INTRODUCTION
Kidney transplantation is the treatment of choice for patients with end-stage kidney disease, in which healthy kidneys are transplanted from a donor to a recipient. According to the Global Observatory on Donation and Transplantation, in 2019, a total of 102,090 kidney transplants were performed worldwide [1].
Currently, approximately 7,500 kidney transplants are performed annually in India, among which 90% are from living donors and 10% are from deceased donors [2]. After an organ transplant surgery, there is a need for the administration of immunosuppressants for life, to prevent graft rejection. The currently accepted first-line immunosuppressive dosage regimen in renal transplant patients is tacrolimus combined with mycophenolate with or without adding corticosteroids [3].
Tacrolimus, an Immunosuppressant, belongs to the calcineurin inhibitor group. It is considered a cornerstone in drug therapy maintenance after kidney transplantation [4,5]. By forming a complex along with an Immunophilin FK506-binding protein, it results in immunosuppression [6]. The pharmacokinetic characteristics of tacrolimus are well characterized. After tacrolimus enters systemic circulation, it binds to erythrocytes (about 99%) [4], resulting in reduced bioavailability in patients awaiting kidney transplantation [7]. Tacrolimus is primarily metabolized by the CYP3A enzyme system. Tacrolimus metabolites are mainly excreted by bile (Approximately 95%) and only 2% by urinary excretion [4]. Tacrolimus trough ranges from about 4 to 15 ng/ml for a kidney transplant recipient, exhibiting a narrow therapeutic index [8]. As tacrolimus shows significant inter-/intra-patient variation in its pharmacokinetics, narrow therapeutic index, and poor bioavailability(oral), therapy with tacrolimus is challenging. Minor variations in its exposure, result in a reduction of immunosuppression or drug toxicity along with potentially fatal consequences [9].
Underdosing may result in insufficient immunosuppression, thereby increasing the risk of organ rejection, whereas over-dosing can cause toxic side effects, such as nephrotoxicity, neurotoxicity, and gastrointestinal disturbances. Chronic toxicity can also lead to long-term complications, including an increased risk of infections and malignancies. Hence, it is extremely important to monitor tacrolimus levels on a routine basis [10].
Tacrolimus levels can be measured using immunochemical assays. One of the most popular methods for this is liquid chromatography–tandem mass spectrometry (LC-MS/MS) and chemiluminescent microparticle immunoassay. Currently, 53% of therapeutic drug monitoring (TDM) laboratories use LC-MS/MS to monitor tacrolimus concentrations. Whole blood is the most suitable matrix for measuring tacrolimus because of its extensive distribution into red blood cells (erythrocytes) [11].
Polymorphisms in gene CYP3A5 explain 40% to 50% variability in the dose requirement of tacrolimus [4]. The therapeutic drug concentration and efficacy of tacrolimus in transplantation can be influenced by various factors such as the type of organ transplanted, the time since the transplant, the patient’s age, the method used to monitor drug levels, the type of sampling (trough, area under curve, or limited sample strategy), the patient’s race, and any concurrent immunosuppression [10]. The genetic polymorphism assessment for CYP3A5 enzyme is not routinely performed in many clinical settings in our country. Dosage adjustments are based on the clinical judgment of the treating physician along with the drug-level estimates.
The aim of this study was to understand the dosing protocol of tacrolimus in renal transplant patients and to assess the relation between dosage and concentration levels in the absence of information on CYP3A5 genetic polymorphism in patients in a tertiary care setup.
METHODOLOGY
Study participants and study design
This study was planned as a retrospective design by accessing data of relevant patients from the medical records department. Data from patients who underwent kidney transplantation and visited the study tertiary care setting for follow-ups between January 2019 to December 2021 were included in this study. Case files of all patients who underwent kidney transplantation surgery and were treated with tacrolimus were included in the study, whereas patient files with insufficient data were excluded. Ethical approval from the Institutional Ethical Committee of Kasturba Hospital, Manipal, was obtained before the start of data collection (IEC2: 386/2022). This study was a retrospective observational study.
Data collection
Data of 90 patients, including demographic details (age, weight, and height), transplantation details (date of transplant), immunosuppressant drugs given to patients, and their medications, were collected from the in-patient files from the medical records department and out-patient files from the hospital electronic medical records.
Blood sample timings were documented from the case records and tacrolimus blood levels reported from the external laboratories were also collected from the case files. Information like doses of tacrolimus with corresponding tacrolimus levels for up to 10 visits and other parameters such as serum creatinine and hematocrit values, biopsy dates, and their results were also collected. The information on graft function and other comorbidities was insufficient; therefore, we excluded this information from this study.
Data analysis
The collected data were analyzed using the R software package version 4.2.1 [12], to understand the relationship between the tacrolimus doses and levels. No statistical method was used in this study and no tests for significance were included. Only descriptive statistics were used to explain the data. The data were classified into two categories–patients whose tacrolimus levels were monitored right followed from the transplantation date (FFT) and those whose levels were monitored later followed from the transplantation date (nFFT).
In the current study, patient files were screened, and it was noted that the data were available from the transplant time for many patients (FFT).
RESULTS
Case records of 108 renal transplant patients were available during the time-period, and records of 90 were included according to the inclusion and exclusion criteria. The included data of patients (n = 90) were categorized into two groups: patients who were FFT (n = 62) and patients who were followed later nFFT(n = 28). The patient data selection criteria are shown in Figure 1. There were 79 males and 11 females, with a mean age of 41 years and a mean body weight of 63.4 kg. The demographic and clinical characteristics of the patients are presented in Table 1.
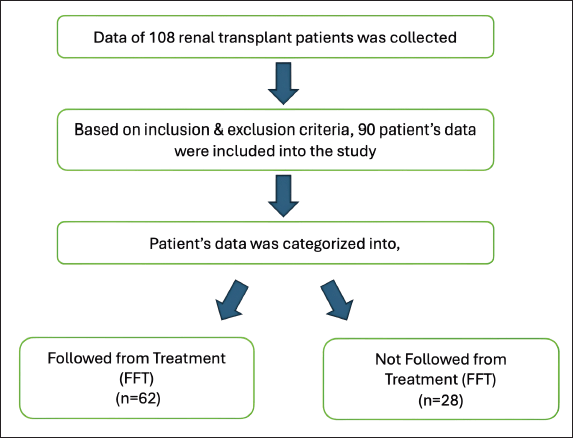 | Figure 1. Patient data collection criteria. [Click here to view] |
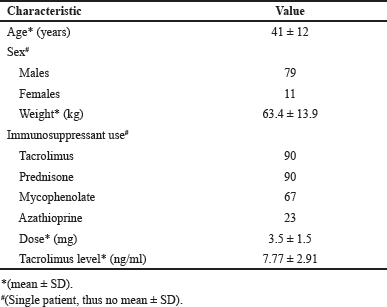 | Table 1. Demographic and clinical characteristics. [Click here to view] |
The tacrolimus doses given were ranged from 1 to 9.5 mg. The tacrolimus concentrations were found to be within the therapeutic window of tacrolimus, i.e., 4–15 ng/ml. The values are presented in Table 2. When the tacrolimus levels were evaluated, the mean concentrations for the group, which followed on a later date nFFT had lower concentrations (5.38 ± 2.43) compared with the group which was FFT (7.80 ± 2.85). The initial doses for the FFT group were found to be higher than for the nFFT group. The mean ± SD of tacrolimus levels for different starting doses was calculated, and the values are presented in Table 3. The average initial and maintenance doses of tacrolimus for the total sample, FFT and nFFT groups were calculated separately and the values are presented in Table 4, respectively. The plots were generated to understand the relation between different follow-up visits and the corresponding tacrolimus doses and concentrations. A scatter plot showing various doses given to the patients during their visits was generated, and it showed that there was a gradual tapering of doses from initial visits until it was maintained at 2–3 mg. (Fig. 2).
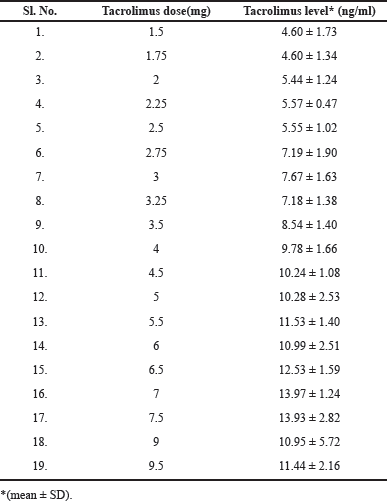 | Table 2. Mean tacrolimus levels observed for the different doses. [Click here to view] |
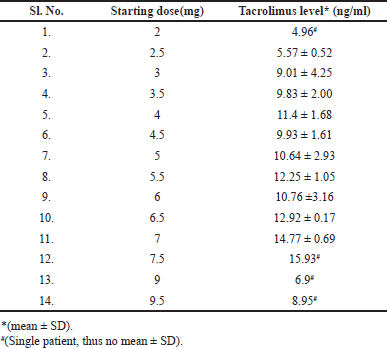 | Table 3. Mean tacrolimus levels for the initial doses given right after the transplantation. [Click here to view] |
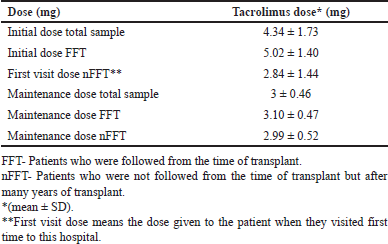 | Table 4. Average initial and maintenance doses of tacrolimus. [Click here to view] |
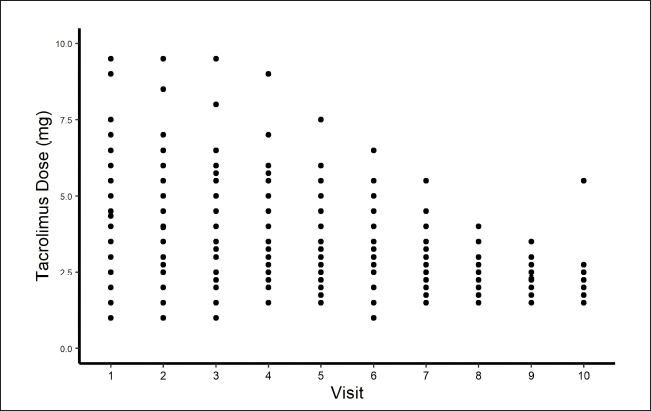 | Figure 2. Visit versus tacrolimus dose plot. [Click here to view] |
When tacrolimus levels versus visits were plotted, a gradual decline was noticed in the tacrolimus trough concentration levels in the body, as doses are tapered off with time (Fig. 3). A linear rise in the levels was noticed when doses were plotted against tacrolimus levels, showing that tacrolimus concentrations in the body decrease proportionally as tacrolimus doses were reduced, and vice-versa (Fig. 4). It was observed that the initial tacrolimus dose was ranged from 0.07 to 0.12 mg/kg in about 65% of patients. It was observed that the tacrolimus levels (ng/ml) after the initial doses (in mg) were twice the value of the doses. For, e.g., if a 5 mg dose was administered, the resultant concentration observed was around 10 ng/ml. Overall, this ratio between the dose and concentration was between 1.6 and 2.4 in 56% of the patients. In the rest of the cases, the ratios were above or below these values. Initial trough levels were around 9.5 ng/ml. The doses were then brought down gradually till the trough levels were maintained between 4 and 6 ng/ml and this reduction in doses was observed in around 80% of the patients.
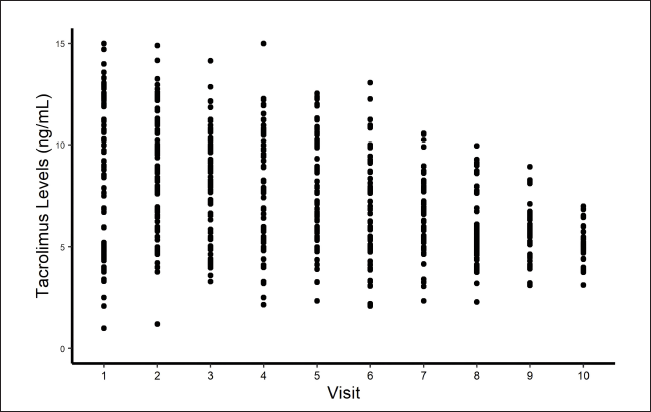 | Figure 3. Visit versus tacrolimus levels plot. [Click here to view] |
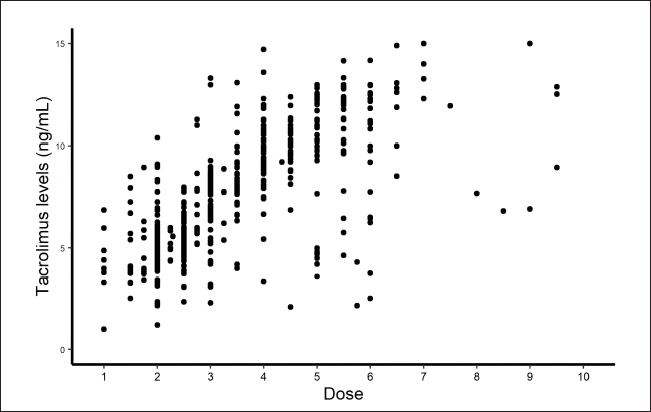 | Figure 4. Tacrolimus dose versus levels plot. [Click here to view] |
In 11.29% of the patients (n = 8), higher trough concentrations were observed, than anticipated and as compared with the mean trough concentrations/dose. In 4 out of the 62 patients (6.4%), trough concentrations were much lower than anticipated (Table 2). This could be due to multiple reasons, such as low hematocrit levels, administration of concurrent interacting medications, genetic polymorphism, and so on.
DISCUSSION
This retrospective study aimed to understand the dosing protocols of tacrolimus and its resulting trough concentrations. Tacrolimus exhibits wide variability in its pharmacokinetics and pharmacodynamics among individuals, which can lead to ineffective treatment. It is important to monitor the correlation between blood drug concentrations and clinical outcomes, supporting the use of TDM. In the early years of its use, the initial target ranges for tacrolimus were broad, ranging between 5 and 40 ng/ml. Subsequently, lower trough concentrations were adopted, typically ranging between 10 and 20 ng/ml [13]. The initial consensus conference on TAC optimization, convened at Lake Louise in 1995, proposed that tacrolimus whole blood concentrations should be maintained between 5 and 20 ng/ml for all transplant populations [13]. During the first year post-transplantation, the trough level of tacrolimus is typically maintained at 3 to 5 ng/ml, provided the recipient’s renal transplant condition remains stable [4,14].
In this study, it was noted that the tacrolimus doses were started with a higher dose range (2.5–9.5 mg) and then tapered down to lower doses (1–2 mg). Initially, after the renal transplant, the doses were given in the range of 4 to 12 mg [6,11,15]. The usual trough concentrations were maintained around 15–20 ng/ml during the first 14 days, and thereafter, the concentrations were reduced to 4 to 10 ng/ml over a period of 6 months [6,11,15,16]. The doses will also be brought to around 1 mg [6]. The literature reports say that higher or lower levels of trough concentrations than the anticipated level may be due to CYP3A5 genetic polymorphisms. Based on this, there is a difference in metabolism among individuals who can be classified as fast, intermediate, and slow metabolizers, depending on which initial doses can be decided. In the present study, initial doses based on genetic polymorphism were not done, as the genetic data were not available.
In a setting where genetic polymorphism assays are performed, patients will be generally classified into rapid and intermediate metabolizers of CYP3A5, the target concentrations were attained with a delay. On the contrary, the target concentrations are achieved faster in patients who are classified as slow metabolizers of CYP3A5 [5,8,17,18]. Generally, genetic phenotypes will be considered for initial dosing, and the body weight of the patient alone may not be sufficient for precise dosing [5,18]. In the current setting, clinicians implemented careful titration of doses based on the tacrolimus levels in the absence of information on the genetic polymorphisms [5,8,17,18]. We observed variations in tacrolimus concentrations from the anticipated levels in a certain number of patients initially post-transplantation. This demonstrates the need to adopt genotype testing before tacrolimus dosing to appropriately decide the initial doses.
In most clinical settings, the starting dose of tacrolimus is exclusively based on body weight. Many population pharmacokinetic (PopPk) studies have been reported in the literature over the years from different patient populations [8–10]. However, very few studies have evaluated PopPK models prospectively. Few such models that incorporated genotyping of CYP3A5 demonstrated better target attainment compared to the body weight-based dosing [15,16]. As per the literature, we have found that genetic polymorphisms of Cyp3A5 can cause high variability in tacrolimus trough levels. By including these data in the study, we can infer key inputs regarding the variations in the patient’s Tacrolimus trough levels based on the type of metabolizers they are, and can provide a better dosing protocol for tacrolimus. Some software platforms have been developed for the model-informed precision dosing of many drugs, including tacrolimus. Some of such tools are available as open-source tools and many of them are available with subscriptions [19]. We did not get much information on the comorbidities and concurrent medications from the medical records as this included information from multiple patient visits. There might be variations in the tacrolimus trough levels due to these factors; however, in this study, we could not include this information.
Apart from the body weight, tacrolimus levels are reported to be influenced by hematocrit values, and duration post-transplantation resulting in inter- and intra-patient variabilities [15,20]. These factors have to be considered in designing the dosage regimen of tacrolimus.
CONCLUSION
This study assessed the protocol and titration approach of tacrolimus dose. Based on patient’s body weight, the doses ranged from 2.5 to 9.5 mg, and the trough concentrations were maintained within 4–15 ng/ml. The current study explained the decision-making of clinicians in the absence of pharmacogenetic information to fine tune the dosing based on the levels of tacrolimus. The use of this type of data along with the pharmacogenetic data can potentially be used to develop a PopPk model for dosage adjustments to recommend precise dosing in this population.
ACKNOWLEDGMENTS
The authors extend their gratitude to Kasturba Medical College, Manipal, for their support in facilitating this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding received to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details is given in the ‘Methodology' section.
DATA AVAILABILITY
Inquiries regarding the data may be directed to the corresponding author.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Statista [Internet]. Number of kidney transplants worldwide by region 2022. [cited 2024 Jul 6]. Available from: https://www.statista.com/statistics/398657/kidney-transplants-by-world-region/
2. Shroff S. Current trends in kidney transplantation in India. Indian J Urol. 2016;32(3):173.
3. Greanya ED, Poulin E, Partovi N, Shapiro RJ, Al-Khatib M, Ensom MHH. Pharmacokinetics of tacrolimus and mycophenolate mofetil in renal transplant recipients on a corticosteroid-free regimen. Am J Health Syst Pharm. 2012;69(2):134–42.
4. Yu M, Liu M, Zhang W, Ming Y. Pharmacokinetics, pharmacodynamics and pharmacogenetics of tacrolimus in kidney transplantation. Curr Drug Metab. 2018;19(6):513–22.
5. Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmacogenomics Pers Med. 2018; 11:23–33.
6. Li L, Li CJ. Tacrolimus in preventing transplant rejection in Chinese patients —optimizing use. Drug Des Devel Ther. 2015;9:473–85.
7. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53.
8. Campagne O, Mager DE, Brazeau D, Venuto RC, Tornatore KM. Tacrolimus population pharmacokinetics and multiple CYP3A5 genotypes in black and white renal transplant recipients. J Clin Pharmacol. 2018;58(9):1184–95.
9. Liu XL, Guan YP, Wang Y, Huang K, Jiang FL, Wang J, et al. Population pharmacokinetics and initial dosage optimization of tacrolimus in pediatric hematopoietic stem cell transplant patients. Front Pharmacol. 2022;13:891648.
10. Bansal S. Therapeutic drug monitoring of tacrolimus in kidney transplantation. Indian J Transplant. 2020;14(1):8.
11. Kocur A, Marsza?ek D, Rubik J, Czajkowska A, Pawi?ski T. Therapeutic drug monitoring of tacrolimus based on volumetric absorptive microsampling technique (VAMS) in renal transplant pediatric recipients—LC-MS/MS method development, hematocrit effect evaluation, and clinical application. Pharmaceutics. 2023;15(1):299.
12. Nguyen TD, Smith NM, Attwood K, Gundroo A, Chang S, Yonis M, et al. Bayesian optimization of tacrolimus exposure in stable kidney transplant patients. Pharmacother J Hum Pharmacol Drug Ther. 2023;43(10):1032–42.
13. Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31(2):139–52.
14. Hwang YH, Kim H, Min K, Yang J. Tacrolimus trough levels in kidney transplant recipients. BMC Nephrol. 2021;22(1):405.
15. Lu Z, Bonate P, Keirns J. Population pharmacokinetics of immediate- and prolonged-release tacrolimus formulations in liver, kidney and heart transplant recipients. Br J Clin Pharmacol. 2019;85(8):1692–703.
16. Schagen MR, Volarevic H, Francke MI, Sassen SDT, Reinders MEJ, Hesselink DA, et al. Individualized dosing algorithms for tacrolimus in kidney transplant recipients: current status and unmet needs. Expert Opin Drug Metab Toxicol. 2023;19(7):429–45.
17. MacPhee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4(6):914–9.
18. Niioka T, Kagaya H, Saito M, Inoue T, Numakura K, Habuchi T, et al. Capability of utilizing CYP3A5 polymorphisms to predict therapeutic dosage of tacrolimus at early stage post-renal transplantation. Int J Mol Sci. 2015;16(1):1840–54.
19. Kantasiripitak W, Van Daele R, Gijsen M, Ferrante M, Spriet I, Dreesen E. Software tools for model-informed precision dosing: how well do they satisfy the needs? Front Pharmacol. 2020;11:620.
20. Andrews LM, Hesselink DA, Van Schaik RHN, Van Gelder T, De Fijter JW, Lloberas N, et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus in adult renal transplant recipients. Br J Clin Pharmacol. 2019;85(3):601–15.