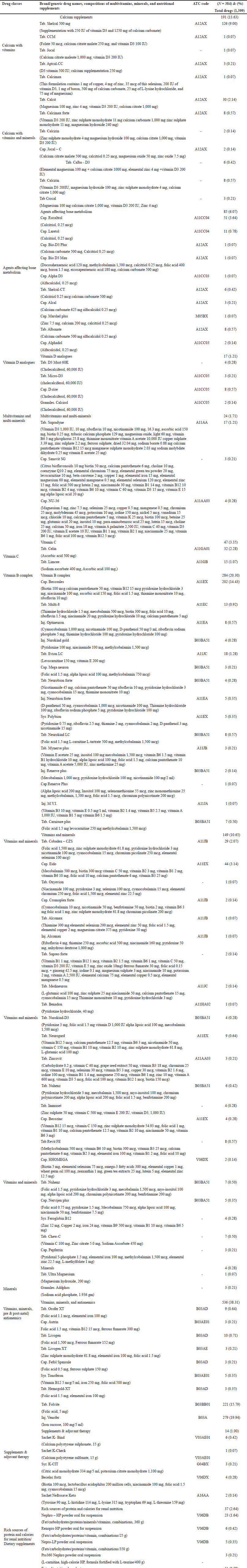
Vitamins, minerals
In vitamins and minerals, cap. Eido is the highly prescribed drug 44 (3.14%), followed by Tab. Cobadex – CZS 29 (2.07%), Tab. Neurogard (0.64%), Tab. Revit FE (0.57%), and Tab. Chew-C (0.50%). Figure 2 shows the percentage of vitamins and minerals used in hemodialysis patients.
 | Figure 2. Percentage of different vitamins and minerals medications used in hemodialysis patients. [Click here to view] |
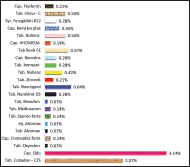
Vitamin B complex
In vitamin B complex Cap., Becosules is the highly prescribed drug (14.43%), followed by Inj. Nuro kind gold (1.28%), Tab. Multi-8 (0.92%), Inj. Optineuron (0.57%), Tab. Neurokind LC (0.57%), and Tab. Carnisure plus (0.50%). Figure 3 shows the percentage of B complex vitamin medications employed on patients receiving dialysis.
 | Figure 3. Percentage of different B complex vitamins employed on patients receiving dialysis. [Click here to view] |
Calcium with vitamins
In calcium with vitamins Tab., Shelcal 500 mg is the highly prescribed drug (9.00%), followed by Tab. Aptcal-CC (0.21%), Tab. CCM (0.07%), and Tab. Jocal (0.07%).
Calcium with vitamins and minerals
Tab. Calcit is the highly prescribed drug (2.14%), followed by Tab. Calcimax forte 0.57%, Tab. calcirin 0.57%, Tab. Calbo-D3 (0.42%), and Tab. Crocal (0.21%).
Agents affecting bone metabolism
Cap. Rocaltrol is the highly prescribed drug (2.14%), followed by Cap. Laretol 0.78%, Tab. Albonate (0.57%), Tab. Shelcal-CT (0.42%), and Cap. Alcal (0.21%).
Vitamin D analogues
Cap. D-rise is the highly prescribed drug (0.57%), followed by Tab. D3 60K (0.28%), Tab. Micro-D3 (0.21%), and Granules Calcirol (0.14%).
Multivitamins and multi-minerals
Tab. Supradyne is the highly prescribed drug (1.21%), followed by Cap. NU-36 (0.28%), and Cap. Sanovit SG (0.21%).
Vitamin C
Tab. Celin 2.28% is the highly prescribed drug (2.28%), followed by Tab. Limcee (1.07%).
Minerals
Granules. Addphos is the highly prescribed drug (0.21%), followed by Tab. Ultra magnesium (0.07%).
Rich sources of protein and calories for renal nutrition
Nephro–high protein (HP) powder is the highly prescribed drug (0.21%), followed by Renopro HP powder (0.42%), Nepro-low protein (LP) powder (0.35%), and Pro360 Nephro powder (0.21%).
Enteral/nutritional products
Tab. Renolog is the highly prescribed drug (0.35%), Tab. Alfalog (0.21%), Cap. Beneficiale (0.14%), and Biscuit threptin (0.07%).
Vitamins, minerals, and antianemics
Inj. Venofer (19.94%) and Tab. Folvite (15.79%) is the highly prescribed drug, followed by Tab. Livogen (0.71%), Tab. Orofer XT (0.64%), Syr. Tonoferon (0.35%), Tab. Hemogold-XT (0.35%), Tab. Livogen XT (0.21%), and Cap. Autrin (0.21%).
DISCUSSION
This study analyzes the prescribing patterns and usage of multivitamins, multi-minerals, and dietary supplements among HD patients in South Karnataka, India. The discussion below highlights vital findings, contextualizes them within the broader medical landscape, and provides insights into the clinical implications and potential directions for future research.
The study identified significant variability in prescribing patterns among healthcare providers treating HD patients. A key observation was the inconsistency in the types and combinations of supplements prescribed, highlighting the absence of standardized guidelines. This underscores the urgent need for evidence-based protocols and a unified consensus on supplement prescriptions, ensuring that supplementation aligns with the nutritional requirements and clinical needs of HD patients.
Patient perspectives offered valuable insights into the multifaceted aspects of supplement use. Many patients reported a positive outlook toward supplement use, citing improved energy levels, overall well-being, and symptom management. However, challenges related to financial constraints and the burden of adhering to a complex medication regimen were also voiced. Addressing these patient-centered concerns is vital for enhancing adherence and optimizing the potential benefits of supplementation.
Patients with longer HD durations and higher comorbidity burdens appeared more likely to receive supplements. This could reflect a clinical strategy to mitigate potential nutrient deficiencies due to prolonged HD and compromised renal function. However, caution must be exercised to avoid excessive supplementation, which could lead to adverse effects.
The study’s nutritional status and outcomes examination revealed intriguing correlations between supplement use and certain biomarkers. HD patients receiving specific supplements demonstrated improved levels of crucial nutrients, such as iron, vitamin D, and B vitamins. These findings suggest the potential efficacy of targeted supplement interventions in ameliorating nutritional deficiencies commonly observed in this patient population. Moreover, the study’s findings open avenues for investigating the association between improved nutrient status and enhanced clinical outcomes, including reduced morbidity and mortality.
Collaborative efforts involving clinical pharmacists, nephrologists, dietitians, and other healthcare professionals are pivotal for devising patient-specific supplement regimens that address nutritional deficiencies and avoid potential risks. Future studies should investigate the long-term impact of supplement use on patient outcomes, focusing on dietary improvements, quality of life, hospitalization rates, and mortality.
Several studies have analyzed the prescription patterns of vitamins and minerals in HD patients, revealing significant variability in supplementation practices. Abhisek et al. [26] reported that oral iron (90.43%) was the most frequently prescribed supplement, followed by folic acid (89.56%), parenteral iron (68.69%), multivitamins and minerals (66.95%), and vitamin B12 (64.34%). Similarly, Narayana Murthy et al. [27] found that 15% of patients received iron supplements (e.g., livogen, folic acid, and iron sucrose), while 11% were prescribed multivitamins, with methylcobalamin being the preferred choice for diabetic patients.
In a multidisciplinary review, Battistella et al. [28] reported 18,710 annual prescriptions for calcitriol, highlighting its widespread use. Bajait et al. [29] found that vitamins and minerals (24.71%) were the most commonly prescribed, followed by multivitamins and minerals (14.82%), iron (8.65%), folic acid (8.55%), calcitriol (5.60%), and vitamin D3 (4.27%). In addition, Oommen et al. [30] noted that 94.66% of medical facilities and 65.06% of tertiary hospitals recommended vitamin and mineral supplementation.
Tadvi et al. [31] reported iron (92.68%) as the most prescribed drug, followed by multivitamins and minerals (87.8%) and calcitriol (63.41%). Konduru et al. [32] found that calcium carbonate and vitamin D (66.66%) were highly prescribed, followed by multivitamins (43.80%). Pothen et al. [33] observed a 13% prescription rate for multivitamins, while Anil et al. [34] reported that among hematinics, parenteral iron (67%) was the most prescribed, followed by folic acid (26%) and oral iron (7%). Commonly prescribed multivitamin brands included Polybion (44%), Neurobion Forte (18%), Revit Fe (15%), and others [34].
Chundu et al. [35] noted that vitamin supplements were prescribed at 0.79%, while electrolytes were prescribed at 0.53%. Raja et al. [36] found that vitamin B complex was prescribed to 85% of patients at the initial visit and 78.5% at the last visit, with iron supplements given to 58% and 57% of patients, respectively. Other frequently recommended supplements included α-cholecalciferol, folic acid, and niacin [36].
Kumar et al. [37] reported that minerals and vitamins accounted for 32.03% of total prescriptions, while Chakraborty et al. [38] found that mineral and vitamin supplements were among the most commonly recommended medications (12.29%), followed by multivitamins and multi-minerals (5.56%), vitamin D3 (4.55%), and calcitriol (2.18%). Al-Ramahi et al. [39] highlighted that vitamin B complex and folate were the second most frequently prescribed medications, with 42.3% and 54.3% of patients receiving them in two study phases, while overall vitamin prescriptions increased from 71.0% in phase one to 78.0% in phase two [39].
The differences in MMHDS prescribing patterns across hospitals may stem from institutional policies, physician preferences, patient demographics, and resource availability. Some hospitals may adhere to international guidelines (e.g., KDIGO and KDOQI), while others rely on clinician discretion due to the lack of standardized national protocols in India. Variations in patient populations, dietary habits, comorbid conditions, and access to nutritional counseling may also influence prescribing trends. In addition, differences in healthcare infrastructure and availability of supplements can impact prescribing decisions, leading to inconsistencies in MMHDS use.
Excessive supplementation can lead to toxicity, metabolic imbalances, and adverse drug interactions. Iron overload from excessive supplementation increases the risk of oxidative stress, cardiovascular disease, and infections, while excessive vitamin B6 can cause neuropathy. Polypharmacy, common in dialysis patients due to multiple comorbidities—raises concerns about drug-supplement interactions, reduced adherence, and increased pill burden, potentially affecting treatment efficacy. To mitigate these risks, routine biochemical monitoring, patient-specific dosing, and evidence-based prescribing guidelines are essential for ensuring safe and effective MMHDS use in HD care.
Pharmacist-led interventions
For HD patients, pharmacists are essential in the proper selection, monitoring, and optimization of nutritional supplements. Because this population has complex nutritional needs and is at risk for deficiencies, pharmacists can offer evidence-based advice on supplement use that minimizes potential interactions and ensures compatibility with prescribed medications. Pharmacists assist in customizing supplementation regimens to meet the needs of each patient through medication therapy management, patient counseling, and coordination with nephrologists and dietitians. Pharmacists can also evaluate adherence, keep an eye out for side effects, and inform patients about the significance of eating the right foods, all of which can help HD patients’ general health.
Recommendations and future research
Recommendations
- MMHDS prescriptions should be individualized based on patient comorbidities, dialysis duration, and nutritional status.
- Findings support the need for regional guidelines for MMHDS use in Indian HD patients.
- Nephrologists should ensure MMHDS prescriptions align with clinical needs.
- Clinical pharmacists should monitor supplement interactions and adjust dosages accordingly.
- Dietitians should be involved in nutritional assessments to prevent over-supplementation.
- Hospitals should adopt standardized prescription protocols to minimize variability in MMHDS use.
Future research
Researchers can aim at longitudinal studies to evaluate the long-term effects of MMHDS on patient outcomes, including mortality and quality of life. Future research should explore the long-term impact of pharmacist-led interventions on nutritional supplement adherence, clinical outcomes, and quality of life in HD patients, as well as the development of standardized guidelines for pharmacist involvement in renal nutrition management.
Limitations of the study
The study was limited to three hospitals, which may not fully represent other regions in India; therefore, a larger multicentric study including hospitals from different states is needed to enhance generalizability. As a cross-sectional study, it captures prescribing patterns at a single time point, making it essential for future research to include long-term follow-up studies to assess the clinical outcomes of MMHDS use. In addition, patient-reported supplement adherence may be subject to recall bias, highlighting the need for future studies to incorporate objective biochemical assessments of nutrient levels for more accurate evaluations. Variability in prescribing practices across hospitals, influenced by institutional policies and prescriber preferences, further underscores the necessity of developing a standardized MMHDS prescription guideline to ensure consistency and optimize patient care.
CONCLUSION
The study’s findings highlight diverse prescribing practices, reflecting a cautious approach to supplement use in addressing nutritional deficiencies while minimizing unnecessary polypharmacy. Given the complex health challenges faced by HD patients, optimizing supplement prescriptions is crucial for improving treatment outcomes and preventing complications.
To enhance patient care, immediate steps should include the development of evidence-based guidelines specific to the Indian healthcare context. Standardized protocols will help streamline prescription practices, improve patient safety, and reduce variability across healthcare centers. In addition, collaborative strategies among nephrologists, clinical pharmacists, and dietitians should be implemented to ensure personalized and effective supplementation.
Hospitals and healthcare policymakers should prioritize training programs for healthcare providers to enhance awareness of rational supplement use in HD patients. Furthermore, future longitudinal studies should assess the long-term impact of supplementation on clinical outcomes, morbidity, and mortality.
This multi-centric study provides a strong foundation for improving MMHDS prescribing practices. By integrating evidence-based decision-making, patient-centered approaches, and ongoing guideline refinement, healthcare professionals can significantly enhance the quality of life and overall well-being of HD patients in South Karnataka and beyond.
Pharmacist-led interventions in the use of nutritional supplements for HD patients enhance patient safety, optimize therapy, and improve adherence, highlighting the critical role of pharmacists in multidisciplinary renal care teams.
LIST OF ABBREVIATIONS
ATC, anatomic therapeutic chemicals; CKD, chronic kidney disease; DOPPS, global dialysis outcomes, and practice patterns study; ESRD, end-stage renal disease; HD, hemodialysis; KDIGO, kidney disease improving global outcomes; KDOQI, kidney disease outcome quality initiative; MMHDS, multivitamins, multi-minerals, hematinics, and dietary supplements.
ACKNOWLEDGEMENTS
We express our sincere gratitude to the hemodialysis patients from the Renal Medicine Divisions at Father Muller Medical College Hospital Mangalore, Kasturba Medical College Hospital Manipal, and Dr. TMA Pai Hospital Udupi for their active involvement and continuous support throughout the data-gathering process.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This diligent investigation has been recognized and supported by the Indian Council of Medical Research (ICMR) under the Senior Research Fellowship (SRF) scheme, with the author awarded a fellowship (Reference No. 3/1/2 (4) Nephro/2021-NCD-II). It is important to emphasize that the funding agency had no role in report writing, data collection, study design, data interpretation, analysis, or the decision to submit the article for publication. The researchers thank the Indian Council of Medical Research, New Delhi, for financial assistance in carrying out this study and the Manipal Academy of Higher Education for providing the required studies.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Institutional Ethics Committee approved this study before its initiation. Everyone who participated provided cautious approval, ensuring anonymity, confidentiality, and true engagement. Throughout the study, strong security measures were used to preserve data and participant confidentiality. The study was duly approved by multiple Institutional Committees (IEC: 471/2019 from KMC Manipal, covering both TMA Pai Hospital Udupi and KMC Manipal; and FMIEC/CCM/294/2021 from FMMCH Mangalore). In addition, the study was registered with the Clinical Trial Registry-India (CTRI) under the registration number CTRI/2019/08/020874.
DATA AVAILABILITY
The researchers retain full access to the data and will make it available after your request is submitted.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Jankowska M, Szupryczynska N, Debska-Slizien A, Borek P, Kaczkan M, Rutkowski B, et al. Dietary intake of vitamins in different options of treatment in chronic kidney disease: is there a deficiency? Transplant Proc. 2016;48(5):1427–30. CrossRef
2. Steiber AL, Kopple JD. Vitamin status and needs for people with stages 3-5 chronic kidney disease. J Ren Nutr. 2011;21(5):355–68. CrossRef
3. Jakimowicz-Tylicka M, Chmielewski M, Kuzmiuk-Glembin I, Skonieczny P, Dijakiewicz G, Zdrojewska G, et al. Dietary supplement use among patients with chronic kidney disease. Acta Biochim Pol. 2018;65(2):319–24. CrossRef
4. Tucker BM, Safadi S, Friedman AN. Is routine multivitamin supplementation necessary in US chronic adult hemodialysis patients? A systematic review. J Ren Nutr. 2015;25(3):257–64. CrossRef
5. Makoff R. Vitamin replacement therapy in renal failure patients. Miner Electrolyte Metab. 1999;25(4–6):349–51. CrossRef
6. Waxman S, Corcino JJ, Herbert V. Drugs, toxins and dietary amino acids affecting vitamin B12 or folic acid absorption or utilization. Am J Med. 1970;48(5):599–608. CrossRef
7. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. CrossRef
8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130. CrossRef
9. Goldsmith DJ, Covic A, Fouque D, Locatelli F, Olgaard K, Rodriguez M, et al. Endorsement of the kidney disease improving global outcomes (KDIGO) chronic kidney disease-mineral and bone disorder (CKD-MBD) guidelines: a european renal best practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25(12):3823–31. CrossRef
10. Fissell RB, Bragg-Gresham JL, Gillespie BW, Goodkin DA, Bommer J, Saito A, et al. International variation in vitamin prescription and association with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(2):293–9. CrossRef
11. Bossola M, Di Stasio E, Viola A, Leo A, Carlomagno G, Monteburini T, et al. Dietary intake of trace elements, minerals, and vitamins of patients on chronic hemodialysis. Int Urol Nephrol. 2014;46(4):809–15. CrossRef
12. Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: an update. Kidney Int. 2006;70(3):417–22. CrossRef
13. Kalantar-Zadeh K, Kopple JD. Trace elements and vitamins in maintenance dialysis patients. Adv Ren Replace Ther. 2003;10(3):170–82. CrossRef
14. Marumo F, Kamata K, Okubo M. Deranged concentrations of water-soluble vitamins in the blood of undialyzed and dialyzed patients with chronic renal failure. Int J Artif Organs. 1986;9(1):17–24. CrossRef
15. Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;7:25. CrossRef
16. Kasama R, Koch T, Canals-Navas C, Pitone JM. Vitamin B6 and hemodialysis: the impact of high-flux/high-efficiency dialysis and review of the literature. Am J Kidney Dis. 1996;27(5):680–6. CrossRef
17. Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–55. CrossRef
18. Moustapha A, Naso A, Nahlawi M, Gupta A, Arheart KL, Jacobsen DW, et al. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation. 1998;97(2):138–41. CrossRef
19. Perna AF, Castaldo P, Ingrosso D, De Santo NG. Homocysteine, a new cardiovascular risk factor, is also a potent uremic toxin. J Nephrol. 1999;12(4):230–40.
20. Robinson K, Gupta A, Dennis V, Arheart K, Chaudhary D, Green R, et al. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation. 1996;94(11):2743–8. CrossRef
21. Sunder-Plassmann G, Födinger M, Buchmayer H, Papagiannopoulos M, Wojcik J, Kletzmayr J, et al. Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. J Am Soc Nephrol. 2000;11(6):1106–16. CrossRef
22. Tremblay R, Bonnardeaux A, Geadah D, Busque L, Lebrun M, Ouimet D, et al. Hyperhomocysteinemia in hemodialysis patients: effects of 12-month supplementation with hydrosoluble vitamins. Kidney Int. 2000;58(2):851–8. CrossRef
23. Elian KM, Hoffer LJ. Hydroxocobalamin reduces hyperhomocysteinemia in end-stage renal disease. Metabolism. 2002;51(7):881–6. CrossRef
24. Kaplan LN, Mamer OA, Hoffer LJ. Parenteral vitamin B12 reduces hyperhomocysteinemia in end-stage renal disease. Clin Invest Med. 2001;24(1):5–11.
25. Billion S, Tribout B, Cadet E, Queinnec C, Rochette J, Wheatley P, et al. Hyperhomocysteinaemia, folate, and vitamin B12 in unsupplemented haemodialysis patients: effect of oral therapy with folic acid and vitamin B12. Nephrol Dial Transplant. 2002;17(3):455–61. CrossRef
26. Abhisek PA, Panda R, Samal R, Mohapatra N, Mohanty S. Drug utilisation pattern and adverse events in patients with chronic kidney disease undergoing maintenance haemodialysis at a tertiary care hospital of Odisha. JCDR. 2017;11(10):FC11–6. CrossRef
27. Narayana Murthy BV, Satyanarayana V. Prescribing pattern of drugs in chronic kidney disease patients on hemodialysis at a tertiary care hospital. Int J Basic Clin Pharmacol. 2017;6(4):928–32. CrossRef
28. Battistella M, Jandoc R, Ng JY, McArthur E, Garg AX. A province-wide, cross-sectional study of demographics and medication use of patients in hemodialysis units across Ontario. Can J Kidney Health Dis. 2018;5:1–10. CrossRef
29. Bajait CS, Pimpalkhute SA, Sontakke SD, Jaiswal KM, Dawri AV. Prescribing pattern of medicines in chronic kidney disease with emphasis on phosphate binders. Indian J Pharmacol. 2014;46(1):35–9. CrossRef
30. Oommen JM, Nerurkar DP, Sajith M, Jawale S, Ambike S. Prescription pattern of chronic kidney disease patients undergoing hemodialysis in tertiary and private hospital. J Young Pharm 2019;11(2):202–6. CrossRef
31. Tadvi NA, Hussain S. Analysis of prescription pattern in patients on maintenance hemodialysis. Indian J Pharm Pharmacol 2020;7(2):125–9. CrossRef
32. Konduru SS, Kumar JN, Siva KL, Girija K. Assessment of drug use patterns and quality of life in hemodialysis patients. EJPMR. 2018;5(6):628–37.
33. Pothen C, Baby B, Ashokan A, Chacko C, Shenoy P, Nandakumar UP. Drug usage pattern in chronic kidney disease patients undergoing maintenance hemodialysis. Res J Pharm and Tech 2019;12(10):5024–8. CrossRef
34. Anil A, Joseph J, Varghese MA, Chacko S, Abraham E. Assessment of the prescription pattern of drugs used in chronic kidney disease patients undergoing haemodialysis in a tertiary care hospital. IAJPR. 2020;10(4):681–8.
35. Chundu S, Kaki H, Karyamsetty D, Midathana S, Krishna R, Kommanaboina H, et al. Prescribing patterns of medicines and medication adherence in CKD patients on maintenance of hemodialysis. IJPPR. 2018;12(3):251–64.
36. Raja V, Palatty PL, Sajan S, Abraham S, George T, D’Silva P, et al. Drug-prescribing pattern in chronic kidney disease patients on maintenance haemodialysis and audit of cardiovascular complications in them: pilot study from a tertiary care hospital. Hamdan Med J. 2020;13(2):88–92. CrossRef
37. Kumar CR, Jyotsna B, Jaya D. A descriptive analysis of prescribing patterns of drugs in chronic kidney disease patients on maintenance hemodialysis. JMSCR. 2019;7(5):785–92. CrossRef
38. Chakraborty S, Ghosh S, Banerjea A, De RR, Hazra A, Mandal SK. Prescribing patterns of medicines in chronic kidney disease patients on maintenance hemodialysis. Indian J Pharmacol. 2016;48(5):586–90. CrossRef
39. Al-Ramahi R. Medication prescribing patterns among chronic kidney disease patients in a hospital in Malaysia. Saudi J Kidney Dis Transpl. 2012;23(2):403–8. CrossRef