INTRODUCTION
Cardiotoxicity refers to substances that harm the heart, potentially leading to conditions such as arrhythmia, heart attacks, or enlargement of the heart muscle [1]. The harmful effects of anthracyclines on the heart are well-known and alkylating agents have been associated with heart damage as well. This group includes drugs like cyclophosphamide (CP), ifosfamide, cisplatin, busulfan, and mitomycin. These medications can lead to heart-related issues including fluctuations in blood pressure, irregular heartbeats, inflammation of the heart muscle or the surrounding sac, heart compression, heart attacks, shock, and chronic heart muscle disease [2].
Worldwide, the primary cause of mortality and premature death is still cardiovascular disease (CVD). Of the 18.6 million fatalities globally in 2019 attributed to CVD, the majority of 58% occurred in Asia [3]. CP cardiotoxicity varies from 7% to 28%, with abrupt heart failure occurring in 7%–33% of instances following high-dose intravenous therapy. The mortality rate ranges from 11% to 43%, usually occurring in 7–21 days. Acute toxicity is seen in 22% of individuals at IV dosages of 170–180 mg/kg over 4–7 days, with 11% experiencing severe side effects [4].
CP, a medication from the nitrogen mustard family, is used to treat various cancers, including both malignant and non-malignant types [5]. It is a cytotoxic bi-functional alkylating agent. It has a broad range of therapeutic applications, but in both people and laboratory animals it shows significant cytotoxicity to normal cells [6]. CP is metabolized by the liver to produce aldophosphamide, which then breaks down into phosphoramide mustard and acrolein. Acrolein is a toxic metabolite that possesses treatment for endothelial cells and the myocardium [7].
CP disrupts heart mitochondrial function leading to oxidative stress and increased harmful oxygen radicals, ultimately damaging the heart. Therefore, antioxidant therapy could be beneficial in managing cardiotoxicity caused by CP [8].
The Apocynaceae family, which includes Carissa carandas (Karonda) is known for its wide range of biological activities beneficial in treating various health issues, including rheumatism, enhancing appetite, fever reduction, serving as an astringent and managing brain diseases [9], antipyretic, anthelmimintic, antidiabetic, aphrodisiac, analgesic, anti-inflammatory, bitter stomachic, vermifuge, and anorexia [10]. Additionally, it possesses anthelmintic, cardiotonic, and blood pressure-lowering properties [11]. The roots have strong cardiotonic action in addition to containing cardiac glycosides and salicylic acid, which produce a slight drop in blood pressure. Studies have also detected volatile compounds in the roots, including lignan, carinol, sesquiterpenes like carissone and carindone, along with lupeol, β-sitosterol, 16β-hydroxybetulinic acid, α-amyrin, β-sitosterol glycoside, and des-N-methylnoracronycine, an acridone alkaloid [12].
This study is to explore the pharmacological assessment of the hydroalcoholic root extract of C. carandas L. in relation to CP-induced cardiotoxicity in male Wistar rats by explaining the effects of CP on the heart in rats by examining histology, metabolic markers, and electrocardiogram (ECG) data, and investigating the protective properties of C. caranda and the underlying processes. The study’s conclusions also have significance for the creation of fresh therapy strategies to lessen the negative effects of CP on heart function, enhancing cancer patients’ quality of life and treatment results.
MATERIALS AND METHODS
Collection of plant
The roots of C. carandas Linn were collected from the local region of Belagavi District, Karnataka, India, during the post-monsoon season (October to November), which is known to be the period when the roots are likely to have a higher concentration of phytocompounds. The authentication process was carried out by Dr. Divya Khare at Shri B.M.K Ayurvedic Mahavidyalaya, Belagavi. Following verification, the specimen was officially deposited and cataloged under the CRF code CRF/Auth/204/2023. This meticulous authentication ensures the accuracy and reliability of the botanical material used in the study.
Preparation of extract
The roots C. carandas linn underwent an initial cleaning in running water followed by drying and crushing into a coarse powder. This powder was immersed in a solution of ethanol and water (70:30v/v) for 5 days with regular manual stirring. The solution was then filtered using a rotary evaporator to evaporate. The resulting extract was diluted in distilled water to suitable concentrations daily for application in animal experiments [13].
In silico methodology
Target identification, network construction, and cluster analysis
The canonical SMILES were used to anticipate the targets for the C. carandas from the superpred database. The targets for CP-induced cardiotoxicity were retrieved from the gene card database using the keyword CP-induced cardiotoxicity. The targets identified in CP-induced cardiotoxicity were compared with proteins known to be modulated by C. carandas with the aid of Venny 2.1.0. The protein-protein interaction network and KEGG-enriched pathways were then constructed using the common targets as input into STRING version 11.5 [14]. Furthermore, KEGG analysis was performed to determine pathways related to cardiotoxicity that are impacted by C. carandas. These results were then included in version 3.7.0 of Cytoscape to produce compound-pathway-protein interactions. The K-means clustering module of STRING with a clustering coefficient of five clusters was used to conduct the cluster analysis.
Gene ontology analysis
Through the use of the STRING server, gene ontology analysis was carried out in order to obtain GO terms, such as molecular function (MF), biological process (BP), and cellular component (CC), which help predict the components of a cell where molecules may act and the response the cell may deliver. The test agent’s impact at the cellular level is predicted by integrating GO keywords. An analysis was conducted on the top 10 items of BP, CC, and MF [15].
In-vivo study
Experimental animals
The study was conducted on healthy male Wistar rats, weighing between 150 and 200 g. They were kept under standard laboratory conditions with free access to a pelleted diet and water. After a 7-day acclimatization period, the rats were randomly divided into different groups. Ethical approval for the experiments was granted by the animal ethics committee of KLE College of Pharmacy, Belagavi. (Ethical number 221/Po/Re/S/2000/CPSCEA).
Experimental treatment
A total of six rats were placed into each of the five groups at random. For 21 days, food and water were provided daily to Group I, which acted as the control. On the first day of the trial, Group II, the CP control, received a single intraperitoneal dosage of CP (100 mg/kg). Group III received a single dose of CP on the first day (100 mg/kg) and continuous oral administration of Simvastatin (10 mg/kg) for 21 days. Group IV received a single dose of CP on the first day (100 mg/kg) and continuous oral administration of C. carandas linn root extract (500 mg/kg) for 21 days. Group V was given 1,000 mg/kg of C. carandas linn root extract orally continuously for 21 days, along with a single dosage of CP (100 mg/kg) on the first day. On the final day of the experimental paradigm, the animals were starved for 18 hours. Ether anesthesia was used to put the animals to sleep. After being collected in tubes, blood samples were centrifuged for 10 minutes at 4,000 RPM. The resultant serum was kept at −20°C in a refrigerator until additional biochemical research was conducted.
Determination of cardiac markers enzymes
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and creatine kinase-MB (CK-MB) were measured using commercial kits obtained from AGD Biomedicals (P) Ltd.
Antioxidant parameters
The levels of reduced glutathione (GSH) and cardiac malondialdehyde (MDA), indicative of lipid peroxidation, were determined [16]. The superoxide dismutase (SOD) in cardiac tissue homogenate was assessed following the protocol [17].
Body weight assessment
Throughout the study, the BW of the animals was measured on a weekly basis. The average change in body weight was calculated and reported in grams.
ECG pattern
Every animal received anesthesia through intraperitoneal injections of ketamine and xylazine, dosed at 45 mg/kg and 5 mg/kg, respectively. Following this, their ECG readings were captured using a Power Lab 26T system. Once fully anesthetized, the animal was positioned on a table and ECG electrodes were attached to the upper left limb, lower left limb, and right forelimb. The ECG recordings were then collected for a minimum duration of 30 seconds [18].
Histopathological studies
Small samples of heart tissue were excised from each experimental group and then immersed in a 10% formalin solution for fixation. After fixation, the specimens underwent embedding in paraffin wax following the standard protocol. Subsequently, serial sections with a thickness of 5 µm were sliced and stained with hematoxylin and eosin (H&E). These stained sections were later scrutinized and photographed under light microscopy to detect and evaluate any histopathological variances or alterations between the experimental groups and the control.
Statistical analysis
GraphPad Prism version 8.02 was used to enter and analyze the data. The results were statistically analyzed using one-way ANOVA and Tukey’s Multiple Comparison Test to assess significance. The results were reported as Mean ± Standard Error of Mean (SEM). A criterion of p < 0.05 was used to establish significance.
RESULTS
In silico methodology
Target identification, network construction, and cluster analysis
The gene database was used to identify 234 targets that were thought to be related to cardiotoxicity. According to the super pred database, the chemical was also projected to modify 119 targets, 3.1% of which overlapped with targets linked to cardiotoxicity. By adjusting 21 proteins, it was discovered that 3.1% of the targets were shared. Among these common targets, 21 proteins were modulated (Fig. 1). Protein-protein interaction analysis was conducted via STRING (Fig. 2a). Cluster analysis revealed 3 clusters with cluster 1 has the maximum genes, i.e., 14 CDK2, EP300, ESR1, HIF1A, HSP90AA1, MAPK1, NFE2L2, NOS3, RXRA, SLC2A1, STAT3, TERT, TOP2A, and TP53 (Fig. 2b). Analysis of pathways associated with cardiotoxicity identified 14 pathways given in Table 1. A compound-pathway-protein interaction network was constructed, highlighting MAPK1, NOS3, EP300, and STAT3 having the highest edge count of 14, 11, 9, and 9. Among six phytocompounds, cardenolides exhibited the highest modulation, with an edge count of 8, neighborhood connectivity of 7.625, and radiality of 0.70731707 (Fig. 3). Cytohubba analysis of the merged network is shown in Figure 4. The PI3K-AKT signaling pathway (hsa04151) emerged as a main pathway modulated by the compound, having a false discovery rate of 2.78E-05, strength of 1.21, and gene ratio of 0.171 (Table 1 and Fig. 5).
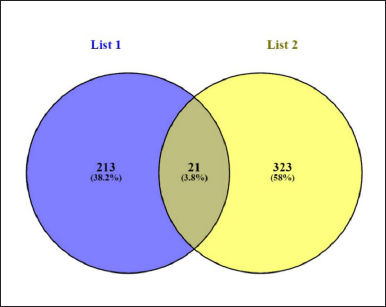 | Figure 1. The Venn diagram representation of common genes between C. carandas linn and cardiotoxicity. [Click here to view] |
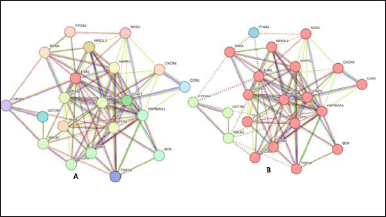 | Figure 2. (a) STRING identified protein-protein interaction for C. carandas Linn, and (b) Cluster analysis where cluster 1: red; cluster 2: green; cluster 3: blue. [Click here to view] |
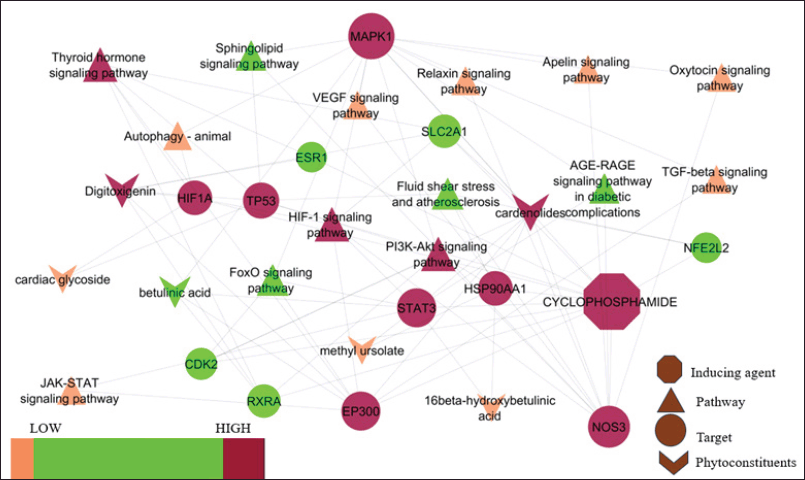 | Figure 3. Protein pathway interaction for C. carandas Linn phytoconstituents. [Click here to view] |
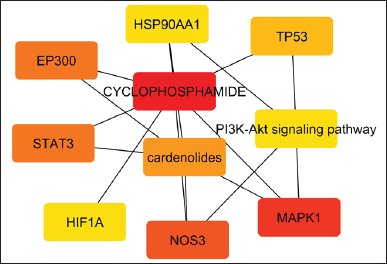 | Figure 4. Cyto hubb analysis of merged network. [Click here to view] |
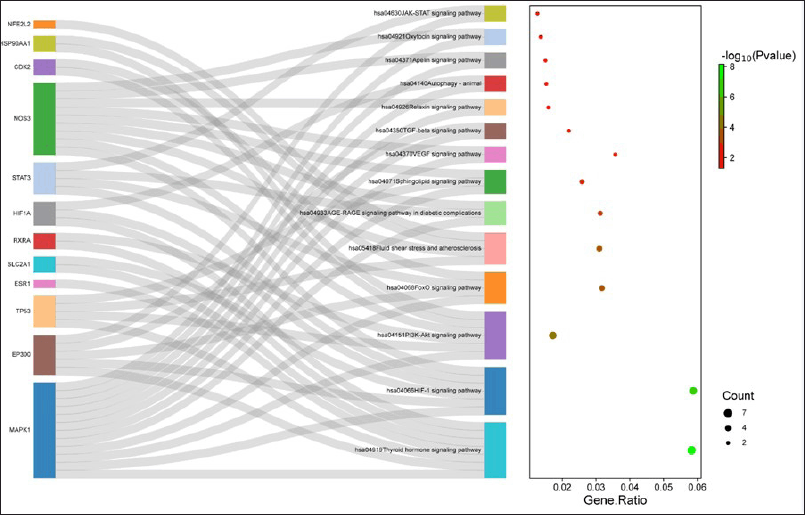 | Figure 5. KEGG pathway analysis. [Click here to view] |
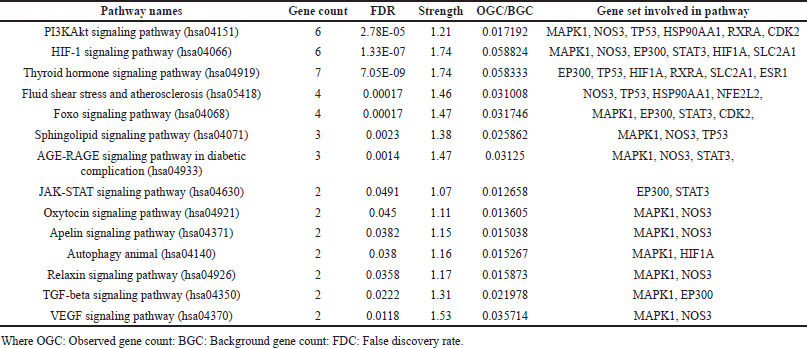 | Table 1. KEGG pathways analysis. [Click here to view] |
Gene ontology analysis
GO analysis revealed 16 CC with Cytoplasm (GO:0005737) showing the lowest false discovery rate of 0.0442, modulating 24 observed genes, i.e., MAPK1, EP300, STAT3, CDK2, TP53, CCR5, NOS3, BCR, UGT2B7, TERT, PGR, SP1, HSP90AA1, PTGS1, PTGS2, FOXO4, NFE2L2, CXCR4, ESR1, TOP2A, SLC2A1, RXRA, HIF1A, and CYP3A4 against 12,056 background genes and strength of 0.18. A total of 44 MFs were identified, with Transition metal ion binding acting on protein (GO:0046914) having the minimal FDR of 0.0436 and involving 7 observed genes: EP300, TP53, NOS3, PGR, ESR1, RXRA, and CYP3A4, against a background of 1,104 genes. Similarly, 325 biological processes (BP) were identified, among which Response to chemical (GO:004221) exhibited the lowest FDR of 1.41E-09. This process was modulated by 23 observed genes: MAPK1, EP300, STAT3, CDK2, TP53, CCR5, NOS3, BCR, TERT, PGR, SP1, HSP90AA1, PTGS1, PTGS2, FOXO4, NFE2L2, CXCR4, ESR1, SLC2A1, RXRA, HIF1A, ABCB1, and CYP3A4, against a BGC of 4010. The selected MF, BP, and CC have been depicted in a bar graph format, as illustrated in (Fig. 6).
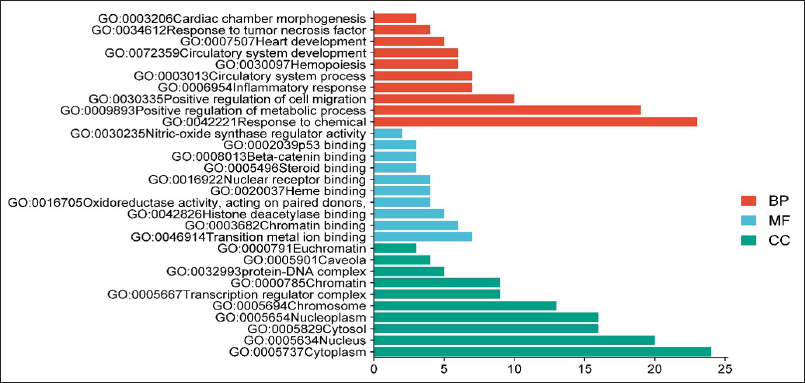 | Figure 6. Gene ontology for the top 10 BP (orange), MF (blue), and cellular component (Green). [Click here to view] |
In-vivo study
Animals body weight
On the 0th, 7th, 14th, and 21st days, the animals in the various groups had their body weight changes observed. The findings indicated reductions in BW following treatment with CP related to the normal rats. However, conversely, the groups administered doses of 500 and 1,000 mg/kg BW exhibited an increase in body weight from day 0th to day 21st when compared to the CP-treated groups, with all increments being statistically significant (p < 0.001). Additionally, the group treated with simvastatin a standard shows a significant increase in BW (p < 0.001) compared to the CP-treated rats, reaching levels comparable to those of the normal group. (Table 2 and Fig. 7).
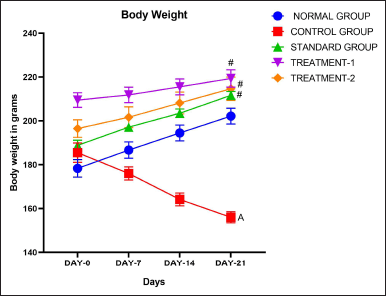 | Figure 7. Body weight of animals. [Click here to view] |
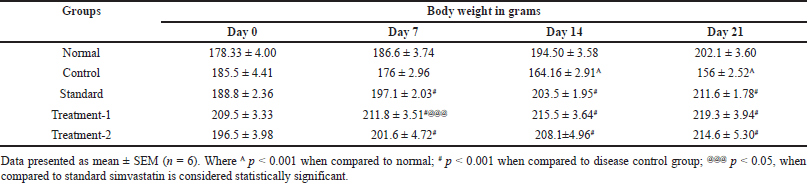 | Table 2. Effect of C. carandas Linn root extract on body weight of CP-induced cardiotoxicity in rats. [Click here to view] |
Cardiac markers
Rats administered with CP had significantly higher levels of cardiac enzymes, especially CK-MB, than the control group; this difference was statistically significant (p < 0.001). In contrast, compared to the CP-treated group, administration with the extract at dosages of 500 and 1,000 mg/kg BW resulted in a substantial decrease in cardiac enzyme levels (p < 0.001, p < 0.001). Comparing the cardiac enzyme levels of the standard therapy group to the CP-treated group, there was a substantial (p < 0.001) drop in the simvastatin group. CK-MB levels were significantly (p < 0.05) higher in the treatment group receiving 500 mg/kg than in the group receiving simvastatin when compared to the standard group. Furthermore, the comparison with the dose of 1,000 mg/kg showed therapeutic value even if it did not yield statistical significance (Table 3 and Fig. 8).
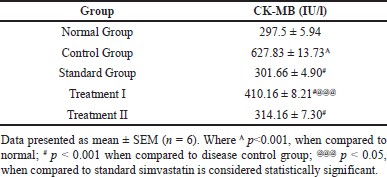 | Table 3. Effect of C. carandas Linn root extract on cardiac markers in CP-induced cardiotoxicity in rats. [Click here to view] |
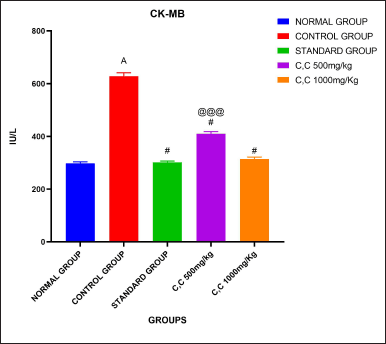 | Figure 8. Cardiac markers analysis. [Click here to view] |
Qualitative test of Troponin-I rapid test
It is designed to be used as a screening test by professionals and provide a preliminary test result to aid in the early diagnosis of acute myocardial infarction. The troponin-1 combo rapid test is a lateral flow chromatographic immunoassay designed for the qualitative detection of cardiac troponin I in human serum. Troponin-I is present in the disease group but not in the normal, standard, and treated groups indicating that this qualitative test is used to identify early detection of myocardial infarction.
Oxidative stress markers
The table presents data on antioxidant enzyme activity SOD, lipid peroxidation levels MDA, and GSH levels in different experimental groups. In the Normal group, SOD activity, MDA levels, and GSH levels are within typical ranges. Conversely, the Control group exhibits significantly reduced SOD activity, increased MDA levels, and decreased GSH levels when compared to the normal group thus indicating oxidative stress and decreased antioxidant capacity. The Standard group shows restoration of anti-oxidant enzyme activity and maintenance of lipid peroxidation and GSH levels (231.01 ± 1.67#), suggesting potential therapeutic effects. However, Treatment-I displays further improvement in SOD activity and reduction in MDA levels compared to the Control group, though still lower than the Normal group. Treatment II demonstrates restored SOD activity and comparable MDA and GSH levels to the Standard group implying effective mitigation of oxidative stress. Overall while the Control group presents oxidative stress markers, both exhibit improvements, with Treatment II achieving outcomes comparable to the Standard group indicating potential therapeutic efficacy in ameliorating oxidative stress-related conditions. The results are depicted in Table 4 and in Figure 9a, 9b, and 9c, respectively.
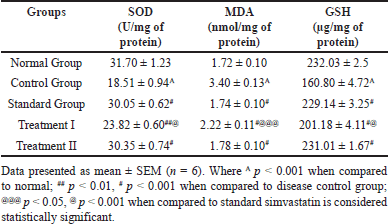 | Table 4. Effect of C. carandas Linn root extract on Oxidative stress markers in CP-induced cardiotoxicity in rats. [Click here to view] |
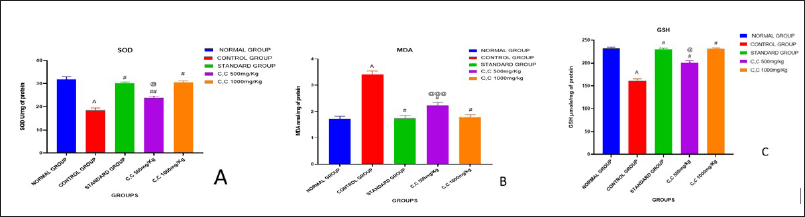 | Figure 9. Oxidative stress markers in CP-induced cardiotoxicity in rats. (a). SOD (b). MDA (c). GSH. [Click here to view] |
Effect of C. carandas Linn root extract on heart weight (HW) and relative heart weight (RHW)
The results indicate that in the normal group, both HW (721 ± 0.009) and relative heart weight (0.3570 ± 0.005) are within typical ranges. However, the Control group exhibits significantly increased HW (0.9036 ± 0.018A) and RHW 0.5799 ± 0.014A compared to the Normal group, suggesting potential cardiac hypertrophy (Fig. 10 (a) and (b)). Contrastingly, the Standard group displays reduced heart weight and relative heart weight when compared to the Control group, indicating potential therapeutic effects in mitigating cardiac hypertrophy. The group treated with 500 mg/kg and 1,000 mg/kg reduced heart weight when compared to the control group. While the control group exhibits cardiac hypertrophy, both Treatment groups show potential therapeutic effects, with Treatment II achieving outcomes similar to the Standard group, indicating promising efficacy in addressing cardiac hypertrophy (Table 5).
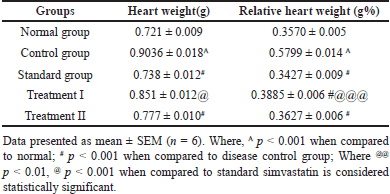 | Table 5. Effect of C. carandas Linn root extract on heart weight(g) and relative heart weight (gm%) changes in CP-induced cardiotoxicity in rats. [Click here to view] |
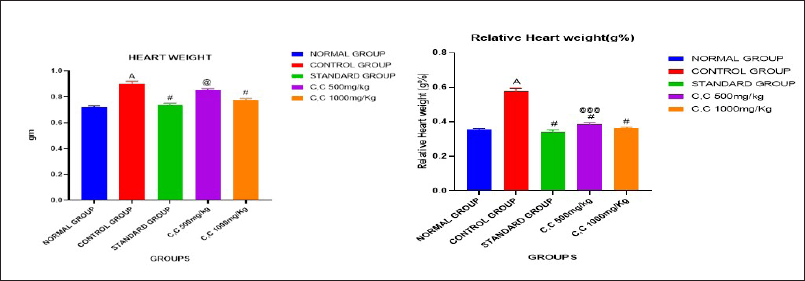 | Figure. 10 (a). Heart weight (b). Relative heart weight. [Click here to view] |
Effect of C. carandas Linn root extract on serum enzyme changes in CP-induced cardiotoxicity in rats
It was noticed that in the Normal group, enzyme levels are reported within typical ranges, serving as a baseline comparison. Conversely, the Control group exhibits significantly increased levels of AST, ALT, and ALP when compared to the normal group suggesting potential heart dysfunction or damage in this group. Contrastingly, the Standard group displays lower enzyme levels relative to the Control group indicating a potential therapeutic effect or mitigation of heart damage. Notably, both Treatment-I and Treatment II groups showcase reductions in enzyme levels (Table 6).
 | Table 6. Effect of C. carandas Linn root extract on serum enzyme changes in CP-induced cardiotoxicity in rats. [Click here to view] |
Electrocardiogram of rats
The results indicate that the normal group exhibited a baseline ECG pattern characterized by a heart rate of 360.83 ± 4.17 beats per minute (BPM), a P-R interval of 0.047 ± 0.0001 milliseconds (msec), a Q-T interval of 0.046 ± 0.0005 msec, and an R wave amplitude of 0.35 ± 0.008 millivolts (mV). Animals induced with CP showed significant deviations in these parameters compared to the normal group (heart rate: 264.83 ± 3.06 BPM; P-R interval: 0.064 ± 0.0001 msec; Q-T interval: 0.068 ± 0.0003 msec; R wave amplitude: 0.24 ± 0.004 mV), indicative of cardiotoxicity. However, treatment with C. carandas Linn root extract (Treatment-I) resulted in notable improvements in ECG parameters when compared to the control group, albeit not fully restored to normal levels (heart rate: 335.16 ± 1.78 BPM; P-R interval: 0.054 ± 0.0002 msec; Q-T interval: 0.056 ± 0.001 msec; R wave amplitude: 0.31 ± 0.003 mV). This suggests a potential protective effect of the extract against CP-induced cardiotoxicity, though not as effective as the standard treatment or Treatment II, which showed closer resemblances to the normal group’s ECG pattern (Table 7 and Fig. 11a–e).
 | Table 7. Effect of C. carandas Linn root extract on ECG pattern CP-induced cardiotoxicity in rats. [Click here to view] |
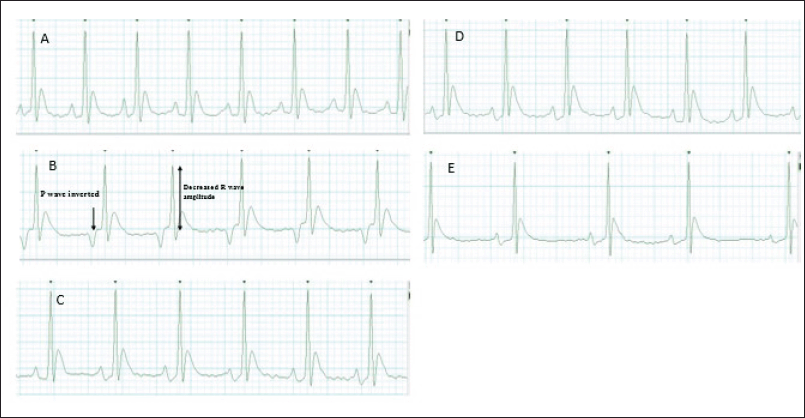 | Figure 11. ECG pattern. (a). Normal group, (b) Control group, (c). Standard group, (d). Treatment I (500 mg/kg), and (e) Treatment II (1,000 mg/kg). [Click here to view] |
Histopathological studies
Histological analysis of normal hearts revealed typical cardiac muscle fibers with regular transverse striations. Connective tissues and intercalated disks were observed between muscle bundles. Fibroblast nuclei and Lipofuscin granules were also present (Fig. 12a). CP-treated animals show changes in cardiac muscle fibers, including increased interfiber spaces, interstitial edema, and inflammation. The endomysium was thickened, with infiltration of inflammatory cells (Fig. 12b). Similarly, standard treatment with simvastatin and the extract at a dose of 1,000 mg/kg demonstrated mild congestion and maintained similar structural integrity compared to normal animals (Fig. 12c).
Heart sections from CP-treated rats receiving 500 mg/kg and 1,000 mg/kg BW showed restoration of muscle fibers, with reduced congestion related to the control group (Fig. 12d). Most cardiomyocyte nuclei appeared centrally positioned with a normal shape (Fig. 12e). Treatment with 1,000 mg/kg effectively mitigated CP-induced cardiac damage by reducing infiltration of inflammatory cells and fragmentation of myofibril.
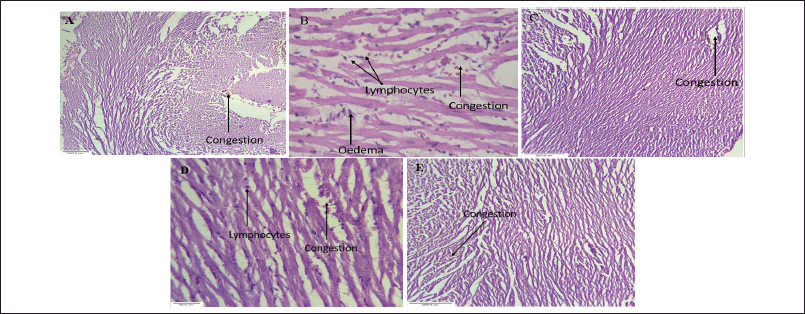 | Figure 12. Histopathological studies (a). Normal group, (b). Control group, (c). Standard group, (d). Treatment(500 mg/kg), and (e). Treatment(1,000 mg/kg). [Click here to view] |
DISCUSSION
The results obtained from the study on the effects of C. carandas Linn root extract on CP-induced cardiotoxicity in rats reveal significant findings across various parameters like Target Identification, network analysis, gene ontology analysis, KEGG Pathway analysis, In Vivo Studies and Troponin-I Rapid Test.
The in-silico methodology employed identified several targets implicated in cardiotoxicity and those modulated by C. carandas Linn root extract. A protein-protein interaction network was constructed, revealing key pathways involved, such as the PI3K-Akt signaling pathway among all 6 phytocompounds cardenolides is highly modulated by possessing the highest edge count 8, neighborhood connectivity 7.625 and radiality 0.70731707 (Fig. 3). Cluster analysis revealed 3 clusters with cluster 1 having the maximum number of proteins, i.e., 14 CDK2, EP300, ESR1, HIF1A, HSP90AA1, MAPK1, NFE2L2, NOS3, RXRA, SLC2A1, STAT3, TERT, TOP2A, and TP53. The cluster analysis further elucidated the clustering of genes/proteins, highlighting clusters with significant gene counts, suggesting potential key players in the regulatory network. Gene Ontology analysis provided insights into the cellular components, MFs, and biological processes affected by C. carandas Linn root extract. Significant modulation of various genes involved in cellular processes and signaling pathways were observed. The KEGG pathway analysis identified specific pathways related to cardiotoxicity, including the PI3K -Akt signaling pathway, HIF-1 signaling pathway, and others. These pathways possess crucial roles in cardiotoxicity and are modulated by the extract, indicating its potential therapeutic effects [19,20].
Treatment with C. carandas Linn root extract led to increases in BW compared to the CP-treated animals., indicating potential protective effects against weight loss associated with cardiotoxicity. Body weight increases in the treatment group due to its antioxidant, anti-inflammatory, and enhanced metabolic function. The extract showed significant reductions in cardiac enzyme levels CK-MB compared to the CP-treated group, suggesting protective effects against cardiac damage.
Oxidative stress markers: Treatment with the extract resulted in the restoration of antioxidant enzyme activitySOD, reduction in lipid peroxidation levels MDA, and maintenance of GSH indicating its antioxidant properties and ability to mitigate oxidative stress. The extract showed significant reductions in serum enzyme levels (AST, ALT, and ALP), indicating potential protective effects against heart damage associated with cardiotoxicity [21,22]. The extract demonstrated potential in reducing heart weight and relative heart weight, suggesting its efficacy in preventing cardiac hypertrophy induced by CP. The increase in heart weight could be attributed to several factors such as heightened vascular hemorrhage, edema, and necrosis of cardiac muscle fibers. This progression may subsequently lead to the infiltration of damaged tissues by inflammatory cells. These combined effects could contribute to the overall increment in heart weight [23].
The administration of CP results in an increase in cellular sodium content and reduced potassium(K+) content, potentially contributing to the prolongation of the QT interval. This elongation may primarily be attributed to the loss of potassium. Additionally, CP-induced atrioventricular (AV) block, leading to an extended PR interval, could be a significant factor in QT interval prolongation. The underlying reasons for AV block include alterations in parasympathetic tone and deformations in the conduction system. These factors collectively contribute to the prolongation of both PR and QT intervals in CP-induced cardiotoxicity [24–26]. Treatment with the extract showed improvements in ECG parameters when compared to the CP-treated group, indicating potential protective effects against cardiotoxicity.
Histological analysis showed that the extract treatment mitigated degenerative changes in cardiac muscle fibers caused by CP. Most fibers regained their normal shape and arrangement, with mild congestion, significantly less than the CP-treated group. Additionally, treatment with 1,000 mg/kg effectively prevented CP-induced cardiac damage by reversing inflammatory cell infiltration and myofibril fragmentation. This action of C. carandas Linn could be attributable to its phytoconstituents such as cardenolide and betulinic acid which are known to its cardiotonic effects on myocardial [27–29]. The phytoconstituents found in C. carandas L, along with others, likely contribute to its protective effect against CP-induced cardiotoxicity. Qualitative testing using the Troponin-I rapid test indicated the absence of cardiac Troponin I in the normal, standard, and treated groups, while it was present in the disease group, suggesting its utility in early detection of myocardial infarction.
Overall, the findings suggest that C. carandas Linn root extract possesses significant cardioprotective effects against “CP-induced” cardiotoxicity, mediated by modulation of key pathways, attenuation of oxidative stress, preservation of cardiac structure and function, and maintenance of physiological parameters such as body weight and cardiac enzyme levels.
CONCLUSION
Carissa carandas Linn root extract demonstrates significant potential in ameliorating CP-induced cardiotoxicity in rats. Through comprehensive analysis, it was observed that the extract effectively countered the detrimental effects of CP on body weight, cardiac markers, oxidative stress markers, serum enzymes, ECG patterns, and histopathological changes. Notably, the treatment group led to improvements in body weight, cardiac enzyme levels, oxidative stress markers, and ECG parameters, suggesting its protective role against cardiotoxicity. Additionally, histological examination revealed that the extract mitigated degenerative changes in cardiac muscle fibers and reduced inflammation. These findings underscore the therapeutic potential of C. carandas Linn root extract in protecting CP-induced cardiotoxicity, potentially through modulation of various molecular targets and pathways implicated in cardiac dysfunction. Further research is required to elucidate the underlying mechanisms and optimize therapeutic strategies for clinical translation.
ACKNOWLEDGMENTS
The authors are grateful to KLE College of Pharmacy, KAHER Belagavi for providing the instruments and software to accomplish the research work.
LIST OF ABBREVATIONS
ALT, Alanine transaminase; AST, Aspartate transaminase; CK-MB, creatine kinase isoenzyme MB; CVD, Cardiovascular disease; GSH, Reduced glutathione.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was conducted without external financial support or funding from any agencies or resources.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee of KLE College of Pharmacy, KLE Academy of Higher Education and Research, Belagavi, India [Approval No. 221/Po/Re/S/2000/ CPSCEA].
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ma W, Wei S, Zhang B, Li W. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front Cell Dev Biol. 2020 Jun 3;8:434.
2. Simbre VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy: implications for children. Pediatric Drugs. 2005 May;7:187–202.
3. Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC: Asia. 2021 Jun 1;1(1):1–3.
4. Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, Ali SM, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019 Feb 1;218:112–31.
5. Tripathi DN, Jena GB. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology. 2008 Jun 27;248(2-3):96–103.
6. Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity: characterising and avoiding the problem. Drugs. 1991 Nov;42:781–95.
7. Alhumaidha KA, Saleh DO, Abd El Fattah MA, El-Eraky WI, Moawad H. Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Canadian J Physiol Pharmacol. 2016;94(2):131–9.
8. El Kiki SM, Omran MM, Mansour HH, Hasan HF. Metformin and/or low dose radiation reduces cardiotoxicity and apoptosis induced by cyclophosphamide through SIRT-1/SOD and BAX/Bcl-2 pathways in rats. Mol Biol Rep. 2020 Jul;47:5115–26.
9. Bint-e-Sadek Y, Choudhury N, Shahriar M. Biological investigations of the leaf extracts of Carissa carandas. Int J Pharm Res Technol. 2013;5:97–105.
10. Galipalli S, Patel NK, Prasanna K, Bhutani KK. Activity-guided investigation of Carissa carandas (L.) roots for anti-inflammatory constituents. Nat Prod Res. 2015 Sep 2;29(17):1670–2.
11. Tesfaye T, Ravichadran YD. Traditional uses, pharmacological action and phytochemical analysis of Carissa carandas Linn. A review. Nat Prod Chem Res. 2018;6(5):1–20.
12. Bhosale SV, Shete RV, Adak VS, Murthy K. A review on Carissa carandas: traditional use, phytochemical constituents, and pharmacological properties. J Drug Deliv Ther. 2020 Dec 15;10(6-s):145–50.
13. Gudasi S, Gharge S, Koli R, Patil K. Antioxidant properties and cytotoxic effects of Oxalis corniculata on human Hepatocarcinoma (Hep-G2) cell line: an in vitro and in silico evaluation. Fut J Pharm Sci. 2023 Mar 28;9(1):25.
14. Gudasi S, Gharge S, Koli R, Kagawad P. Exploring in-silico, in-vitro antioxidant, and cytotoxic potential of valerian wallichii by on cervical epithelial carcinoma (HeLa) cell lines. Chem Biodivers. 2024;21:e202302072.
15. Ternikar SG, Patil MB, Pasha I, Dwivedi PS. Gene ontology enrichment analysis of PPAR-γ modulators from Cassia glauca in diabetes mellitus. J Diabetes Metab Disord. 2021 Dec;20(2):1239–46.
16. Khan HA, Abdelhalim MA, Al-Ayed MS, Alhomida AS. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi J Biol Sci. 2012 Oct 1;19(4):461–4.
17. Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem. 2001 Oct 19;276(42):38388–93.
18. Swamy AV, Patel UM, Koti BC, Gadad PC, Patel NL, Thippeswamy AH. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013 Jan 1;45(1):44–8.
19. Cui G, Shan L, Hung M, Lei S, Choi I, Zhang Z, et al. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol. 2013 Sep 30;168(2):1349–59.
20. Deng RM, Zhou J. The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int Immunopharmacol. 2023 Oct 1;123:110714.
21. Attia AA, Sorour JM, Mohamed NA, Mansour TT, Al-Eisa RA, El-Shenawy NS. Biochemical, histological, and ultrastructural studies of the protective role of vitamin E on cyclophosphamide-induced cardiotoxicity in male rats. Biomedicines. 2023 Jan 28;11(2):390.
22. Tesfaye BA, Berhe AH, Wondafrash DZ, Berhe DF. Cardioprotective effect of crude extract and solvent fractions of Urtica simensis leaves on cyclophosphamide-induced myocardial injury in rats. J Exp Pharmacol. 2021;13:147–60.
23. Ayza MA, Balasubramanian R, Berhe AH. Cardioprotective effect of Croton macrostachyus stem bark extract and solvent fractions on cyclophosphamide-induced cardiotoxicity in rats. Evid Based Complement Alternat Med. 2020;2020:1–13.
24. Levine ES, Friedman HS, Griffith OW, Colvin OM, Raynor JH, Lieberman M. Cardiac cell toxicity induced by 4-hydroperoxycyclophosphamide is modulated by glutathione. Cardiovasc Res. 1993 Jul 1;27(7):1248–53.
25. Chakraborty M, Bhattacharjee A, Kamath JV. Cardioprotective effect of curcumin and piperine combination against cyclophosphamide-induced cardiotoxicity. Indian J Pharmacol. 2017 Jan 1;49(1):65–70.
26. Kolikpinar Y, Yusuf DA, Tekin S, Yildirim S, Azman PD, Atasever A, et al. Investigation of protective effects of Naringin on ECG, cardiac enzymes, cardiac histopathology and 8-OHdG expression in cyclophosphamide-induced cardiotoxicity in rats. Atatürk Üniv Vet Bilim Derg. 2021 Oct 31;16(2):196–203.
27. Salim SM, Yunos NM, Jauri MH, Kamisah Y. Cardiotonic effects of cardiac glycosides from plants ofApocynaceae family. Chulalongkorn Med J. 2020;64(4):459–66.
28. Alruhaimi RS. Betulinic acid protects against cardiotoxicity of the organophosphorus pesticide chlorpyrifos by suppressing oxidative stress, inflammation, and apoptosis in rats. Environ Sci Pollut Res. 2023 Apr;30(17):51180–90.
29. Xia A, Xue Z, Li Y, Wang W, Xia J, Wei T, et al. Cardioprotective effect of betulinic acid on myocardial ischemia reperfusion injury in rats. Evid Based Complement Alternat Med. 2014;2014:573745.