INTRODUCTION
Polycystic ovary syndrome (PCOS) is a multifactorial endocrine disorder affecting 11%–13% of women worldwide [1]. PCOS is characterized by clinical features such as hyperandrogenism, chronic anovulation, and polycystic ovaries which lead to menstrual irregularities, infertility, and metabolic disturbances [2]. Women with PCOS typically experience elevated levels of luteinizing hormone (LH) and androgens (such as testosterone) with relatively low levels of follicle-stimulating hormone (FSH). These together contribute to menstrual irregularities and hyperandrogenic symptoms like hirsutism and acne [1,3,4]. Elevated prolactin levels and abnormal insulin secretion are often observed leading to insulin resistance and a heightened risk of type 2 diabetes [5]. These hormonal disturbances complicate the management of PCOS, exacerbate symptoms, and influence metabolic health. Women with PCOS are also at an elevated risk of developing obesity, type 2 diabetes, and future cardiovascular disease (CVD). This highlights its significance for both research and therapeutic approaches [6].
Endothelial dysfunction is one of the key cardiovascular risks in women with PCOS. It is often assessed by measuring biomarkers like asymmetric dimethylarginine (ADMA) [7]. ADMA is an endogenous inhibitor of nitric oxide (NO) synthesis. It plays a pivotal role in vascular health by promoting vasodilation [7,8]. Elevated ADMA levels in PCOS women are associated with impaired endothelial function. It contributes to increased blood pressure and the progression of atherosclerosis [9]. ADMA levels are also linked to insulin resistance, another major hallmark of PCOS. It suggests that ADMA could be a crucial factor connecting metabolic and cardiovascular dysfunction in these patients [9].
In addition to cardiovascular dysfunction, PCOS is associated with disruptions in mitochondrial function and fatty acid metabolism. Both of which play critical roles in energy balance [10–12]. Mitochondrial dysfunction in PCOS contributes to increased oxidative stress, inflammation, and insulin resistance [13]. This disturbance is partly attributable to the buildup of toxic intermediates, including carbonyl, during fatty acid oxidation and energy metabolism [14]. These abnormalities in fatty acid metabolism compound the metabolic dysfunction in women with PCOS, resulting in additional weight gain, high levels of lipids, and greater pre-disposition to CVD [15]. Thus, aiming at ADMA and carbonyl pathways can be quite effective in enhancing the metabolic and cardiovascular health of women with PCOS. Acetyl-L-carnitine (ALC) is an acetyl ester of L-carnitine, which enhances the transport of long-chain fatty acids across the mitochondrial membrane for β-oxidation and contributes to cellular energy synthesis. It also possesses antioxidant components and optimizes tissue sensitivity to insulin, thus decreasing inflammation and increasing mitochondrial formation [16]. L-arginine is considered a semi-essential amino acid that has the function of the precursor of NO, which leads to vasodilation and better endothelial function [17]. Coenzyme Q10 (Co-Q10) is part of the mitochondrial electron transport increases the cell’s energy potential and has antioxidant effects [18]. ALC together with arginine, and Co-Q10 can synergistically enhance mitochondrial function, lower ADMA levels, reduce oxidative stress, and decrease the accumulation of toxic metabolites like carbonyl. This combination provides a comprehensive approach to addressing metabolic and cardiovascular dysfunction in women with PCOS [19].
Studies provide information on how combinations of drugs may not only improve cardiovascular function through the increase of NO synthesis and decrease of ADMA, but also influence fatty acid oxidation and energy production. Since it works on both, metabolic and cardiovascular components of PCOS [20,21]. The combined intervention may perhaps serve as a new form of therapy for treating PCOS, and its potential benefits to curb oxidative stress and lipid and hormonal changes and decrease total CVD risk in PCOS. Hence, we evaluated the effects of a combination of ALC+Arg+Co-Q10 on oxidative stress, and endocrine and metabolic anomalies in PCOS women. Therefore, this research intends to facilitate future therapeutic interventions that can help reduce the cardiovascular risks and other metabolic dysfunctions linked to PCOS by reducing oxidative stress, and endocrine and metabolic abnormalities.
MATERIALS AND METHODS
Study design
This clinical trial was a double-blind randomized control study. It was carried out for 12 weeks to assess the impact of ALC+Arg+Co-Q10 supplementation. Oxidative stress markers together with levels of metabolic and endocrine indices in women with PCOS were to be measured. The recruited participants were randomly assigned in a 1:1 ratio. One group received a combination of ALC+Arg+Co-Q10 while the other group received metformin. The study was approved by the advanced study and research board (Approval No. DIR/KMU-AS&RB/EC002067). Ethical approval was obtained from the Institutional Review Board for Ethics (Approval No. KMU/IBMS/IRBE/7th meeting/2023/209-29). All the participants provided written informed consent before enrollment. The trial was registered on www.clinicaltrials.gov NCT05653895 and conducted in accordance with the CONSORT guidelines.
Participants
Women aged 16–45 years diagnosed with PCOS according to ESHRE/ASRM 2003 criteria were recruited for the study. In the inclusion criteria of the participants, BMI was required to be between 25 and 40 kg/m2. PCOS diagnosis was confirmed by at least two criteria: oligo-ovulation or anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries observed on ultrasound. Exclusion criteria included a history of diabetes, CVD, liver or kidney disease. Women taking hormonal contraceptives, insulin-sensitizing agents, or lipid-lowering drugs within 3 months before the study were also excluded.
Intervention
Participants in the treatment group received a daily oral dose of 2,000 mg ALC, 2,000 mg L-arginine, and 200 mg of Co-Q10 for 12 weeks. The control group received a daily oral dose of 1,000 mg of metformin. The participants as well as the investigators were blinded to the treatment allocation throughout the study period. The dosages were based on prior research indicating the efficacy of ALC+Arg+Co-Q10 in improving metabolic, endocrine parameters, and vascular function [22–24].
Comprehensive analysis of biomarkers and metabolic health
The primary outcomes of the study were changes in plasma levels of ADMA. It is a marker of endothelial dysfunction, and carbonyl, an indicator of fatty acid metabolism and mitochondrial function. Biomarkers of oxidative stress, malondialdehyde (MDA), were also examined. Additionally, various metabolic and endocrine indicators were evaluated.
Anthropometric measurements
Anthropometric evaluations, including body weight, height, waist circumference (WC), and hip circumference (HC), were carried out both at baseline and following the intervention. BMI was calculated by dividing body weight in kilograms by the square of height in meters. The waist and HCs were calculated by using a measuring tap in centimeters, and thus, central adiposity, which is often associated with PCOS, was calculated.
Blood collection and sample handling
Blood samples were collected from participants in the morning after an overnight fast to control for diurnal variations and dietary influences on metabolic markers. Each participant’s blood was drawn via venipuncture by a trained phlebotomist, following standard aseptic techniques to minimize contamination. The collected blood samples were immediately placed in anticoagulant-treated tubes and kept at 4°C until processing. Samples were centrifuged at 2,000 × g for 10 minutes at 4°C to separate the plasma. The resulting plasma was aliquoted into sterile tubes and stored at −80°C till analyses. All procedures were performed under blinded conditions to reduce bias.
Enzyme linked immuno-sorbent assay (ELISA) assay
ELISA was used for the analysis of LH (Catalog No. H326), FSH (Cat. No. H327), and levels with kits purchased from Bioactive Diagnostic System Germany; for total testosterone, an ELISA kit obtained from the microwell diagnostic system (Cat No. 00230710); and for prolactin (Cat. No. MB-PRL-96) from Multi-Bio were utilized to assess the concentrations of serum levels as suggested by the manufacturer. The serum levels of oxidative markers MDA (Cat. No. RE10165), ADMA (Catalogue No. E1887Hu), and carbonyl (Cat. No. E1426Hu) were purchased from BT Lab Technology and were used according to manufacturer instructions. All the tests were with intra- and inter-assay variability controlled to remain below 10%.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Software version 6. The Shapiro–Wilk test was applied to assess data normality. Baseline comparisons between the groups were made using independent sample t-tests for normally distributed data and the Mann-Whitney U-test for non-normally distributed data. Comparisons between the groups were made using paired sample t-tests for normally distributed data and the Wilcoxon sign-ranked test for non-normally distributed data. Changes in both primary and secondary outcomes from baseline to post-intervention were analyzed using t-tests depending on data distribution. A p-value below 0.05 was considered statistically significant.
Sample size calculation
Based on prior studies evaluating ADMA and metabolic markers in PCOS, a sample size of 30 participants per group was calculated to provide 80% power to detect a 10% reduction in ADMA levels with an alpha level of 0.05. This calculation was made assuming a standard deviation of 15% in ADMA measurements and a dropout rate of 10%, as demonstrated in the study by Orio et al. [25] Battaglia et al. [26] and Genazzani et al. [20] which examined metabolic markers in obese and overweight women with PCOS [21–24].
RESULTS
A total of 87 PCOS women were screened for eligibility. Out of these 17 participants, for various reasons, they were considered ineligible. A total of 60 participants were recruited and randomized into 2 groups: 30 participants received a combination of ALC, L-arginine, and Co-Q10 (ALC+Arg+Co-Q10), while the other 30 participants received metformin. The study duration was 12 weeks, and the dropout rates were relatively low in the ALC+Arg+Co-Q10 group, with five participants dropped due to conception. The drop-out ratio was a bit higher in the metformin group, where nine participants were lost to follow-up with only a single pregnancy. The CONSORT flow chart of the participant’s recruitment and randomization is shown in Figure 1.
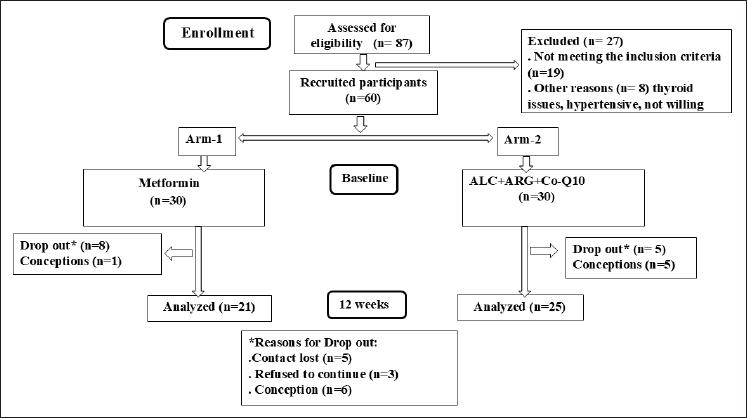 | Figure 1. Flow diagram of the participants in the groups. [Click here to view] |
Baseline characteristics
At baseline, the mean age of participants in the ALC+Arg+CoQ10 group was 27.68 ± 4.83, and 26.15 ± 5.58 years in the metformin group, with no statistically significant difference (p = 0.33). The ALC+Arg+CoQ10 group had a significantly higher BMI (33.30 ± 2.37 kg/m2) compared to the metformin group (29.85 ± 4.76 kg/m2, p = 0.0028). Similarly, the WC was notably higher in the ALC+Arg+CoQ10 group (117.6 ± 5.83 cm) than in the metformin group (102.3 ± 10.50 cm, p < 0.0001). No significant differences were observed in other baseline parameters, including height, weight, HC, systolic blood pressure (SBP), fasting glucose, FSH, MDA, and right and left ovary volumes (Table 1).
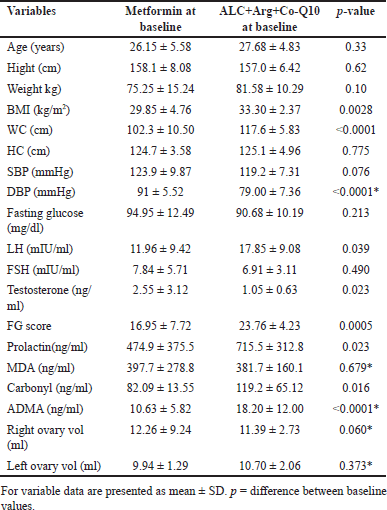 | Table 1. Baseline parameters in individual groups. [Click here to view] |
ALC+Arg+CoQ10 treatment led to significant improvements in anthropometric measures
After 12 weeks of intervention, both treatment groups showed significant reductions in weight, BMI, WC, and HC. In the ALC+Arg+CoQ10 group, weight decreased from 81.58 ± 10.29 kg to 78.28 ± 9.84 kg (p < 0.0001), and BMI reduced from 33.30 ± 2.37 kg/m2 to 31.67 ± 2.31 kg/m2 (p < 0.0001). The WC also significantly decreased in the ALC+Arg+CoQ10 group (from 117.6 ± 5.83 cm to 113.3 ± 5.91 cm, p < 0.0001), showing a greater percentage reduction as compared to the metformin group (Table 2).
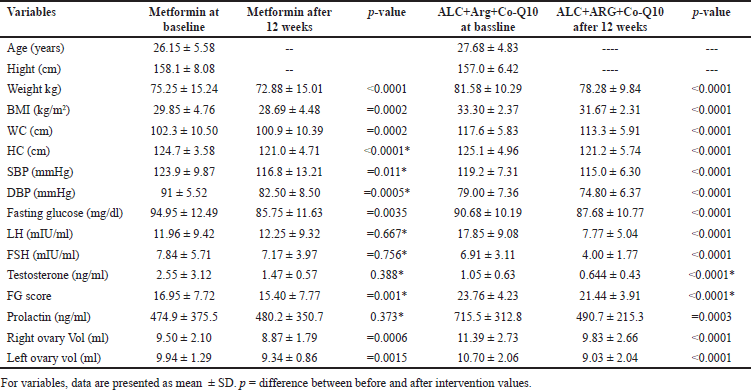 | Table 2. Comparison of parameters at baseline and after 3 months of treatment in both groups. [Click here to view] |
ALC+Arg+CoQ10 therapy significantly improved hormonal and metabolic profiles
The ALC+Arg+CoQ10 group showed significant reductions in LH, FSH, and testosterone levels. LH decreased from 17.85 ± 9.08 to 7.77 ± 5.04 (p < 0.0001), FSH decreased from 6.91 ± 3.11 to 4.00 ± 1.77 (p < 0.0001), and testosterone levels reduced from 1.05 ± 0.63 to 0.644 ± 0.43 (p < 0.0001). Additionally, prolactin levels significantly decreased in the ALC+Arg+CoQ10 group (from 715.5 ± 312.8 to 490.7 ± 215.3, p = 0.0003), whereas no significant change was observed in the metformin group. Fasting glucose levels also showed a significant reduction in the ALC+Arg+CoQ10 group, decreasing from 90.68 ± 10.19 to 87.68 ± 10.77 (p < 0.0001). While the metformin group also exhibited a decrease in fasting glucose levels, the magnitude of reduction was more pronounced in the ALC+Arg+CoQ10 group, indicating a potential beneficial effect on glucose metabolism in PCOS patients (Table 2).
ALC+Arg+CoQ10 treatment improved ovarian morphology
Both right and left ovarian volumes showed a significant decrease in the ALC+Arg+CoQ10 group. The right ovary volume reduced from 11.39 ± 2.73 to 9.83 ± 2.66 ml (p < 0.0001), and the left ovary volume decreased from 10.70 ± 2.06 to 9.03 ± 2.04 ml (p < 0.0001), indicating an improvement in ovarian morphology post-treatment (Table 2).
ALC+Arg+CoQ10 therapy ameliorated oxidative stress markers
The intervention with ALC+Arg+CoQ10 led to a significant decrease in oxidative stress markers. ADMA levels dropped significantly in the integrative therapy (p = 0.0084) with a similar statistically significant decrement in the level of carbonyl (p < 0.0001), suggesting improvements in endothelial function and mitochondrial health compared to the metformin group, where the carbonyl level only dropped significantly (p = 0.0195). However, MDA levels did not show a significant change in either group (Table 2).
ALC+Arg+CoQ10 treatment resulted in a notable reduction in dermatological issues
The study also evaluated the Ferriman–Gallwey (FG) score, which measures the severity of hirsutism in women with PCOS. A significant reduction in FG scores was observed in the ALC+Arg+CoQ10 group, decreasing from 23.76 ± 4.23 to 21.44 3.91 (p < 0.0001). This reduction was more substantial compared to the metformin group, suggesting that the combination therapy may effectively alleviate androgen-related dermatological issues in PCOS patients (Table 2).
Enhanced metabolic and hormonal benefits of ALC+Arg+CoQ10 over metformin in PCOS management: a comparative analysis
Table 3 presents a comparative analysis of the percentage changes in various parameters after 12 weeks of treatment. The ALC+Arg+CoQ10 group experienced a BMI reduction of −4.84%, which was greater than the −3.88% reduction in the metformin group. WC decreased by −3.63% in the ALC+Arg+CoQ10 group, compared to −1.32% in the metformin group. Testosterone levels dropped by −39.04% in the ALC+Arg+CoQ10 group, slightly less than the −42.35% reduction in the metformin group. The FG score declined by −9.76% in the ALC+Arg+CoQ10 group, compared to a −9.14% reduction in the metformin group. Prolactin levels also showed a notable decrease of −31.4% in the ALC+Arg+CoQ10 group, while the metformin group experienced only a minimal increase (+1.11%).
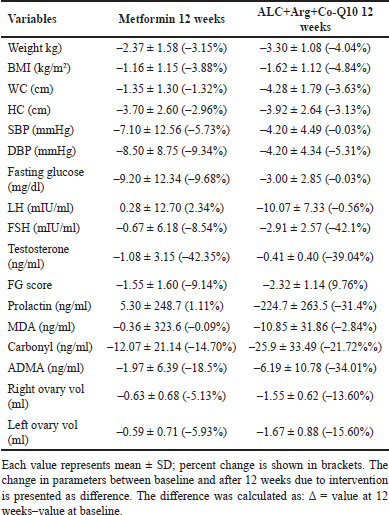 | Table 3. Comparison of the change in parameters with intervention in the two treatment groups. [Click here to view] |
The ALC+Arg+CoQ10 group demonstrated a more substantial reduction in advanced oxidative stress markers, with ADMA levels decreasing by −34.01% compared to −18.5% in the metformin group (Fig. 2). Carbonyl levels also reduced by −21.72% in the ALC+Arg+CoQ10 group, compared to −14.70% in the metformin group. Finally, ovarian morphology improved more favorably in the ALC+Arg+CoQ10 group, with the right ovary volume decreasing by−13.60% and the left by −15.60% compared to the reductions of −5.13% and −5.93%, respectively, in the metformin group. While some markers, such as MDA and fasting glucose, did not show statistically significant differences between the groups, the overall trend suggests a more favorable impact of the ALC+Arg+CoQ10 combination on metabolic and endocrine profiles in women with PCOS.
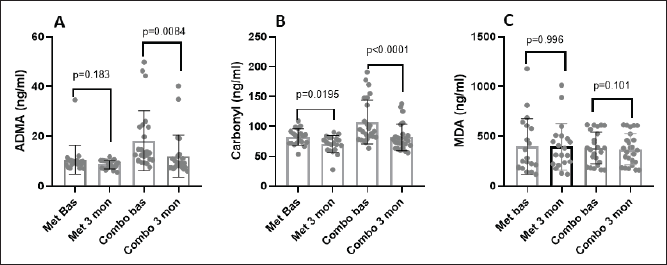 | Figure 2. Shows the comparison of mean ± SD serum levels of ADMA (A), carbonyl (B), and MDA (C) at baseline and after treatment in. [Click here to view] |
DISCUSSION
This study demonstrates that the integrative administration of ALC, arginine, and CoQ10 significantly improves oxidative, metabolic, and endocrine parameters in overweight/obese women with PCOS. Over the 12-week intervention period, notable reductions were observed in BMI, WC, blood pressure, and HC, which are crucial factors in managing PCOS-related metabolic disturbances. At baseline, the group receiving ALC+Arg+Co-Q10 had a higher BMI and WC compared to the metformin group. Despite this initial disadvantage, the combination therapy led to significant reductions in these parameters, highlighting its potential efficacy in addressing central obesity in women with PCOS. We found it consistent with a study done by Martelli et al. [27] on CoQ10 alone. This decrease in anthropometric measures is important, as central obesity is closely linked with an increased risk of CVD in this population [28]. Besides changes in body composition, the therapy resulted in significant improvements in markers of oxidative stress and endothelial function. Plasma levels of ADMA, which is a marker of endothelial dysfunction showed a significant reduction post-intervention which indicates enhanced endothelial function. Carbonyl levels reflect disruptions in fatty acid metabolism and mitochondrial function, also decreased significantly. It suggests an improvement in mitochondrial health. Martelli et al. [27] study on CoQ10 has similar findings. However, MDA levels, another oxidative stress marker, did not show a significant change. This difference in oxidative stress markers suggests that the combined therapy may target specific metabolic pathways. It likely focuses on improving mitochondrial function and enhancing fatty acid oxidation.
The intervention showed positive effects on hormonal profiles. Testosterone and LH levels decreased significantly. These hormones are typically elevated in PCOS. Their reduction may help improve menstrual irregularities and hyperandrogenism. Martelli et al. [27] reported similar outcomes in their study which showed that CoQ10 supplementation reduced oxidative stress and inflammation markers like MDA and C-reactive protein). CoQ10 also lowered inflammatory cytokines such as TNF-alpha and IL-6. These cytokines contribute to endocrine and vascular dysfunction in PCOS. Martelli et al. [27] also observed reductions in LH and testosterone levels. This suggests CoQ10 may influence hormone regulation directly. They noted that combining CoQ10 with alpha-tocopherol might enhance these effects. Samimi et al. [29] found carnitine supplementation reduces weight, BMI, and waist and HC. It also improved glycemic control but did not affect free testosterone levels [29]. This suggests that the decrease in testosterone in our study might be due to CoQ10 rather than carnitine. The intervention also significantly reduced the FG score. This score measures the severity of hirsutism in PCOS. Hirsutism is a symptom of hyperandrogenism. The reduction in FG scores suggests the therapy can reduce androgen-related symptoms. This aligns with Salehpour et al. [30] who reported that L-carnitine supplementation decreased hirsutism in PCOS. Karamali and Gholizadeh [31] found CoQ10 supplementation reduced both hirsutism and testosterone levels. This supports the role of CoQ10 in reducing androgen symptoms [31]. Another study showed that carnitine combined with chromium improved mental health, reduced testosterone, and lowered FG scores in PCOS [32]. Battaglia et al. [26] found no effect of L-arginine on FG scores. The reduction in hirsutism in our study is likely due to L-carnitine or CoQ10. These findings highlight the potential of CoQ10 and carnitine in managing hyperandrogenic symptoms.
Prolactin levels were evaluated in this study. Elevated levels are often associated with various endocrine disorders, including PCOS. The intervention with ALC+Arg+Co-Q10 led to a significant reduction in prolactin levels. It indicates a potential normalizing effect on pituitary function. These findings are consistent with a study that compared L-Carnitine with CoQ10 and Vitamin E for idiopathic male infertility. It demonstrated a reduction in prolactin levels in both groups which highlights the potential impact of this combination therapy in addressing hormonal imbalances in PCOS [33]. However, it is important to note that other studies, such as the one conducted by Krogh et al. [34] on arginine supplementation in lactating sows, reported an increase in serum prolactin concentrations. This difference may suggest that the effect of arginine on prolactin levels could vary depending on the physiological context and the specific population studied. Nonetheless, the reduction observed in our study further supports its multifaceted role. Ovarian volume is a key diagnostic criterion of PCOS and showed a significant reduction in both the right and left ovaries after the 12-week intervention. The decrease in ovarian volume suggests an improvement in ovarian morphology. It could be indicative of restored ovarian function. This aligns with the previous study. A trial found improved reproductive morphology with L-carnitine, CoQ10, and Vitamin E for idiopathic male infertility [33]. This alignment in results further supports the positive impact of the therapy on reproductive aspects of PCOS, in addition to the reduction in hormonal imbalances, such as LH and testosterone.
Improvements in secondary metabolic markers, such as fasting glucose, suggest the therapy's potential for enhancing overall metabolic health. Similar findings were reported in a study on the integrative use of ALC, L-carnitine, L-arginine, and N-acetylcysteine in overweight or obese patients with PCOS. Their results showed significant improvements in metabolic dynamics and hepatic insulin extraction, despite differences in drug composition [19]. The antioxidant properties of ALC and CoQ10 contribute to improvements in metabolic and cardiovascular functions. Arginine also supports these improvements through its vasodilatory effects. This combination is effective not only in managing metabolic and hormonal disturbances in PCOS but also in other conditions. Pathak [35] reported that this combination improves respiratory health through their antioxidant properties and enhanced cellular energy metabolism. CoQ10 supports mitochondrial function and reduces oxidative stress. These properties make it beneficial in chronic inflammation and respiratory dysfunction. The findings in this study align with these observations. The integrative use of these compounds may have broader therapeutic applications, including respiratory diseases.
This study to the best of our knowledge is the first to examine the combined effects of ALC+Arg+Co-Q10 in women with PCOS. It highlights the potential of this combination therapy as a novel treatment strategy. The approach targets multiple metabolic and endocrine pathways. Significant improvements were observed in oxidative stress markers, hormonal profiles, and anthropometric measures. These findings suggest that this intervention offers multifaceted benefits for women with PCOS. The most significant limitation of this study was the absence of separate groups to assess the effects of ALC+Arg+Co-Q10 individually. Separate treatment arms for ALC, L-arginine, and CoQ10 were not included. This omission makes it unclear which drug contributed most to the therapeutic effects. A control group with lifestyle modifications only was also not included. Such a group would have allowed for a clearer distinction between the effects of lifestyle changes and pharmacological treatment. Key metabolic markers like fasting insulin and HOMA-IR were not assessed. Including these markers could have provided better insights into the insulin-sensitizing effects of the therapy. Future randomized controlled trials should be done to cover these gaps. According to the present study, it could be concluded that supplementation with ALC+Arg+Co-Q10 is an effective treatment regimen for PCOS especially for women with overweight and obesity issues. Thereby, routine assessment of oxidative stress parameters like ADMA and carbonyl is suggested to check the response to treatment. Other parameters such as testosterone, prolactin, LH should also be followed. Further long-term follow-up work is required to determine the effectiveness and implication of this therapeutic regime.
CONCLUSION
This study shows that the combined use of ALC+Arg+Co-Q10 can improve metabolic, oxidative, and endocrine markers in overweight or obese women with PCOS. Significant reductions were observed in BMI, WC, and oxidative stress markers such as ADMA and carbonyl. Hormonal imbalances, prolactin levels, and ovarian volume also improved with this treatment. These findings suggest that this combination therapy could be a promising approach for managing PCOS. More studies are needed to identify the specific effects of each component, evaluate long-term outcomes, and refine treatment methods. This research offers a basis for creating focused strategies to reduce the various risks linked to PCOS.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
This project is sponsored by Prince Sattam bin Abdulaziz University (PSAU) as part of funding for its SDG Roadmap Research Funding Programme project number PSAU-2023-SDG-138.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details is given in the ‘Material and Methods section’.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2(1):1–18. CrossRef
2. Bulsara J, Patel P, Soni A, Acharya S. A review: brief insight into polycystic ovarian syndrome. Endocrinol Metab. 2021;3:100085. CrossRef
3. Yang J, Chen C. Hormonal changes in PCOS. J Endocrinol. 2024;261(1):e230342. CrossRef
4. Oliveira TF, Comim FV. Understanding hirsutism in PCOS. Expert Rev Endocrinol Metab. 2024;19(2):103–10. CrossRef
5. Mastnak L, Herman R, Ferjan S, Janež A, Jensterle M. Prolactin in polycystic ovary syndrome: metabolic effects and therapeutic prospects. Life. 2023;13(11):2124. CrossRef
6. Osibogun O, Ogunmoroti O, Michos E. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovas Med. 2020;30(7):399–404. CrossRef
7. Awonuga AO, Camp OG, Abu-Soud H. A review of nitric oxide and oxidative stress in typical ovulatory women and in the pathogenesis of ovulatory dysfunction in PCOS. Reprod Biol Endocrinol. 2023;21(1):111.
8. Dowsett L, Higgins E, Alanazi S, Alshuwayer NA, Leiper FC, Leiper JJ, et al. ADMA: a key player in the relationship between vascular dysfunction and inflammation in atherosclerosis. J Clin Med. 2020;9(9):3026. CrossRef
9. Avci E, Karabulut A, Alp AG, Baba B, Bilgi CJ. Crucial markers showing the risk of coronary artery disease in obesity: ADMA and neopterin. J Med Biochem. 2020;39(4):452. CrossRef
10. Zeng X, Huang Q, Long SL, Zhong Q, Mo ZJ. Mitochondrial dysfunction in polycystic ovary syndrome. DNA Cell Biol. 2020;39(8):1401–9. CrossRef
11. Finsterer J. Mitochondrial dysfunction in polycystic ovary syndrome. Reprod Sci. 2023;30(5):1435–42. CrossRef
12. Sun C, Zhao S, Pan Z, Li J, Wang Y, Kuang H. The Role played by mitochondria in polycystic ovary syndrome. DNA Cell Biol. 2024;43(4):158–74. CrossRef
13. Zhang L, Pornpattananangkul D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17(6):585–94. CrossRef
14. Ribas GS, Vargas CR. Evidence that oxidative disbalance and mitochondrial dysfunction are involved in the pathophysiology of fatty acid oxidation disorders. Mol Cell Neurosci. 2022;42(3):521–32. CrossRef
15. Ferrer MJ, Silva AF, Abruzzese GA, Velázquez ME, Motta AB. Lipid metabolism and relevant disorders to female reproductive health. Curr Med Chem. 2021;28(27):5625–47. CrossRef
16. Virmani MA, Cirulli MJ. The role of l-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int J Mol Sci. 2022;23(5):2717. CrossRef
17. Durante WJ. Amino acids in circulatory function and health. In: Wu G, editor. Amino acids in nutrition and health. Cham, Switzerland: Springer; 2020. pp. 39–56.
18. Khan Z, Khan H, Shah MA. Antioxidant activity of coenzyme-Q; bright and dark side. antioxidants effects in health. Amsterdam, The Netherlands: Elsevier; 2022. pp. 323–40. CrossRef
19. Genazzani AD, Prati A, Genazzani AR, Battipaglia C, Simoncini T, Szeliga A, et al. Synergistic effects of the integrative administration of acetyl-L-carnitine, L-carnitine, L-arginine and N-acetyl-cysteine on metabolic dynamics and on hepatic insulin extraction in overweight/obese patients with PCOS. GREM. 2020;1:56–63.
20. Genazzani AD, Tomatis V, Manzo A, Ressa F, Caroli M, Piccinini M, et al. Treatment with carnitines, L-arginine and N-acetyl cysteine in patients affected by functional hypothalamic amenorrhea (FHA) induces hormonal and metabolic changes. Eur Gynecol Obstet. 2020;2:239–45. CrossRef
21. Tippairote T, Bjørklund G, Gasmi A, Semenova Y, Peana M, Chirumbolo S, et al. Combined supplementation of coenzyme Q10 and other nutrients in specific medical conditions. J Nutr. 2022;14(20):4383. CrossRef
22. Talenezhad N, Mohammadi M, Ramezani-Jolfaie N, Mozaffari-Khosravi H, Salehi-Abargouei A. Effects of l-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin Nutr ESPEN. 2020;37:9–23. CrossRef
23. Pahlavani N, Jafari M, Sadeghi O, Rezaei M, Rasad H, Rahdar HA, et al. L-arginine supplementation and risk factors of cardiovascular diseases in healthy men: a double-blind randomized clinical trial. F1000Res. 2014;3:306. CrossRef
24. Zhang T, He Q, Xiu H, Zhang Z, Liu Y, Chen Z, et al. Efficacy and safety of Coenzyme Q10 supplementation in the treatment of polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Sci. 2023;30(4):1033–48. CrossRef
25. Orio F, Palomba S, Colao A, Russo T, Dentico C, Tauchmanova L, et al. GH release after GHRH plus arginine administration in obese and overweight women with polycystic ovary syndrome. J Endocrinol Invest. 2003;26:117–22.
26. Battaglia C, Mancini F, Battaglia B, Facchinetti F, Artini PG, Venturoli SJ. L-arginine plus drospirenone-ethinyl estradiol in the treatment of patients with PCOS: a prospective, placebo controlled, randomised, pilot study. J Gynaecol Endocrinol. 2010;26(12):861–8. CrossRef
27. Martelli A, Testai L, Colletti A, Cicero A. Coenzyme Q10: clinical applications in cardiovascular diseases. Antioxidant. 2020;9(4):341. CrossRef
28. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–1010. CrossRef
29. Samimi M, Jamilian M, Ebrahimi FA, Rahimi M, Tajbakhsh B, Asemi ZJ. Expression of concern: oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Endocri. 2016;84(6):851–7. CrossRef
30. Salehpour S, Nazari L, Hoseini S, Moghaddam PB, Gachkar LJ. Effects of L-carnitine on polycystic ovary syndrome. JBRA Assist Reprod. 2019;23(4):392. CrossRef
31. Karamali M, Gholizadeh MJ. The effects of coenzyme Q10 supplementation on metabolic profiles and parameters of mental health in women with polycystic ovary syndrome. J Gynaecol. Endocrinol. 2022;38(1):45–9. CrossRef
32. Jamilian M, Foroozanfard F, Kavossian E, Aghadavod E, Amirani E, Mahdavinia M, et al. Carnitine and chromium co-supplementation affects mental health, hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. J Psychosom Obstet Gynecol. 2019:1–9. CrossRef
33. Ma L, Sun Y. Comparison of L-Carnitine vs. Coq10 and vitamin E for idiopathic male infertility: a randomized controlled trial. Eur Rev Med Pharmacol Sci. 2022;26(13):4698–704. CrossRef
34. Krogh U, Farmer C, Huber LA, Theil PK, Trottier NL. Impact of arginine supplementation on serum prolactin and mRNA abundance of amino acid transporter genes in mammary tissue of lactating sows. Anim Sci J. 2020;98(11):skaa335. CrossRef
35. Pathak A. A crucial role of arginine, carnitine, and coenzyme Q20: as a natural treatment for respiratory diseases. WJPPS. 2020;9:1106–29. CrossRef