INTRODUCTION
Cisplatin, a platinum-based chemotherapeutic drug, is widely used for the treatment of various types of cancers, such as lung, cervical, gastric, and ovarian cancers, as well as breast cancer [1]. It is a standard treatment for triple-negative breast cancer (TNBC), used either alone or in combination with other therapies [2]. The molecular mechanism of cisplatin involves crosslinking purine bases in DNA to create platinum–DNA adducts. These adducts block DNA repair, causing damage and triggering cancer cell death [3]. In TNBC, chemo-resistance poses a significant challenge. Continuous exposure to cisplatin can cause breast cancer cells to develop resistance, leading to the emergence of cisplatin-resistant cells [4]. Several molecular mechanisms have been identified in cisplatin resistance, including abnormal gene expression, reduced intracellular drug accumulation, increased drug efflux, enhanced DNA repair, alterations in cellular regulatory pathways, stimulation of abnormal blood and lymphatic vessel formation, and promotion of tumor cell growth, progression, and metastasis, ultimately contributing to the chemotherapeutic failure [5,6].
The overexpression of multidrug resistance (MDR) proteins, or ATP-binding cassette (ABC) transporters, including ABCB1 (P-glycoprotein), ABCC1 multidrug resistance-associated protein 1, (MRP1), and ABCC2 (MRP2), and ABCG2 breast cancer resistance protein, (BCRP) in breast cancer cells is a leading cause of cisplatin resistance. These proteins were overexpressed in cancer cell membranes, facilitating the efflux of cisplatin from the intracellular space to the extracellular environment, which results in decreased cisplatin concentrations within the cells [7]. As a result, high-dose chemotherapy has been employed, but it provides limited therapeutic efficacy and causes severe systemic toxicity [8]. These highlight the urgent need for single drugs or combination therapies with lower toxicity to combat MDR in cisplatin-resistant breast cancer treatment effectively.
α-Mangostin (AMG) is a natural xanthone compound isolated from the pericarps of mangosteen (Garcinia mangostana L). This compound exhibits potent antioxidant, antidiabetic, anti-inflammatory, antimicrobial, and antiparasitic activities [9]. In addition, AMG shows anti-tumor activity in both in vitro and in vivo studies by targeting several cellular factors via many mechanisms [10]. It has been shown to inhibit cancer cell progression through induction of cell cycle arrest, tumor cell apoptosis, and cancer cell autophagy, as well as suppression of angiogenesis, invasion, and metastasis [11]. A previous study has shown that AMG synergizes the effect of a low dose of 5-fluorouracil (5-FU) in breast cancer cells by inhibiting cell proliferation [12]. The combination treatment of doxorubicin and ethanol extract of mangosteen (Garcinia mangostana) pericarp showed a more significant reduction of MCF-7 cell viability than extract or doxorubicin treatment alone [13]. In an in vivo study, the combined therapy of AMG and cis-diamminedichloroplatinum (II) (CDDP) significantly reduced average tumor volume. It prevented nephrotoxicity more effectively than either CDDP or AMG treatment alone [14]. Moreover, the cytotoxic effect of AMG has been reported that it was selective in cancer cells but did not in normal cells by acting synergistically with chemotherapeutic agents, including doxorubicin and 5-FU [14]. These findings indicated that AMG could be a potential neoadjuvant cancer treatment agent. However, no reports have demonstrated the effect of AMG on chemotherapeutic-resistant breast cancer to date.
Here, the cisplatin-resistant breast cancer cell lines (CIS/MCF-7 and CIS/MDA-MB-231)-derived from parental cells were established and characterized. These cell line models were used to investigate the synergistic effects of AMG with cisplatin. This study demonstrated that AMG could improve cisplatin efficiency in cisplatin-resistant breast cancers and its mechanism, possibly via targeting MRP2 induction.
MATERIALS AND METHODS
Cell lines and AMG
Breast cancer cell lines, namely MCF-7 and MDA-MB-231 obtained from the American Type Culture Collection, were cultured in DMEM/F12 (Gibco, Thermo Fisher, USA) containing 10% fetal bovine serum (Gibco), antibiotic-antimycotic solution (Gibco, Thermo Fisher, USA) at 37°C, 5% CO2 in a humidified atmosphere. The cells were sub-cultured every 3–4 days following the standard trypsinization procedure. AMG was purchased from Sigma Chemical Corporation (Sigma-Aldrich, MA, USA). The compound was dissolved in DMSO and kept at −70°C.
Establishment of cisplatin-resistant cells
The cisplatin-resistant cells were established and isolated by the stepwise selection with increasing concentrations of cisplatin. Briefly, 2 × 105 cells/well (parental MCF-7 or MDA-MB-231) were plated in a 6-well plate and cultured for 24 hours. Initially, cells were grown in a culture media containing 8 μM cisplatin (Sigma-Aldrich, St. Louis, MO, USA). When the cells could grow and reach the appropriate confluence at a particular concentration, the cells were sub-cultured, and the concentration of the previous cisplatin concentration was doubled for stepwise selection of resistant cells. The final concentration was reached at the cisplatin concentration of 24 μM. At the time of the experiment, the CIS/MCF-7 and CIS/MDA-MB-231 cells were maintained in the cisplatin-free medium for at least 2 days before the experiments.
Cytotoxicity assay
Cell line at density 5 × 103 cells/well was plated in a 96-well plate for 24 hours. Various concentrations of the test compounds were added to a 96-well plate and incubated for 72 hours. DMSO was used as the diluent control. Cell viability was determined using PrestoBlue® cell viability assay (Invitrogen, Carlsbad, USA) based on the reducing capacity of the living cells. The absorbance at OD570 was measured, whereas OD600 was used as a reference. The changes in absorbance were used to calculate the percentage of cell viability relative to the untreated control (set as 100%). The percentages of cell viability and half-maximal inhibitory concentration (IC50) were analyzed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA).
Real-time RT-PCR
Total RNA was isolated from parental and resistant cells using TRI Reagent® (MRC Inc., US). Briefly, cDNA synthesis was performed using 1 μg of total RNA as a template, employing the ReverTra Ace™ qPCR RT master mix with gDNA remover (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The cDNA template was added to the PCR reaction containing the mixture of specific primer and Luna® Universal qPCR Master Mix (New England Biolabs, Ipswich, Ma, USA). Fold changes in gene expression level were calculated by the 2-ΔΔCT method using a housekeeping gene, GAPDH, to normalized gene expression. The relative gene expression of the treated group was compared with that of the untreated control group. The primer sequences are shown in Table 1.
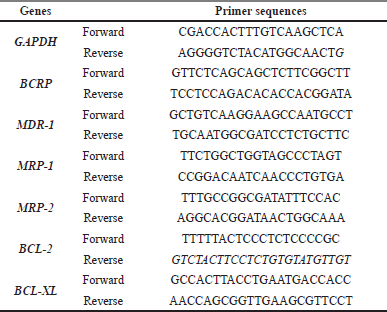 | Table 1. List of primer pair sequences. [Click here to view] |
Molecular docking
The three-dimensional structures of MRP2 were prepared via homology modeling using the Swiss Model (https://swissmodel.expasy.org/) [15] in which the cryo-EM structure of wild-type bovine MRP1 (PDB entry: 6UY0, chain A) was used as the template [16]. The protein receptor was prepared by removing water and other atoms, and then adding a polar hydrogen group using Biovia Discovery studio client 2020 [Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, Release 2017, San Diego: Dassault Systèmes, 2016]. The ligand structure of AMG was derived from MolView (https://molview.org/) and optimized for molecular docking using the UCSF Chimera program [17]. The interaction of AMG and MRP2 was investigated using the SwissDock web-based server (http://www.swissdock.ch/) [18]. The binding modes with the most favorable energies were clustered. The docking analyses were performed by Biovia Discovery Studio client 2020 to demonstrate the interaction in a 2D diagram (Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, Release 2017, San Diego: Dassault Systèmes, 2016).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5. All data from at least three independent experiments were expressed as mean ± standard error of the mean. Student’s t-test was used to compare the results of the control and investigated groups, and a two-way ANOVA with a post hoc test was used to compare multiple treatment conditions. A p-value below 0.05 was considered to be statistically significant.
RESULTS
Characteristics of resistant-breast cancer cell lines
Cisplatin-resistant breast cancer, including CIS/MCF-7 and CIS/MDA-MB-231, were established. The different biological features of cell morphology were observed under the inverted microscope. Similarly, CIS/MCF-7 and CIS/MDA-MB-231 cells had enlarged morphologies, and more prominent cytoplasm contained multiple nuclei than indicated parental cells (Fig. 1A and 1D). The cell viabilities of MCF-7, MDA-MB-231, CIS/MCF-7, and CIS/MDA-MB-231 after 72-hour treatment of the cisplatin at various concentrations were determined by using PrestoBlue™ Cell Viability Reagent. The results revealed that cisplatin-resistant breast cancer (CIS/MCF-7 and CIS/MDA-MB-231) resisted the cisplatin treatment at 100 μM, whereas parental cell lines were sensitive at the same concentrations (Fig. 1B, 1C, 1E, and 1F). The IC50 of cisplatin in CIS/MCF-7 and CIS/MDA-MB-231 was relatively higher than that of parental cells, with the IC50 of 63.87 ± 1.40 and 173.7 ± 1.22 μM, respectively. Compared with the parental cells, the resistance indices of CIS/MCF-7 and CIS/MDA-MB-231 to cisplatin were 1.99-fold and 6.03-fold, respectively (Table 2). These data suggested that the cisplatin-resistant cells were successfully established.
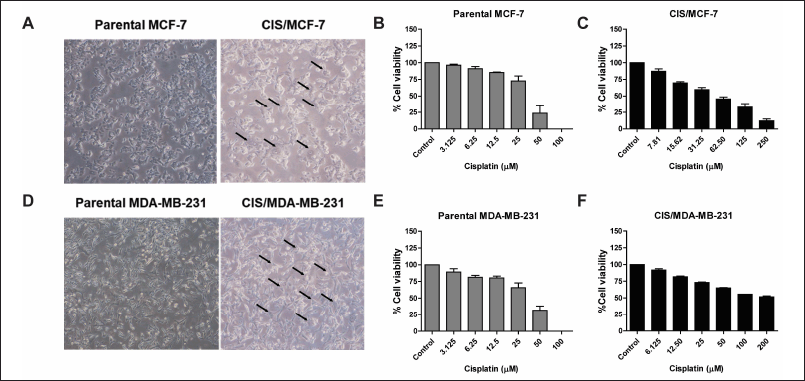 | Figure 1. Establishment of cisplatin-resistant breast cancer cell lines. The morphology alteration of cisplatin-resistant breast cancer cell lines (CIS/MCF-7 and CIS/MDA-MB-231) compared with the parental cells under an inverted microscope. The arrow lines indicate the morphological changes in the cisplatin-resistant breast cancer cell lines (A and D). The parental and cisplatin-resistant breast cancer cell lines were treated with cisplatin at various concentrations for 72 hours. The percentages of cell viability were determined using PrestoBlue™ Cell Viability Reagent. Results are presented as mean ± SEM (error bars) from three independent experiments (n = 3). (B, C, E, and F). [Click here to view] |
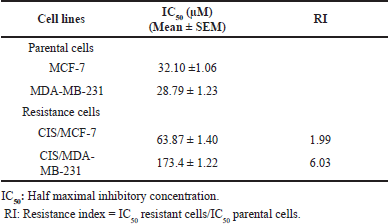 | Table 2. IC50 values and resistance index (RI) of cisplatin for the parental and cisplatin-resistant breast cancer cell lines. [Click here to view] |
The expressions of chemo-resistance-associated genes in resistant breast cancer cells
The modulation in expression levels of chemo-resistance?associated genes, including BCRP, MDR-1, MRP-1, MRP-2, BCL-2, and BCL-XL, has been reported to be associated with acquired chemotherapy resistance in cancers. We, therefore, investigated the expression of these genes in resistant-breast cancer cells compared with those parental cells using real-time PCR. In CIS/MCF-7, the expression of BCRP (fold change; 2.60) and BCL-2 (fold change; 2.54) were up-regulated but did not significantly differ compared with the MCF-7 (Fig. 2A). Interestingly, the MRP2 (fold-change; 4.0) gene increased dramatically in CIS/MDA-MB-231 (Fig. 2B). This result suggested that MRP2 might be the potential target for cancer therapy.
 | Figure 2. The expression level of chemo- resistance-associated genes in cisplatin-resistant breast cancer cell lines. Alterations in the expression level of chemo-resistance and apoptosis-associated genes in cisplatin-resistant breast cancer cells, including CIS/MCF-7 (A) and CIS/MDA-MB-231 (B) relative to parental cells, were determined by real-time PCR. Results are presented as mean ± SEM (error bars) from three independent experiments (n = 3). *p < 0.05 indicated the data significant difference between groups was analyzed by Student’s t-test. [Click here to view] |
Enhancement of cisplatin in resistant breast cancer cells by AMG
Several study reports have shown that AMG could be a potential therapeutic by reversing the function of the multidrug-resistant protein and enhancing the activity of chemotherapeutic drugs. CIS/MCF-7 and CIS/MDA-MB-231 were treated with various concentrations of AMG alone or a combination of 30 μM cisplatin and multiple concentrations of AMG for 72 hours. The percentages of cell viability were determined using PrestoBlue™ Cell Viability Reagent. The results revealed that resistant breast cancer cells treated with 30 μM cisplatin did not affect the cell viability of these cell lines. For CIS/MCF-7, the combination treatment of 30 μM cisplatin with AMG at a concentration of 6.25 and 12.5 μM showed a strongly suppressed cell viability compared with that AMG treatment alone (p < 0.5). The cell viability of the combination of 30 μM cisplatin and 12.5 μM AMG was 51.87% ± 5.01% lower than the 12.5 μM AMG treatment alone was 74.58% ± 9.40 % (Fig. 3B). Similarly, the 30-μM cisplatin combined with 1.56, 3.13, 6.25, 12.5, and 25-μM AMG affected cell viabilities of CIS/MDA-MB-231 more than AMG alone (p < 0.0001). The cell viability of the condition combination of 30 μM cisplatin and 6.25 μM AMG was 84.74% ± 3.09% lower than 6.25 μM AMG treatment alone (111.70% ± 3.36%) (Fig. 3C). These results indicated that AMG could enhance the efficacy of cisplatin in resistant breast cancer cells.
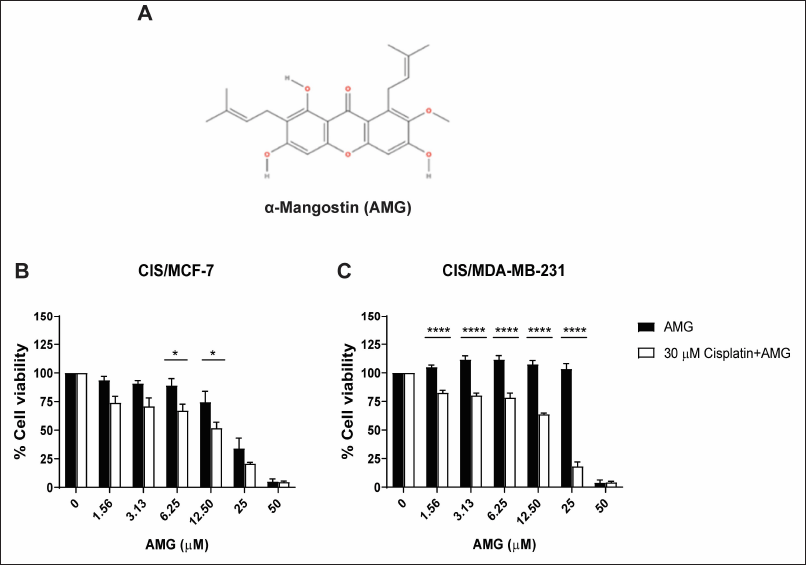 | Figure 3. Enhancement of cisplatin in cisplatin-resistant breast cancer cell lines by AMG treatment. The chemical structure of AMG (A). Cells were treated with 30 μM cisplatin alone or combined with cisplatin and various concentrations of AMG for 72 hours in CIS/MCF- 7 and CIS/MDA-MB- 231 (B and C) . The percentages of cell viability of cisplatin-resistant breast cancer cell lines were determined using PrestoBlue™ Cell Viability Reagent. Results are presented as mean ± SEM (error bars) from four independent experiments (n = 4). ****p < 0.0001 and *p < 0.05 were analyzed by two-way ANOVA. [Click here to view] |
The interaction of AMG and MRP2
A molecular docking study demonstrated the interaction of AMG and MRP2. The three-dimensional structure of MRP was predicted using homology modeling via the SwissModel web-based program AMG, where the currently available bovine MRP1 cryo-EM structure was used as a template (PDB entry: 6UY0) (Fig. 4A). The docking simulation demonstrated that AMG favorably interacted with the transmembrane domain (TMD) of MRP1 (Fig. 4A). Interestingly, 41 of 42 docking clusters were located at the nearby position of TMD (supplementary data). The docking pose with the most favorable docking score of −8.23 is stabilized by hydrogen bonding with SER481 and hydrophobic interactions with surrounding hydrophobic residues LEU441, Val445, Ile448, Ile474, Ala478, Leu596, and Ile 600 (and C). These results suggested that AMG can bind significantly to MRP protein, sensitizing cancer sensitivity to cisplatin.
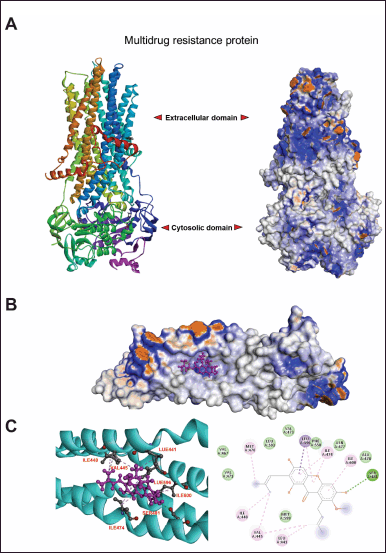 | Figure 4. The interaction between α- mangostin with target protein, MRP, by molecular docking analysis. AMG structure was prepared by using MolView web- based server (https://molview.org/) where the MRP was remodelled using bovine MRP1 cryo-EM structure as a template (PDB entry: 6UY0) via Swiss-Model program (https://swissmodel.expasy.org/) (A). To illustrate the interaction of AMG and MRP2, molecular docking was performed via SwissDock ( http://www.swissdock. ch/). The favourable docking poses were clustered. The details of the ligand-receptor binding mode were analysed (B) , and the interaction of amino acids of MRP2, which is involved in binding stabilisation (C), was demonstrated. [Click here to view] |
DISCUSSION
Cisplatin is a common chemotherapeutic drug that is used as a single or combination treatment for patients with breast cancer [1]. Long-term exposure to chemotherapeutic drugs of cancer cells leads to the development of chemotherapy resistance, which is a serious problem for cancer treatment [2,19,20]. In breast cancer, several MDR-ABC transporters, including ABCB1, ABCG2, ABCC1, and ABCC2, were found to be overexpressed on the cell membrane of resistant cancer cells that can efflux cisplatin from the intercellular to the extracellular and subsequently reduces the intracellular accumulation of the drugs [7,21,22]. Several experimental studies have reported that natural products can reverse the effects of chemotherapy drugs on breast cancer [23]. Therefore, this research aims to use AMG as a co-chemotherapeutic agent combined with a low dose of cisplatin to overcome chemotherapy resistance in breast cancer.
First, breast cancer cells (MCF-7 and MDA-MB-231) were exposed to cisplatin with a relatively low dose for 3 months to establish two cisplatin-resistant cell lines, namely, CIS/ MCF-7 and CIS/MDA-MB-231. The morphologies of the two cell lines under the inverted microscope showed the differences in the size of resistant cells, which were more prominent than the parental cells. These cells had resistance indexes higher than the parental cells, which indicated the ability to resist the cisplatin chemotherapeutic drug. In addition, the expression of the MRP2 gene, the multidrug resistance gene, was dramatically increased in CIS/MDA-MB-231 compared with the control cells. MRP expression modulation response to chemotherapeutic treatment resistance has been reported in other acquired resistant cells. Previously, Kars et al. [24] reported that the upregulation of MDR1 gene expression could be found in docetaxel, paclitaxel, vincristine, and doxorubicin-resistant MCF-7 cells [24]. Furthermore, the tamoxifen-resistant MCF-7 cell line was established by exposing 4-hydroxytamoxifen for 9 months, which increased MRP2 expression greater than control MCF-7 cells [25]. In the model of triple-negative breast cancer, the MDR1 and CYP2C8 genes were up-regulated expressions that involved the potential resistance mechanisms in the paclitaxel-resistant MDA-MB-231 cells [26]. In the other cancers, the oxaliplatin-resistant ovarian cancer cell line showed the alteration of N-linked glycosylation and high expression of MRP1 and MRP4 that involved cell resistance to cisplatin and oxaliplatin [27]. These reports indicated that multidrug resistance-related molecules, including MDR1, MRP2, and MRP4, are associated with the chemo-resistance of cancer cells and may serve as potential target molecules to improve the efficacy of chemotherapy in cancer treatment.
Various natural products have been widely investigated as natural anti-cancer agents, which have served as potential candidates for overcoming the problem of chemo-resistance in cancer cells. AMG is an active compound primarily found in the pericarp of mangosteen. Many studies have shown the biological activities and pharmacological properties of AMG, such as anti-cancer, anti-inflammation, antioxidant, and anti-microbial activities [11]. However, none of the reports demonstrating the effect of AMG in chemotherapeutic-resistant breast cancer has been reported yet. In this study, we reported the effect of AMG as a potent co-adjuvant for cisplatin treatment in cisplatin-resistant breast cancer. Our results revealed that the various concentrations of AMG combined with 30 μM cisplatin treatment showed a higher inhibitory effect on the cell viability of cisplatin-resistant cell lines, CIS/MCF-7 and CIS/MDA-MB-231. In addition, molecular docking results showed a favorable interaction with the TMD of MRP that was up-regulated in the cisplatin-resistant breast cancer cell line. AMG may bind and attenuate the MRP function, making the resistant cell sensitive to cisplatin treatment. Interestingly, our result is in accordance with the study of Wu et al. [28], which showed that AMG modulated BCRP in the ABCG2-overexpressed cell line for reversing MDR function by direct interaction [28]. The co-treatment of AMG with the low concentration of 5-FU showed a synergic effect on the inhibition of breast cancer cell growth, reducing chemotherapy-derived adverse effects [12]. In addition, the combination of sorafenib, a kinase inhibitor, and AMG shows potential as a treatment for advanced HCC in patients who are not responsive to sorafenib therapy, potentially through the mTOR and MAPK pathways [29]. In the cervical cancer mice model, tumor volume was significantly reduced. At the same time, the cytotoxicity of cisplatin was increased dramatically without nephrotoxicity in pre-administration with AMG before cisplatin treatment [14]. Additionally, AMG decreased the stemness and proliferation of cervical cancer stem-like cells while enhancing cisplatin cytotoxicity, likely through mitochondrial apoptosis activation [30]. Similarly, in pancreatic cancer, AMG suppresses epithelial-to-mesenchymal transition and cancer stem cell (CSC) markers by inhibiting Gli signaling and its target genes [31]. These findings highlight the ability of AMG to target CSCs through multiple mechanisms, supporting its potential as a therapeutic agent for cervical and pancreatic cancers. However, despite its promising anti-cancer properties, the clinical application of AMG remains challenging due to poor solubility, low bioavailability, and rapid metabolism. To overcome these limitations, nanoparticle-based drug delivery systems have been explored, offering improvements in solubility, cellular uptake, controlled drug release, and targeted delivery while minimizing off-target effects [32].
Natural compounds could serve as potential candidates for the treatment of resistant cancers. Other natural products, besides AMG, showed high efficacy in modulating multidrug resistance proteins in various cancer cells. For example, myricetin, a flavonoid substance, enhanced the cancer cells’ sensitivity to vincristine chemotherapeutic drug in MRP2-transfected MDCKII cells by modulating the function of MRP2 [33]. In addition, tetrahydrocurcumin, a major metabolite of curcumin, is a potential chemosensitizer that could inhibit three ABC drug transporters, including P-glycoprotein, MRP1, and mitoxantrone resistance protein in a multidrug-resistant MCF-7 cell line [34]. Doxorubicin-resistant MCF-7 and MDA-MB-231 cells showed the ABCB4 or MDR3 overexpression that was a target for curcumin to overcome the chemotherapy resistance effect by inhibiting the activity of ABCB4 ATPase of this protein [35]. Moreover, the molecular docking experiment revealed that polyoxypregnanes isolated from M. tenacissima could bind into the cavity of P-gp, leading to inhibiting the activities of P-gp transporter and then increasing the concentrations of the anti-cancer drug in intracellular cancer cells [36]. In addition, the high concentration of mangiferin could be used as a chemosensitizer for doxorubicin therapy. It might be due to the inhibitory effects of mangiferin on P-glycoprotein expression [37]. Accordingly, many natural compounds have emerged as potential alternatives for cancer treatment.
To address the limitations of this study, further research is needed to investigate the interaction between AMG and MRP2, confirming whether AMG directly inhibits cisplatin efflux. Additionally, the underlying mechanisms of AMG induce cancer cell death should be explored as well as its role in drug resistance modulation. These investigations will help clarify the role of AMG in a cisplatin-resistant breast cancer model.