INTRODUCTION
Free radicals and reactive oxygen species (ROS) have an impact on oxidative stress in the cells [1]. Under homeostasis conditions, the human body eliminates these free radicals through the antioxidant defense system [2]. However, if there is an imbalance between the generation and elimination of free radicals, it causes damage to the cells. This has short-term and long-term implications, with numerous studies indicating a significant relationship between free radicals in the body and the occurrence of various diseases such as heart disease, cancer, diabetes, and Parkinson’s disease [3]. As previously stated, this emphasizes the importance of antioxidants in preventing damage caused by oxidative stress.
Natural antioxidants have been extensively studied by scientists through in vivo, in vitro, and clinical trials [4]. To name just a few examples, antioxidants of plant origin, such as curcumin, scavenge free radicals and inhibit the insulin pathway and glucose transport in adipocytes [5]. Similarly, natural polyphenols like resveratrol have exhibited a protective effect against 4-hydroxynonenal-induced oxidative stress and apoptotic death of fibroblasts [6], while mangiferin has shown antioxidant effects and attenuated oxidative stress in renal cells [7]. These examples represent only a fraction of the diverse array of natural antioxidants, underscoring the need for further exploration.
Research has shown that Mitragyna spp., which are abundant in Southeast Asia and belongs to the Rubiaceae family, are a good source of polyphenols and antioxidants. For instance, catechin from M. rotundifolia (Roxb.) Kuntze leaf extract exhibited the antioxidant activity [8]. The acetone leaf extract of M. parvifolia (Roxb.) Korth. accumulates flavonols and condensed tannin, which demonstrated anti-proliferative activity in HeLa cells [9]. An in vitro study has demonstrated that derivatives of flavonoids and polyphenols from the methanol leaf extract of Mitragyna speciosa Korth. possess potential antioxidant and anti-diabetic properties [10]. Additionally, the methanol root extract of M. inermis (Willd.) Kuntze exhibited hepatoprotective activity in acetaminophen-induced hepatic injury in Wistar rats, attributed to its antioxidant properties [11].
Mitragyna diversifolia (Wall. ex G. Don) Havil. (MD), commonly found in the southern region of Thailand, is traditionally used in Thai traditional medicine to treat diarrhea. The antidiarrheal activity of ethanolic bark extract of MD has been confirmed in animal models [12]. However, research on its pharmacological activity remains limited. Monoterpene indole alkaloids from the stem bark of MD have been shown to have a moderate inhibition of acetylcholinesterase [13]. Triterpenes such as rotundic acid, and pololic acid, isolated from the stem bark of MD, exhibited potent cytotoxicity, and inhibited the growth of MCF-7 and HT-29 cells [14]. In the present study, antioxidant activity will be screened from leaves, branches, stem barks, and fruits. Phytochemical profiles will be searched using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS), and thin-layer chromatography. Additionally, the protective effect of MD on H2O2-induced oxidative stress using L6 myotubes muscle cells as a model is also investigated. The results of this study could provide insight into the use of MD as a natural antioxidant.
MATERIALS AND METHODS
Materials
Catechin, epicatechin, gallic acid, rutin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), linoleic acid, thiobarbituric acid (TBA), dimethyl sulfoxide (DMSO), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), vanillin, and Toyopearl® HW-40 were obtained from Sigma-Aldrich (St. Louis, USA). Ferric chloride was obtained from Ajax Finechem (Sydney, Australia). Phosphoric acid was purchased from J.T. Baker (Tyco, USA). Formic acid and Folin-Ciocalteau’s reagent were obtained from Loba Chemie Pvt. Ltd. (Mumbai, India). Aluminum chloride was purchased from M&B Laboratory Chemicals Ltd. (London, UK). Solvents were analytical grade. Acetone, methanol, toluene, ethanol, and glacial acetic acid were purchased from RCI LabScan Limited (Bangkok, Thailand). High-performance thin-layer chromatography (HPTLC) (silica gel 60 F254) glass plate was purchased from Merck (Darmstadt, Germany). (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Invitrogen (CA, USA). Gibco (NY, USA) provided high-glucose Dulbecco’s Modified Eagle Medium (DMEM-high), fetal bovine serum (FBS), horse serum (HS), penicillin, streptomycin, and 0.25% trypsin-EDTA. The hydrogen peroxide solution (35%) was from Vidhyasom Co., Ltd. (Bangkok, Thailand).
Plant materials
All parts of MD (Wall. ex G. Don) Havil. were collected from Walailak University, Nakhon Si Thammarat Province, Thailand, and the herbarium was prepared. The specimen was identified by Associate professor Dr. Tanomjit Supavita from the School of Pharmacy at Walailak University. Voucher specimen SKP165130401 was deposited at the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Prince of Songkhla University, Thailand. Leaves, branches, stem barks, and flowers were collected, cleaned, and dried at 50°C in a hot-air oven for 24 hours, then crushed with a grinder.
Preparation of crude extracts
Ten grams of the sample were macerated in methanol at a ratio of 1:15 for 3 days at room temperature. The filtrates were collected and evaporated to dryness. The extracts were designated as leaf crude extract (LCE), branch crude extract (BCE), stem bark crude extract (SCE), and flower crude extract (FCE) for the crude extracts from leaves, branches, stem barks, and fruits, respectively.
Size-exclusion chromatography
LCE was fractionated using size-exclusion chromatography. Column filled with Toyopearl® HW-40 (2.5 · 60 cm, Sigma, Germany), equilibrated with methanol, was prepared. The LCE (0.5 g) was dissolved in methanol and loaded on top of the column. The column was eluted with methanol at a flow rate of 1 ml/min. Fractions were collected. TLC was used to help pool the fractions. Fractions were obtained (Fr 1–Fr 5).
Determination of chemical constituents
Total phenolic content
The total phenolic content was assessed using the Folin-Ciocalteu method [15]. Five milligrams of the sample were weighed and dissolved in methanol. The volume was adjusted to 10 ml, and the sample was then diluted to a concentration between 0.156 and 5 mg/ml. The phenolic content analysis was performed on a 96-well plate. Fifteen microliters of the sample were pipetted, and 125 µl of 10% v/v Folin-Ciocalteu reagent in water was added. The mixture was left to stand for 5 minutes, followed by the addition of 125 µl of 7.5% w/v sodium carbonate solution. The mixture was thoroughly mixed and incubated in the dark for 60 minutes. The absorbance was measured at 760 nm using a microplate reader. For the blank, the sample was mixed with distilled water. The phenolic content was expressed as mg gallic acid equivalents (GAEs) per gram of extract. The analysis was conducted in triplicate.
Total condensed tannin content
The total condensed tannin content was determined using the acidified vanillin method [16]. The 4% w/v vanillin-hydrochloric acid reagent was prepared in methanol. The sample was prepared in methanol at a concentration ranging from 0.156 to 5 mg/ml. For the total phenolic content analysis, 20 µl of the sample was mixed with 225 µl of vanillin-hydrochloric acid reagent. The absorbance was measured at a wavelength of 500 nm. For the blank sample, the sample was mixed with methanol. The analysis was conducted in triplicate, and the total condensed tannin content was expressed as mg catechin equivalent (CE) per gram extract.
Total flavonoid content
The total flavonoid content was measured using a modified aluminum chloride colorimetric method [17,18]. A 10% w/v aluminum chloride solution and a 5% w/v sodium nitrite solution were prepared in water. The sample was dissolved in methanol at concentrations ranging from 0.156 to 5 mg/ml. For the flavonoid content analysis, 20 µl of the sample was pipetted into a 96-well plate. Then, 80 µl of distilled water and 6 µl of sodium nitrite solution were added and left to stand for 6 minutes. Afterward, 6 µl of aluminum chloride solution was added and left for another 6 minutes. Subsequently, 40 µl of 4% w/v sodium hydroxide and 48 µl of distilled water were added. The mixture was thoroughly mixed, and the absorbance was immediately measured at 510 nm. For the blank, the sample was mixed with distilled water. The total flavonoid content was expressed as mg rutin equivalents per gram of extract. The analysis was performed in triplicate.
Assays for antioxidant activity
DPPH assay
The antioxidant activity was determined using the DPPH radical scavenging assay [19]. The DPPH solution was freshly prepared at a concentration of 150 mM in methanol. The sample was prepared in methanol at concentrations ranging from 0.078 to 1.25 mg/ml, and the standard gallic acid was prepared in methanol at concentrations ranging from 1.56 to 25 µg/ml. For the antioxidant activity analysis, 20 µl of the sample was mixed with 180 µl of DPPH solution. The mixture was then incubated at room temperature for 30 minutes. The absorbance was measured at a wavelength of 517 nm. For the blank sample, the sample was mixed with methanol, while the control contained only the DPPH solution. The percentage of inhibition (IC50) was calculated using the following equation.
The IC50, expressed in mg/ml, was computed from the plot of the % inhibition versus concentration of the standard or sample.
Ferric ion reducing antioxidant power (FRAP)
The total antioxidant power was measured using the FRAP assay [20]. The FRAP reagent was prepared by mixing 28 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tris TPTZ solution in 40 mM HCl, and 20 mM ferric chloride hexahydrate (FeCl3·6H2O) in a 10:1:1 v/v ratio. The sample was prepared in methanol at concentrations ranging from 0.039 to 1.25 mg/ml For the FRAP analysis, 30 µl of the sample was mixed with 270 µl of FRAP reagent. The absorbance was measured at 593 nm using a microplate reader. For the blank sample, the sample was mixed with distilled water. Antioxidant power was expressed as GAEs (mg) per gram of extract. The sample was analyzed in triplicate.
Lipid peroxidation
The assay was performed based on the reaction between TBA and malondialdehyde (MDA). The MDA level was determined using the previous method as described in [21,22] with a slight modification. The linoleic emulsion stock solution was prepared [20 mM linoleic acid, tween 20, and 100 mM Tris-HCl, pH 7.5] and sonicated for 30 minute. Samples and butylated hydroxytoluene (BHT) were prepared at concentrations of 37.5, 75, 150, 300, and 600 µg/ml. One milliliter of linoleic emulsion (20 mM) was mixed with 200 µl of samples; subsequently, 100 µl of 2 mM ascorbic acid was added, and the mixture was vortexed and sonicated for 10 minutes. The linoleic acid peroxidation was initiated by adding 100 µl of 4 mM FeSO4, vortexed, and sonicated for 60 minutes. The reaction mixture was terminated by adding 1 ml of 10% w/v trichloroacetic acid in 0.5% v/v HCl. Next, 2 ml of 1% w/v TBA in 50 mM NaOH was added, and it was heated at 90°C in a water bath for 30 minutes. The reaction was cooled in an icebox for 10 minutes, and 1 ml of reaction was taken and centrifuged at 7,000 rpm for 10 minutes. The supernatant (200 µl) was pipetted and measured using a microplate reader in a 96-well plate at 532 nm. The percentage of linoleic acid peroxidation inhibition was calculated using the equation below. The IC50 value was analyzed using linear a regression analysis of graphs plotted between inhibition percentages and sample concentrations.
where ODcontrol was the absorbance of reaction without sample and ODsample was the absorbance of reaction with sample solution.
Phytochemical profiling
LC-ESI-MS/MS
The fraction that has the best antioxidation was identified for chemical profiles by LC-ESI-MS/MS (The Office of Scientific Instrument and Testing, Prince of Songkla University, Thailand). The analysis was performed by using an Agilent UHPLC coupled to a tandem MS instrument (Agilent Technologies, CA, USA). The UHPLC was equipped with a binary pump, an autosampler, and a column oven. The column, ZORBAX Eclipse Plus C18 (150 mm · 2.1 i.d., 1.8 µm) was used. The column temperature was maintained at 35°C. The mobile phases consisted of (A) 0.1% v/v formic acid in water and (B) 0.1% v/v formic acid in acetonitrile. The gradient elution profile was at t = 0.00 minute, 35% B; 14.00 minute, 35% B; 22.00 minute, 60% B; 28.00 minute, 80% B; 34.00 minute, 100% B; 40.00 minute, 100% B; 45.00 minute, 10% B. The flow rate was maintained at 0.2 ml/minute. The injection volume was 5 µl. Fr 5 (10.7 mg) was dissolved in ethanol, centrifuged at 10,000 rpm, and filtered through a nylon membrane before subjection.
Mass spectrometry detection was performed using an MS 6500 series Q-TOF, G6545A model with an electron spray ionization source operating in negative mode. LC-ESI-MS/MS data were collected and processed by Agilent MassHunter workstation software.
High-performance thin-layer chromatography
Samples obtained from the fractionation on the Toyopearl® HW-40 column were applied to an HPTLC plate for fingerprinting. Samples at a concentration of 10 mg/ml were prepared in methanol. Catechin and epicatechin, used as chemical markers, were prepared in methanol at a concentration of 1 mg/ml. The HPTLC glass plates (silica gel G 60 F254), 20 × 10 cm, were activated by washing with methanol and heating at 100°C in a hot-air oven for 1 hour. The plates were kept in a drying chamber. The samples and markers (5 µl of each) were applied on the HPTLC plate using CAMAG® Linomat 5 with an 8 mm band width. The plates were developed in the CAMAG® Twin Trough Chamber filled with a mobile phase (toluene: ethyl acetate: chloroform: formic acid; 4:8:1:3). The running distance was 8 cm. The HPTLC plates were visualized under UV at 254 and 366 nm, sprayed with anisaldehyde/H2SO4 reagent, and heated at 100?C. To detect antioxidative constituents, the HPTLC plate was sprayed with 0.02% w/v DPPH/methanol and then incubated in the dark for 1 hour. The pictures were captured using CAMAG® TLC visualizer 2. The HPTLC chromatogram was developed using CAMAG® TLC 4, and the data were analyzed using Vision CATS software (Muttenz, Switzerland).
In vitro H2O2-induced oxidative stress and cell viability assay
Cell line and culture conditions
The L6 myoblast cell line (CRL-1458, Manassas, VA, USA) was cultured in high-glucose DMEM-high supplemented with 10% FBS and 1% antibiotics (penicillin-streptomycin), and incubated in a humidified atmosphere with 5% CO2 at 37°C. The L6 myotubes cell line was differentiated by changing the media to DMEM-high, supplemented with 2% HS for 2 days. Fresh media were added every 2 days, and the L6 myotubes cell line was ready on the 8th day after the initiation of differentiation.
Treatments and induction of oxidative stress
L6 myotubes cells (2 · 103) in a 96-well plate were treated with the fractionated fractions at concentrations of 12, 25, 50, and 100 µg/ml, or epicatechin (positive control) at concentrations of 50, 100, 200, 500, and 1,000 µM for 24 hours. The oxidative stress was then induced with 1.2 mM H2O2 for 6 hours. After treatment, the media was removed, and viability cells were evaluated.
Cell viability assay
Viable cells were stained with a 0.5 mg/ml MTT assay. After being incubated at 37° for 3 hours, the MTT reagent was removed. DMSO (100 µl) was added to dissolve the formazan crystal product and measured at 570 nm with a microplate reader. The viability of living cells was calculated and presented as the percentage of cell viability using the following equation:
Statistical analysis
The results were exhibited as the mean ± standard deviation (SD) of three repeated measurements of the samples. A statistically significant difference of data was performed by using one-way ANOVA followed Duncan’s multiple-range test or pair t-test (SPSS software V26, IL, USA). Microsoft Excel was employed to calculate the IC50 value. Data on the H2O2-induced oxidative stress were expressed in the relative amount of the control (1% DMSO). Groups were analyzed using a paired sample test (2-tailed). Significance was considered at p < 0.01 and p < 0.05 at 99% and 95% confidence, respectively.
RESULTS AND DISCUSSION
Antioxidant activity of MD plant extracts
Mitragyna spp., including M. speciosa, M. rotundifolia, M. parvifolia, M. hirsuta, and MD, are natively distributed in the Southeast Asia region [23]. Nowadays, scientists have particularly focused on M. speciosa, as it is a psychoactive plant and possesses a variety of pharmacological activities [24]. Nevertheless, the use of M. speciosa causes addiction and withdrawal symptoms. This has made it intriguing to explore the use of other species of Mitragyna to avoid psychoactive effects. In this study, we aimed to investigate MD and evaluate its antioxidant potential on protection against oxidative stress.
Firstly, we performed a screening test to see what chemical contents and antioxidants were in the methanol extracts, prepared from leaves (LCE), branches (BCE), stem barks (SCE), and fruits (FCE). Table 1 summarizes the total phenolic, total tannin, total flavonoid contents, and antioxidant powers of MD extracts. Polyphenolic compounds are distributed in different profiles in plant parts of MD. Total phenolic content was LCE > BCE > SCE > FCE, while total tannin content was LCE ~ BCE > FCE > SCE, and total flavonoid content was LCE ~ FCE > BCE > SCE. Notably, MD leaves contained the highest amounts of phenolic, tannin, and flavonoid contents. Polyphenolic compounds in LCE reflected their antioxidant activities. The antioxidative capacity of LCE exhibited IC50 values of 459.19 ± 9.08 µg/ml for DPPH assay and 150.93 ± 7.59 µg/ml for lipid peroxidation assay, with an FRAP value of 279.88 ± 2.54 µM GAE/g extract (Table 1).
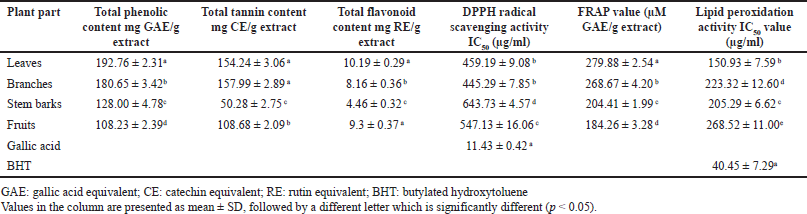 | Table 1. Total phenolic, total tannin, and total flavonoid contents and antioxidant powers from methanol extracts of MD. [Click here to view] |
Comparatively, M. speciosa leaf methanol extract contained a total phenolic content of 105.6 mg GAE/g and a total flavonoid content of 91.1 mg CE/g and had a DPPH IC50 value of 37.08 µg/ml [25]. It seemed that MD leaves contained higher phenolics (198.08 ± 3.83 mg GAE/g) but less flavonoids (16.44 ± 0.67 mg RE/g) than M. speciosa leaves (Table 2). In contrast to MD, M. rotundifolia accumulated phenolics and flavonoids mostly in bark rather than leaves and showed a high FRAP value in n-butanol extract [8].
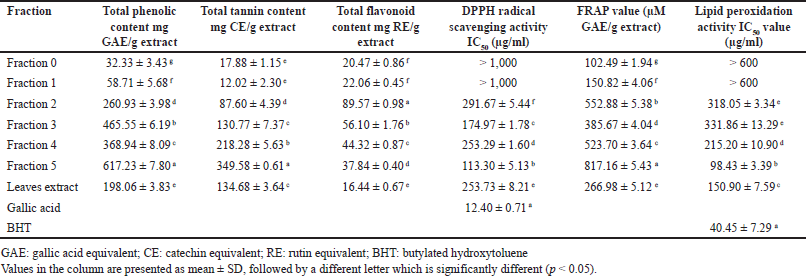 | Table 2. Total phenolic, total tannin, and total flavonoid contents, as well as antioxidant powers from fractionation on Toyopearl® HW-40. [Click here to view] |
To enhance the antioxidant activity, LCE was further fractionated based on molecular weight on the size-exclusion column. Toyopearl® HW-40 is commonly used to produce polyphenols- and tannins-enriched extracts [26,27]. Our study used the Toyopearl® HW-40 column to fractionate the polyphenols, which resulted in five fractions collected based on TLC patterns. Notably, Fr 0 represents the flow-through collected after 1/3 of the total column volume (100 ml), followed by collecting fractions. As shown in Table 2, Fr 5 contained the highest total phenolics (617.23 ± 7.80 mg GAE/g) and total tannin contents (349.58 ± 0.61 mg CE/g). In addition, Fr 2 contained the highest content of flavonoids (89.57 ± 0.98 mg RE/g). Focusing on Fr 5, the size-exclusion column could increase the contents of phenolics, tannins, and flavonoids by about 3.1-, 2.6-, and 2.3-fold, respectively, when compared to LCE. Interestingly, the IC50 values of Fr 5 decreased to 113.30 ± 5.13 µg/ml (DPPH) and 98.43 ± 3.39 µg/ml (lipid peroxidation). The FRAP value was increased from 266.98 ± 5.12 to 817.16 ± 5.43 µM GAE/g. This experiment was easily fractionated through Toyopearl® HW-40 and could enrich polyphenolic compounds and enhance antioxidant activity about three times from the original LCE.
Phytochemical profile in the enriched polyphenolic fraction
Qualitative analysis using LC-ESI-MS/MS was conducted to identify the compounds in Fr 5. Sixteen compounds were identified using a negative mode based on MS-MS identifier peaks. Table 3 summarizes the potential candidate compounds in Fr 5. Polyphenols, alkaloids, sesquiterpenes, and triterpenes were reported. The polyphenols found in Fr 5 include procyanidin B2, procyanidin C1, and epicatechin. Indole alkaloids such as uncarine C, uncarine E, strictosidine, isorhynchophylline, desacetylvindoline, cinchonidinone, mitragynine, and quinine were identified. Surprisingly, our results found that mitragynine accumulates in MD leaves, albeit in small quantities. Mitragynine is a chemical marker that accumulates abundantly in M. speciosa leaves [24]. The presence of indole alkaloids has been reported from the MD bark, including specionoxeine-N(4)-oxide, 7-hydroxyisopaynantheine, 3-dehydropaynantheine, and 4-isopaynantheine-N(4)-oxide, which is reported to exhibit moderate acetylcholinesterase inhibitory activity [13]. Fr 5 also contains the triterpenoid compound betulonic acid. Recently, this compound has become a lead molecule for anticoronaviral agents [14].
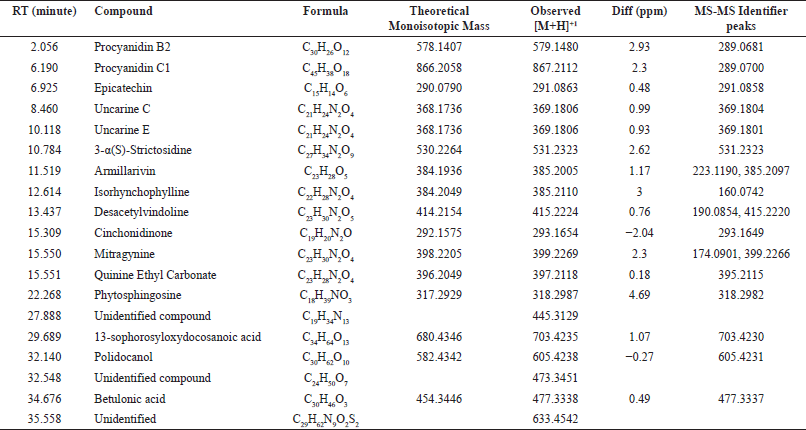 | Table 3. LC-ESI-MS/MS results (negative mode). [Click here to view] |
Focusing on the antioxidant activity, the presence of epicatechin in Fr 5 was promising for antioxidant potential in MD fractions. We then examined all fractions on the HPTLC plate, using catechin and epicatechin as chemical markers. As shown in Figure 1, under the separation conditions, catechin has an Rf-value of 0.60, while epicatechin has a value of 0.58. The bands of catechin and epicatechin were distinguished after detection under UV 254 nm and using the anisaldehyde/H2SO4 spray reagent. The scavenging effect of compounds in the fractions was confirmed after spraying the HPTLC with a DPPH/methanol solution. The positive bands appeared white on a violet background. It can be noted that all fractions contained components with scavenging activity. After scanning at 280 nm, the TLC chromatogram was developed (Fig. 2A). The results clearly showed a peak corresponding to epicatechin in Fr 2–Fr 5 among various impurities. The absorbance spectra, scanned at wavelengths of 200–500 nm, revealed the presence of epicatechin in those fractions (Fig. 2B). The amount of epicatechin in Fr 5 was higher than in other fractions because Toyopearl® HW-40 got rid of high molecular weight or polymerized polyphenol.
 | Figure 1. HPTLC fingerprints of MD extracts. Visualized under A. White light; B. 254 nm UV light; C. 366 nm UV light; D. Sprayed with an anisaldehyde/H2SO4 spray reagent, heated at 100?C for 5 minutes; E. Sprayed with 0.02% w/v DPPH/methanol, kept in dark for 1 hour. LM: MD leaves extract, C: Catechin, EC: Epicatechin, F5–F0: Fractionated Fr 5–Fr 0, respectively. [Click here to view] |
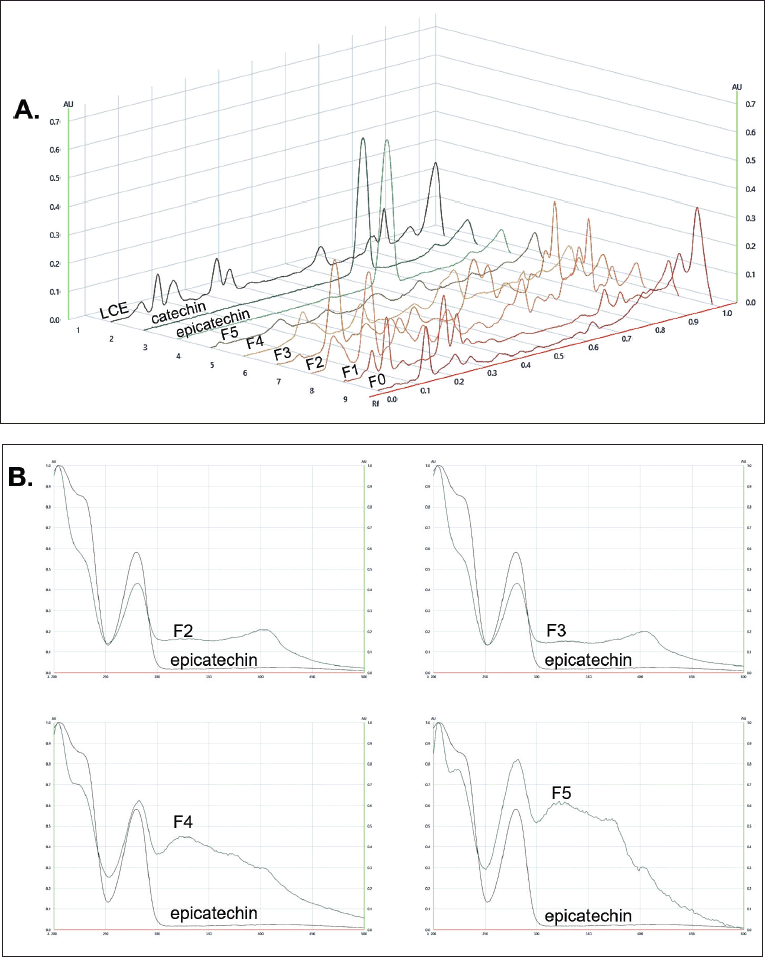 | Figure 2. HPTLC chromatograms and absorbance spectra. A. HPTLC– densitometric chromatograms, scanned at 280 nm; B. Absorbance spectra of spot, located epicatechin at Rf of 0.58. [Click here to view] |
Protective effect on H2O2-induced oxidative stress in L6 myotubes
The protective effect of the fractionated fractions of MD against oxidative stress was investigated. In this study, the L6 myotubes cell line was used as a model, which is commonly used to study cellular responses to oxidative stress. The L6 myotubes were obtained after inducing L6 myoblast cells with HS for 8 days, involving the fusion of myoblasts into multinucleated myotubes. The L6 myotubes were employed to investigate the effects of oxidative stress after exposing cells to hydrogen peroxide (H2O2). To find the appropriate concentration of H2O2 for inducing oxidative stress without being toxic to the cells, the cells were cultured and treated with H2O2 at concentrations ranging from 0.05 to 10 mM. Cell viability was measured, and the experiment results indicated that the appropriate concentration of H2O2 was 1.2 mM (data not shown).
The addition of 1.2 mM H2O2 for 6 hours resulted in significant cell death, reducing cell viability to only 40% compared to the untreated group. This indicates that H2O2 induces ROS, which damage cellular activity (Fig. 3). In this study, the viability of myotube cells was assessed after pre-treatment with various fractions isolated from Toyopearl HW-40, including Fr 1 – Fr 5, for 24 hours prior to H2O2 induction.
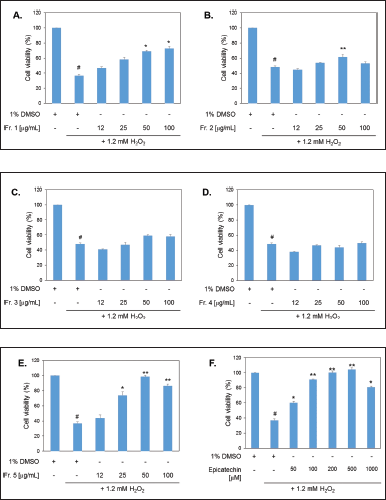 | Figure 3. Cell viability using a MTT assay. Pretreated cells with the fractionated fractions of MD extracts for 24 hours before induction of oxidative stress (1.2 mM H2O2). A–E. represent treatments with Fr 1–Fr 5 (12–100 µg/ml), F. treatment with epicatechin (50–1,000 µM). Statistical analyses were performed by paired sample test (2-tailed). # designates a significant difference from a control (1% DMSO) (p < 0.05); *, ** designate significant differences from 1.2 mM H2O2-treated cells at p < 0.05 and p < 0.01, respectively. [Click here to view] |
The experimental results showed that Fr 1, Fr 2, and Fr 5 were able to protect cells from H2O2-induced damage. Compared to the standard compound epicatechin, Fr 5 at a concentration of 25 µg/ml was able to protect the cells from H2O2 as effectively as epicatechin about 70% cell viability. Moreover, Fr 5 at concentrations of 50 and 100 µg/ml provided 100% protection against 1.2 mM H2O2. Notably, Fr 1 and Fr 2 at concentrations above 50 µg/ml were also able to protect the cells from H2O2, while Fr 3 and Fr 4 showed no protective effect.
Phytochemical profiling revealed that all fractions contained epicatechin, but in varying amounts. The epicatechin content was relatively high in Fr 5, likely due to fewer contaminants, as observed on the HPTLC plate. Fr 1, which also provided cell protection, is hypothesized to contain polymeric polyphenolics with large molecular sizes, which were eluted from the column earlier. The protective effect of Fr 2 may be attributed to the presence of flavonoids. However, further quantitative analysis is necessary to support these hypotheses.
The antioxidant properties of catechin and epicatechin have been shown to be excellent natural antioxidants in many studies. Tea catechins (epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate) have been claimed to have anti-aging effects due to their ability to combat oxidative stress [28]. An in vitro study on the effect of quercetin, catechin, and betaine on oxidative stress induced by ethanol demonstrated that all agents prevented oxidative stress, reduced MDA levels, and increased the expression of glutathione peroxidase 4 [29]. Using a model of H2O2-induced apoptotic cell death in fibroblasts, a study found that catechin protected fibroblasts from oxidative stress-induced cell death. This might be because it stopped the phosphorylation of Jun N-terminal kinases and p38 mitogen-activated protein kinases [30].
The discovery of epicatechin in the methanol extract of MD leaves supports its antioxidant effects and its ability to protect cells from H2O2-induced oxidative stress. The properties of MD leaves as a source of antioxidants are beneficial for use in treating diseases related to ROS, which are causes of various conditions. This is the first report of MD leaf extract’s effect against ROS in myotubes. The MD leaf can be used as an alternative source of antioxidants without any psychoactive effects. The results of this study could provide insight into the use of MD against oxidative stress, which causes several metabolic diseases.
CONCLUSION
The antioxidant activity of MD leaf extract has been explored and demonstrated to be a good source of antioxidants. The abundance of phenolics, tannins, and flavonoids contributes to its antioxidant power. The polyphenol-enriched fraction containing epicatechin was obtained by fractionating the leaf extract using size exclusion chromatography on Toyopearl® HW-40. It exhibited a protective effect against H2O2-induced oxidative stress.
ACKNOWLEDGMENT
This research was supported by the Faculty of Pharmaceutical Sciences, Prince of Songkla University (grant No. PHA6404029S).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Abdull Razis AF, Modu B, et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;10(11):1158198. CrossRef
2. Engwa GA. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases [Internet]. In: Asao T, Asaduzzaman M, editors. Phytochemicals—source of antioxidants and role in disease prevention. London, UK: InTech; 2018. [cited 2024 June 8]. CrossRef
3. Gospodaryov D, Lushchak V. Oxidative stress: cause and consequence of diseases [Internet]. In: Asao T, editor. Oxidative stress and diseases. London, UK: InTech; 2012 [cited 2024 June 8]. CrossRef
4. Mendonça JDS, Guimarães RCA, Zorgetto-Pinheiro VA, Fernandes CDP, Marcelino G, Bogo D, et al. Natural antioxidant evaluation: a review of detection methods. Molecules. 2022;27(11):3563. CrossRef
5. Zhang X, Liang D, Guo L, Liang W, Jiang Y, Li H, et al. Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol Med Rep. 2015;12(1):1347–55. CrossRef
6. Kutuk O, Adli M, Poli G, Basaga H. Resveratrol protects against 4-HNE induced oxidative stress and apoptosis in Swiss 3T3 fibroblasts. Biofactors. 2004;20(1):1–10. CrossRef
7. Saha S, Sadhukhan P, Sinha K, Agarwal N, Sil PC. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem Biophys Rep. 2016;14(5):313–27. CrossRef
8. Kang WY, Li CF, Liu YX. Antioxidant phenolic compounds and flavonoids of Mitragyna rotundifolia (Roxb.) Kuntze in vitro. Med Chem Res. 2010;19:1222–32. CrossRef
9. Ghatak AA, Bhembre ND, Kamath AA, Mehta SS, Mendonca MR, D’souza AW, et al. Antiproliferative, antioxidant activity and total phenolic content of Mitragyna parvifolia (roxb.) Korth. Int J Pharm Pharm Sci. 2014;1;6(4):632–7.
10. Zailan NF, Sarchio SN, Hassan M. Evaluation of phytochemical composition, antioxidant and anti-diabetic activities of Mitragyna speciosa methanolic extract (MSME). Malays J Med Res. 2022;4:18.
11. Abdulhamid A, Ukwuani-Kwaja AN, Umar ZB, Zubairu A, Sani I, Fakai IM. Protective effects of Mitragyna inermis roots methanol extract on acetaminophen-induced hepatic injuries in Wistar rats. J Med Sci. 2022;22(1):13–21.
12. Uddin SB, Mahabub-Uz-Zaman M, Akter R, Ahmed NU. Antidiarrheal activity of ethanolic bark extract of Mitragyna diversifolia. Bangladesh J Pharmacol. 2009;4(2):144–6.
13. Cao XF, Wang JS, Wang XB, Luo J, Wang HY, Kong LY. Monoterpene indole alkaloids from the stem bark of Mitragyna diversifolia and their acetylcholine esterase inhibitory effects. Phytochemistry. 2013;96:389–96. CrossRef
14. Xing-Fen CA, Jun-Song WA, Peng-Ran WA, Ling-Yi KO. Triterpenes from the stem bark of Mitragyna diversifolia and their cytotoxic activity. Chin J Nat Med. 2014;12(8):628–31. CrossRef
15. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299;152–78. CrossRef
16. Rebaya A, Belghith SI, Baghdikian B, Leddet VM, Mabrouki F, Olivier E, et al. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J Appl Pharm Sci. 2015;5(1):052–7. CrossRef
17. Santiago-Adame A, Medina-Torres L, Gallegos-Infante JA, Calderas F, Gonzãlez-Laredo RF, Rocha-Guzmán NE, et al. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT-Food Sci Technol. 2015;64:571–7.
18. Jiao Z, Liu J, Wang S. Antioxidant activities of total pigment extract from blackberries. Food Technol Biotechnol [Internet]. 2005 [cited 2024 June 08];43(1):97–102. Available from: https://hrcak.srce.hr/110451
19. Seiquer I, Rueda A, Olalla M, Cabrera-Vique C. Assessing the bioavailability of polyphenols and antioxidant properties of extra virgin argan oil by simulated digestion and Caco-2 cell assays. Comparative study with extra virgin olive oil. Food Chem. 2015;188:496–503. CrossRef
20. Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. CrossRef
21. Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–40. CrossRef
22. Kada S, Bouriche H, Senator A, Demirta? I, Özen T, Çeken Toptanci B, et al. Protective activity of Hertia cheirifolia extracts against DNA damage, lipid peroxidation and protein oxidation. Pharm Biol. 2017;55(1):330–7. CrossRef
23. Ngernsaengsaruay C, Leksungnoen N, Boonthasak W, Utharatsamee S, Racharak P, Leetanasakskul K, et al. Additional knowledge on the genus Mitragyna (Rubiaceae) in Thailand. Thai Forest Bull Bot. 2022;50(1):20–39.
24. Prevete E, Kuypers KPC, Theunissen EL, Esposito G, Ramaekers JG, Pasquini M, et al. Clinical implications of kratom (Mitragyna speciosa) use: a literature review. Curr Addict Rep. 2023;10(2):317–34. CrossRef
25. Parthasarathy S, Bin Azizi J, Ramanathan S, Ismail S, Sasidharan S, Said MI, et al. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules. 2009;14(10):3964–74. CrossRef
26. Iwai K, Narita Y, Fukunaga T, Nakagiri O, Kamiya T, Ikeguchi M, et al. Study on the postprandial glucose responses to a chlorogenic acid-rich extract of decaffeinated green coffee beans in rats and healthy human subjects. Food Sci Technol Res. 2012;18(6):849–60.
27. Iwaoka Y, Suzuki S, Kato N, Hayakawa C, Kawabe S, Ganeko N, et al. Characterization and identification of bioactive polyphenols in the Trapa bispinosa Roxb. pericarp extract. Molecules. 2021;26(19):5802. CrossRef
28. Maurya PK, Rizvi SI. Protective role of tea catechins on erythrocytes subjected to oxidative stress during human aging. Nat Prod Res. 2009;23(12):1072–9. CrossRef
29. Oliva J, Bardag-Gorce F, Tillman B, French SW. Protective effect of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Exp Mol Pathol. 2011;90(3):295–9. CrossRef
30. Tanigawa T, Kanazawa S, Ichibori R, Fujiwara T, Magome T, Shingaki K, et al. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement Altern Med. 2014;14:133. CrossRef