INTRODUCTION
Bacterial cellulose or biocellulose (BC) is an exopolymer composed of β-1,4-D-glucopyranose units. It is usually produced by aerobic bacteria, such as Gluconacetobacter, Sarcina, Enterobacter, Pseudomonas, and Achromobacter genera [1,2]. The biomaterial is biodegradable, environmentally friendly, nontoxic, nonallergenic, chemically stable, and biocompatible. BC film has high purity, crystallinity, degree of polymerization, tensile strength and Young’s modulus, small size diameter of fiber, swelling capacity, and high hydrophilicity, compared to plant cellulose [3]. Bacteria synthesize BC as an extracellular polysaccharide, forming a protective sheath around the cell. Based on its morphological structure, BC is a ribbon-like cellulose nanofiber [4]. BC does not contain components that are difficult to separate in plants, i.e., lignin, pectin, and hemicellulose as found in plant cellulose [5]. After the microbial fermentation process, the BC pellicle is harvested. It contains bacterial cells, metabolic by-products, and nutrient residues, which can be simply separated from the BC network to produce a highly purified film. These distinct characteristics indicate that BC film is a highly advantageous biomaterial for application in various areas and industries [6].
The diameter of BC fibers is around 100 times smaller than plant cellulose fibers, exceptionally permeable, and highly porous. The characteristic allows for the diffusion of various active ingredients into the wound or skin. It absorbs body fluids, such as exudates, because of its swelling capacity. The BC film functions as a physical barrier that effectively eliminates the infiltration of contaminants. Therefore, BC is widely used for wound healing applications [6]. Many types of injuries, such as traumatic injuries, pressure sores, skin tears, diabetic wounds, and donor skin graft sites, use BC as a temporary dressing. BC enhanced the healing process, leading to decreased pain at the wounded sites [7]. Several investigations have demonstrated that composites of BC enhanced the proliferation of corneal stromal cells [8]. BC has been studied for its potential use as a contact lens as well as for its therapeutic application in ocular surgery as a dressing with ciprofloxacin. It has also been explored for its ability to promote bone regeneration in bone transplants and as an implant in cardiac and vascular surgery [9–11]. In the field of cosmetic and personal care, BC as a sheet mask’s base material is loaded with compounds with cleansing, moisturizing, or anti-inflammatory properties, as well as plant extracts [12–15]. BC exhibited excellent swelling characteristics in various chemical compositions, indicating its potential as a carrier for several useful active compounds employed in cosmetics [16]. Furthermore, in drug delivery, the combination Ag-BC demonstrated significant antibacterial efficacy against both Gram-positive and Gram-negative bacteria [7,17]. BC was employed in the production of film-coated paracetamol [18], as well as in protein delivery, macromolecular prodrug delivery, and transdermal drug administration. It is also used in the food industry for low-cholesterol diets, traditional desserts, vegetarian meat substitutes, and food packaging materials, among other uses [6].
Bacteria type or strain affects BC yield and characteristics [19]. One of the bacteria potentially developed in BC production is Lentilactobacillus parafarraginis (basionym: Lactobacillus parafarraginis). Several Lactobacillus species have been widely used in BC production, i.e., Lactobacillus lactis, Lactobacillus plantarum, Lactobacillus brevis, and Lactobacillus acidophilus [20,21]. Lactobacillus sp. is classified as nonpathogenic, safe, and defined as generally recognized as safe [22]. The co-culture of Lactobacillus mali and Gluconoacetobacter xylinus was reported as an appropriate combination to increase BC production [23]. The combination of cultures between Gluconacetobacter sp. gel_SEA623-2 and L. plantarum KCCM 80077 increased the weight and thickness of dry BC (37.83 ± 6.81 g/l and 10.33 ± 0.58 mm) compared to the single culture of Gluconacetobacter sp. gel_SEA623-2 [24]. Under the same physiological conditions, L. hilgardii IITRKH159 produced more BC with finer and thinner fiber, higher crystallinity index (CrI), and smaller crystal size than Komagataeibacter xylinus [25]. Prior research has demonstrated that L. parafarraginis has been utilized to generate BC using the oven-dried technique without casting, yielding desirable properties [26].
Other factors that affect BC quality are fermentation conditions (production method, fermentation time, and inoculum size), source of nutrition, and so on [19,27,28]. The selected method for BC production is a static condition because it produces BC film with better characteristics compared to other methods [29]. Culture medium is one of the essential factors because it provides essential nutrients for bacterial growth and BC production, and influences BC yield as well as structure significantly, including its mechanical and physical properties [30]. Coconut water was chosen as the primary ingredient in the fermentation medium due to its high mineral content and significant amounts of fructose (32.52 ± 0.227–39.04 ± 0.824 mg/ml), glucose (29.96 ± 0.243–35.43 ± 0.510 mg/ml), and sucrose (6.36 ± 0.06–0.85 ± 0.010 mg/ml) [31]. The medium contained the best type of carbon source for high BC yields [32]. Furthermore, applying coconut water as a medium for fermentation yielded BC with enhanced properties compared to the Hestrin Schramm medium and the combination of Hestrin Schramm-coconut water [33,34]. As one of the ingredients of bacterial culture medium, coconut water also lacks of need for pre-treatment [31].
The coconut water used in this study was sourced from the Bondowoso district in East Java, Indonesia. In 2023, East Java ranked as the third largest province in Indonesia in terms of coconut production, with a total of 233.70 thousand tons. Bondowoso district contributed 2,415 tons of coconut production in 2022 [35,36]. Coconut water has traditionally been regarded as waste in coconut processing industries, such as desiccated coconut factories and coconut milk factories [37]. Consequently, there is an abundance of coconut water that can be used as a bacterial growth medium for BC production. Due to its cost-effectiveness, utilizing coconut water as a fermentation medium enhances the economic value of wasted coconut water, particularly in traditional markets [38].
Nevertheless, there may be quality alteration during coconut water storage because of the fermentation performed by natural bacteria, such as L. plantarum, L. paracasei, and Pediococcus sp. [39]. It increased lactic acid bacteria (LAB) quantity and decreased pH value [40]. The microbes caused a content deterioration of coconut water, including sugar as a carbon source of microbial growth [41]. Previous studies showed that different medium conditions and fermentation times affected the yield of BC, and its characteristics [28,42,43]. Furthermore, the application of the casting method in BC manufacturing involves blending the obtained BC, casting it onto a glass plate, and drying it to form a film layer with uniform fiber mass and thickness. This process allows for easier modification of BC with enhanced properties. Hence, this work produced BC by employing different coconut water storage times and fermentation times through the casting method, resulting in cost-effective BC with good characteristics suitable for various uses.
MATERIALS AND METHODS
Materials
Coconut water was obtained from coconut (Cocos nucifera L.) fruit grown in Bondowoso, Indonesia, and harvested in 11–12 months. Lentilactobacillus parafarraginis was obtained from PT Biotechno, Serang, Indonesia, and identified using molecular analysis of the chromosomal 16S rRNA gene at Professor Nidom Foundation, Surabaya, Indonesia (certificate number of 071122/PNF-XI/2022). The polymerase chain reaction method was applied to isolate and amplify the DNA sample. This result was determined using Sanger sequencing. The sequences were compared to data from NCBI BLAST and identified as L. parafarraginis strain F0439 with a homology of 99.67% (Accession number HM596288.1). KH2PO4, NaOH, sucrose, acetic acid, and ammonium sulfate were supplied by Merck (Darmstadt, Germany).
Preparation of inoculum
Lentilactobacillus parafarraginis, as a starter in producing BC film, was grown in the culture medium consisting of coconut water (94%) as the main ingredient rich in nutrients, suspended culture (2%) as a bacterial source, sucrose (2.5%) as a carbon source, acetic acid (1%) for pH adjustment and BC yield enhancement, and ammonium sulfate (0.5%) as a nitrogen source [26,44,45]. The coconut water was left at 25°C for 48 hours, subsequently filtered, and then boiled at 100°C. The heated coconut water was added with sucrose, ammonium sulfate, and acetic acid. The mixture was then stirred and sterilized at 121°C for 15 minutes. The starter bacteria were introduced into the growth medium and inoculated under static incubation at 26°C–30°C for 7 days.
Preparation of BC film
The BC film production used the same method as the preparation of inoculum with different coconut water storage times and fermentation times, i.e., BC with 1-day storage of coconut water and 5 days fermentation time (BC15), BC with 1-day storage of coconut water and 7 days fermentation time (BC17), BC with 1-day storage of coconut water and 9 days fermentation time (BC19), BC with 2-day storage of coconut water and 5 days fermentation time (BC25), BC with 2-day storage of coconut water and 7 days fermentation time (BC27), and BC with 2-day storage of coconut water and 9 days fermentation time (BC29). However, coconut water was placed at room temperature for 1 day and 2 days before preparation. pH of all mediums was determined using a pH meter. Two percent (v/v) inoculum was utilized for all experimental procedures. The inoculum adjusted its turbidity using a spectrophotometer (Thermo Scientific Genesys, Mississauga, Canada) was equivalent to 0.1 at OD600 (approximately 1 × 107 CFU/ml) [46]. The fermentation was carried out at 26°C–30°C for 5, 7, and 9 days statically. pH of the remnant medium was recorded after fermentation completion, and the BC pellicle was harvested, rinsed in running water, and boiled in distilled water for 60 minutes. The wet BC was treated with purification using 0.5 M NaOH at 100°C for 30 minutes. It was then rinsed with running water and immersed several times in distilled water until it reached a neutral pH of 7.0. BC film was blended until smooth, filtered, weighed 13 g, cast onto a glass plate [47] (width of 14 cm and length of 15 cm with 4 mm thickness), and dried at 60°C for 72 hours.
BC film characterization
Determination of organoleptic properties and pH
The organoleptic test of wet and dry BC film was conducted using a human visual appearance at 25°C to observe its color, smell, and surface flatness. The pH determination was performed using a pH meter by immersing 1% of the dry BC film in distilled water [48].
Determination of weight and thickness
Wet BC weight was determined using an analytical balance. Meanwhile, BC thickness measurement was performed using calipers in 5 distinct positions. Moreover, the weight and thickness of dry BC film were determined using the same procedures [48].
Determination of water vapor transmission (WVTR)
Five grams of anhydrous calcium chloride was placed into a weighing bottle. The dry BC was placed onto the top of the bottle. It was kept in desiccator at 75% RH and 25°C for 24 hours. The WVTR was determined using this specific equation:
WVTR = W / S 1
W represents the weight of the sample (gram), whereas S represents the area of the sample (m2) [48].
Determination of moisture content
The dry BC film of 2 × 2 cm size was heated at 90°C for 24 hours. Afterward, the moisture content was determined utilizing the specified equation:
Moisture content = (m1 - m2) / m2 × 100% 2
m1 is the initial mass of the sample (gram), whereas m2 is the final mass of the sample (gram) [48].
Determination of swelling degree
The BC film with 1.5 × 1.5 cm size was weighted and immersed in a 25 ml solution of phosphate buffer at a pH of 7.4 at 25°C for 1, 4, and 6 hours. After each immersion period, the BC film was promptly moved and dried using filter paper to remove any remaining solution on the surface. The film was weighted and calculated for a swelling degree using the following equation:
Swelling degree = (W2 - W1) / W1 x 100% 3
W1 is the initial weight of the sample (gram), while W2 is the weight of the sample after swelling for certain times (gram) [48].
Determination of mechanical strength
The mechanical strength was measured using a universal testing machine (Huang Ta HT-2328, Taichung City, Taiwan) at room temperature to determine tensile strength, elongation at break, and Young’s modulus value. The instrument was operated at a deformation rate of 10 mm/minute [44]. The BC film was cut in 6 cm × 2 cm size for the evaluation.
Analysis of Fourier Transformed Infra-Red (FTIR) spectroscopy
BC film was evaluated for its functional groups using an FTIR spectrometer (Bruker Alpha Eco-ATR, Esslingen, Germany) in ATR (Attenuated Total Reflection) mode. The FTIR spectra of dry BC film were recorded in 4,000–600 cm-1 wavenumbers and the baseline of all spectra was normalized [49].
Analysis of X-Ray Diffraction (XRD)
The X-ray diffractograms were obtained at room temperature using a diffractometer (Rigaku Miniflex 600, Tokyo, Japan) at the Cu Kα radiation wavelength of 1.54 Å. The voltage and current used for operation were 40 kV and 15 mA, respectively. The data were obtained using reflection mode within the 5–90° 2θ range, with a step of 0.02° 2θ intervals. The scan speed was 10°/minute. The CrI and percentage of crystallinity (%crystallinity) of BC films were determined using the following equations:
I002 is the highest value of intensity observed in the diffraction spectrum of the lattice plane (002) at an angle of 2θ between 22° and 23°. Iam is the minimum intensity at 2θ of 18° to 19° [50].
Morphological analysis of scanning electron microscopy (SEM)
The surface morphology of the dried BC film was examined using SEM (Hitachi TM3000, Tokyo, Japan) imaging. The film was attached to a carbon type and covered with platina using an ion sputter coater (Hitachi E-1045, Tokyo, Japan). Photomicrographs were taken at 5,000 magnifications operated at 5 kV [49].
Texture analysis of atomic force microscopy (AFM)
BC film with the best characteristics (BC29) was observed for its morphology with an AFM (Bruker N8 Neos, Santa Barbara, USA). Ambient atmosphere measurement was performed in tapping mode with rectangular silicon cantilevers. The height mode of the image was 5 × 5 µm2 and scanned with the speed of 1–3 Hz [51].
Data analysis
The tests were conducted in triplicates. The results were reported as the mean of all measurements ± the standard deviation. One-way analysis of variance and the least significance difference method were utilized to analyze significant differences between groups, with a significance level (α) of 5%.
RESULTS AND DISCUSSION
BC film production
As shown in Table 1, it was known that the pH of all mediums was around 3.00. The results followed the previous study, which reported that L. parafarraginis strain A1 (KU495926) exhibited higher growth under acidity at pH 3.00 than at pH 7.00 and pH 9.00 [52]. The pH is important for appropriate nutrient uptake, oxidative reaction, solubility, and enhancement in the production of BC film [53]. pH before fermentation of all mediums was higher than the pH after fermentation. Furthermore, the longer fermentation, the more acidic the pH. Therefore, the medium of BC29 showed the most acidic pH. It indicated that fermentation time affected medium acidity. Several studies reported that LAB, such as L. parafarraginis metabolized sugars, including sucrose through a fermentation process to produce several acidic-by products, i.e., lactic, propionic, formic, and acetic acid causing the pH of the medium to decrease [54]. This result concorded with the previous study that used Acetobacter xylinum and oil palm frond juice as a growth medium for the production of BC film. The study showed that the enhancement of fermentation time increased the acidic-by-product concentration and acidity of the medium [55]. Moreover, the pH of medium from coconut water kept for 2 days was lower than 1 day of coconut water. It indicated that coconut water storage caused initial fermentation that decreased the pH value of the medium.
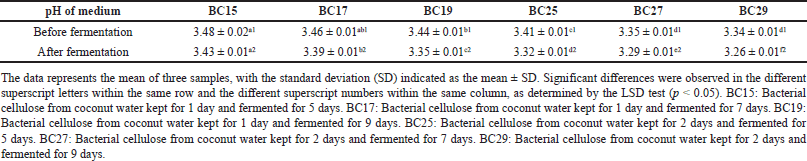 | Table 1. pH of the medium in BC production. [Click here to view] |
The fermentation from the early day to the ninth day is shown in Figures 1 and 2. After several days of fermentation, the pellicles form on the surface of the coconut water medium. This is because L. parafarraginis requires oxygen for its growth (as an aerobic bacteria). The initial stages of pellicle formation varied among media for different times of coconut water storage. Production of BC film was started on the fourth day for coconut water kept for 1 day and the third day for coconut water kept for 2 days. An increase in fermentation time caused an enhancement of the cellulose pellicle’s thickness. It was suggested that optimal BC production was started at the log phase of bacterial growth as the primary metabolite. At this time, sugars as substrates were metabolized to produce energy for its growth and conversion of glucose into cellulose [56].
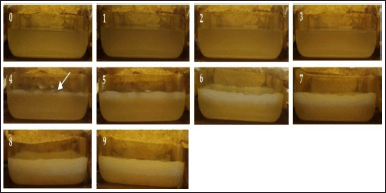 | Figure 1. Fermentation of coconut water kept for 1 day at (0) early day, (1) first day, (2) second day, (3) third day, (4) fourth day, (5) fifth day, (6) sixth day, (7) seventh day, (8) eighth day, and (9) ninth day. The arrow sign indicated the formation of the first cellulose pellicle. [Click here to view] |
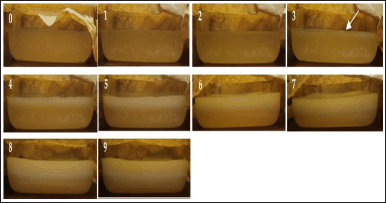 | Figure 2. Fermentation of coconut water kept for 2 days at (0) early day, (1) first day, (2) second day, (3) third day, (4) fourth day, (5) fifth day, (6) sixth day, (7) seventh day, (8) eighth day, and (9) ninth day. The arrow sign indicated the formation of the first cellulose pellicle. [Click here to view] |
Determination of organoleptic properties and pH
The physical appearances of wet and dry BC film were demonstrated in Figures 3 and 4. The wet BC was yellowish–white, odorless, and had a smooth surface. Its organoleptic properties were similar to wet BC produced by L. hilgardii IITRKH159. The harvested BC turned from pale yellow to a yellowish-white film after purification treatment to remove the residual bacterial cells and the medium [25]. BC film fermented for 5 days was slightly more translucent than longer fermented samples. The dry BC films showed brownish color, especially at the edge of the films. The heat from the drying process using the oven probably caused discoloration. Nevertheless, the dry BC was odorless, flat, and smooth. As shown in Table 2, the pH of all dry BC films was in the range of 5.67 ± 0.02–5.89 ± 0.01. The pH value was in the skin pH range (4.5–6.5), indicating that the use of BC films does not cause any irritations, especially for skin usage, such as wound dressing, sheet masks, and so on [57].
 | Figure 3. Wet BC films with various coconut water storage and fermentation times. BC15: Bacterial cellulose from coconut water kept for 1 day and fermented for 5 days. BC17: Bacterial cellulose from coconut water kept for 1 day and fermented for 7 days. BC19: Bacterial cellulose from coconut water kept for 1 day and fermented for 9 days. BC25: Bacterial cellulose from coconut water kept for 2 days and fermented for 5 days. BC27: Bacterial cellulose from coconut water kept for 2 days and fermented for 7 days. BC29: Bacterial cellulose from coconut water kept for 2 days and fermented for 9 days. [Click here to view] |
 | Figure 4. Dry BC film after casting onto glass plates. [Click here to view] |
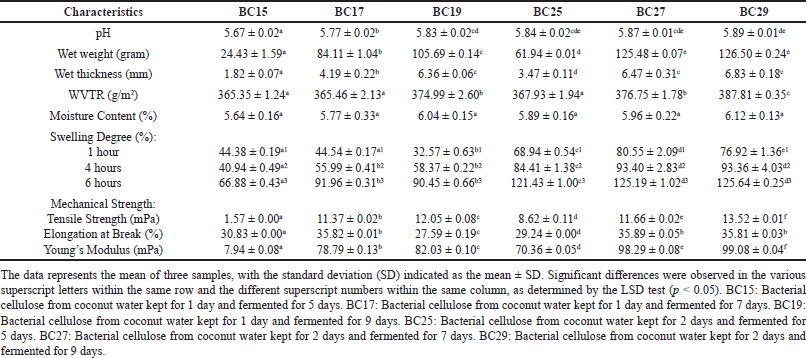 | Table 2. Characterization of BC films with various coconut water storage and fermentation times. [Click here to view] |
Determination of weight and thickness
For equal fermentation time, wet BC film from coconut water kept for 2 days possessed a greater weight and thickness than BC film from 1 day storage time of coconut water (Table 2). The longer the fermentation, the weight and thickness increase. Bacteria produced an increasing amount of cellulose exopolymers as fermentation time increased, which then aggregated to form the fibers of BC. For wet BC with coconut water kept for 1 day, BC19 had the highest weight and thickness (105.69 ± 0.14 g and 6.36 ± 0.06 mm). Whereas, in wet BC film from coconut water kept for 2 days, the weight and thickness of BC27 (125.48 ± 0.07 g and 6.47 ± 0.31 mm) and BC29 (126.50 ± 0.24 g and 6.83 ± 0.18 mm) were not significantly different, but higher than BC25. A previous study also exhibited that BC film produced in 9 days resulted in the highest yield and there was BC yield reduction for 12 and 14 days [55].
Determination of WVTR and moisture content
BC films with coconut water kept for 2 days had a higher WVTR value than BC films from 1 day of coconut water storage (Table 2). The longer the fermentation, the WVTR value increases. Wet BC films produced for 9 days of fermentation (BC19 and BC29) had higher WVTR characteristics than other BCs with equal coconut water storage time. In addition, all BC films showed moisture content characteristics that were not significantly different. The expected value of WVTR was between 300–840 g/m2 to have sufficient occlusive and moisture-retentive characteristics [58]. The expected moisture content value was 5%–10% for dry BC film [59]. If the moisture content was under 5%, the hydrophilic and absorption properties of BC films would decrease. From this study, it was known that all BC films met the requirements of WVTR and moisture content value.
Determination of swelling degree
As shown in Table 2, all dry BCs swelled rapidly in the first hour. The swelling degree value increased until 6 hours, even reaching more than 100% for all BC films with a coconut water storage time of 2 days. Generally, BC films from coconut water kept for 2 days had a higher swelling degree value than BC films from 1 day of coconut water storage. BC19 (90.45% ± 0.66%) and BC29 (125.64% ± 0.25%) resulted in a higher swelling degree for 6 hours than BC films with equal storage time of coconut water. Nevertheless, the values were not significantly different with BC17 (91.96% ± 0.31%) and BC27 (125.19% ± 1.02%). A similar effect of swelling ability was suggested due to similar arrangement and characteristics of fibers and pores that formed the three-dimensional structure of BC films, as shown in SEM images (Fig. 7). BC film with a high swelling degree value showed a high absorption characteristic [60]. Therefore, BC film can be applied as wound dressing preparation that absorbs a lot of exudates or as a cosmetical mask impregnated in serum or essence containing active ingredients [13,60].
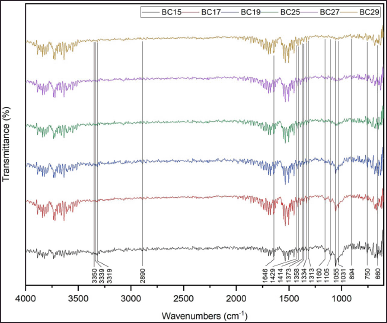 | Figure 5. FTIR spectra of BC films with various coconut water storage and fermentation times. BC15: Bacterial cellulose from coconut water kept for 1 day and fermented for 5 days. BC17: Bacterial cellulose from coconut water kept for 1 day and fermented for 7 days. BC19: Bacterial cellulose from coconut water kept for 1 day and fermented for 9 days. BC25: Bacterial cellulose from coconut water kept for 2 days and fermented for 5 days. BC27: Bacterial cellulose from coconut water kept for 2 days and fermented for 7 days. BC29: Bacterial cellulose from coconut water kept for 2 days and fermented for 9 days. [Click here to view] |
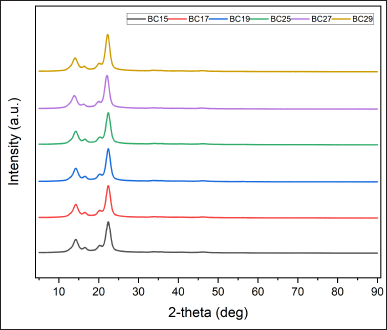 | Figure 6. X-ray diffraction spectra of BC films with various coconut water storage and fermentation times. BC15: Bacterial cellulose from coconut water kept for 1 day and fermented for 5 days. BC17: Bacterial cellulose from coconut water kept for 1 day and fermented for 7 days. BC19: Bacterial cellulose from coconut water kept for 1 day and fermented for 9 days. BC25: Bacterial cellulose from coconut water kept for 2 days and fermented for 5 days. BC27: Bacterial cellulose from coconut water kept for 2 days and fermented for 7 days. BC29: Bacterial cellulose from coconut water kept for 2 days and fermented for 9 days. [Click here to view] |
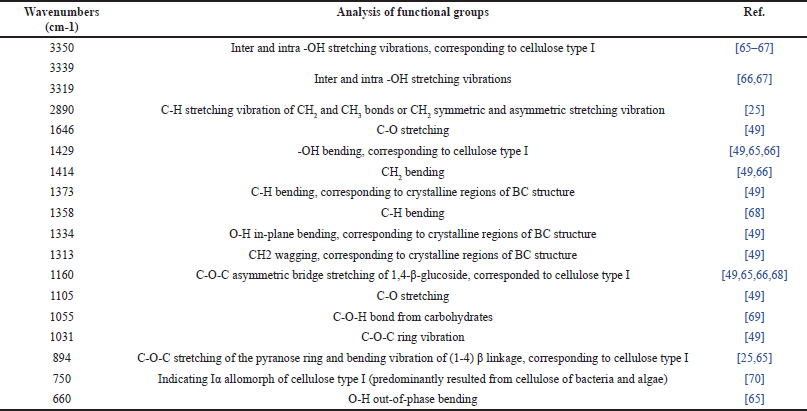 | Table 3. Analysis of FTIR spectra of BC films. [Click here to view] |
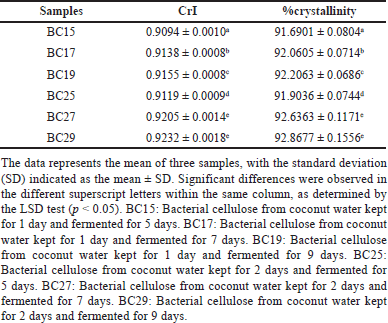 | Table 4. CrI and %crystallinity of BC films. [Click here to view] |
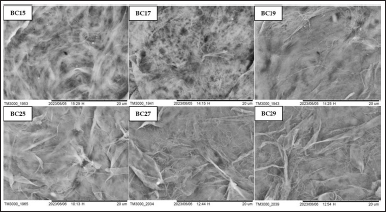 | Figure 7. Scanning electron micrograph of BC films with various coconut water storage and fermentation times. BC15: Bacterial cellulose from coconut water kept for 1 day and fermented for 5 days. BC17: Bacterial cellulose from coconut water kept for 1 day and fermented for 7 days. BC19: Bacterial cellulose from coconut water kept for 1 day and fermented for 9 days. BC25: Bacterial cellulose from coconut water kept for 2 days and fermented for 5 days. BC27: Bacterial cellulose from coconut water kept for 2 days and fermented for 7 days. BC29: Bacterial cellulose from coconut water kept for 2 days and fermented for 9 days. [Click here to view] |
Determination of mechanical strength
The mechanical strength of all BC films is shown in Table 2. In this study, BC film from coconut water kept for 2 days showed higher tensile strength and Young’s modulus value compared to BC film from 1 day of coconut water storage. The longer the fermentation time, the higher the tensile strength and Young’s modulus value. The fermentation duration directly impacts the properties of BC, such as weight and thickness. The weight and thickness of BC increase because of the higher number of fibers produced as fermentation time increases. This will enhance the mechanical strength of BC. BC29 had the highest value of tensile strength and Young’s modulus, whereas BC15 exhibited the lowest value. Additionally, fermentation time enhancement from 5 to 7 days was suggested to increase elongation at break properties. Nevertheless, there was a slight reduction in value for BC film with 9 days of fermentation time. Generally, elongation at break reduction is common for polymers, mainly natural polymers, as it is inversely related to tensile strength [61]. The previous study recommended values of tensile strength and elongation at break for wound dressing were more than 1 mPa and 10% [48]. However, the optimal tensile strength value of the hydrogel mask was more than 0.1 kgf/cm2 [62]. Elongation at break value for sheet mask was more than 30% [63].
Analysis of FTIR spectroscopy
The FTIR spectra of all samples showed several peaks corresponding to the typical BC peaks (Fig. 5). The spectra analysis is shown in Table 3 [25,49,64–69]. Based on its typical peaks, the BC spectra of L. parafarraginis showed similarity with dry BC spectra from L. hilgardii IITRKH159 strain. BC film of L. hilgardii IITRKH159 strain resulted in peaks at around 3,340, 2,900, 1,646, 1,424, 1,360, 1,060, 893, and 750 cm-1 [25]. Dry BC of L. parafarraginis without casting method using 2 days storage time and 10% inoculum size also showed a similar spectrum. Most of the peaks have similar wavenumbers with insignificant shifts [26]. Based on the spectra, it was known that the BC structure had plentiful hydrogen bonds because of many hydroxyl groups and oxygen atoms [70]. The identical spectra of BC film from both bacteria exhibited similar chemical structures. Moreover, the FTIR spectra of BC15, BC17, BC19, BC25, BC27, and BC29 were also identical, implying that the different BC films had similar chemical structures.
Analysis of XRD
The XRD pattern of BC films is presented in Figure 6. The pattern was similar to previous studies that used coconut water as a medium and casting method for its preparation [71,72]. All samples exhibited similar peaks at 2θ of 14.1°, 16.4°, and 22.3°. These diffractions indicated three main peaks representing crystal planes 101, 10?, and 002, respectively. These peaks are typical of the distinctive structure of cellulose I [73]. There was an insignificant shifting of the main peaks for all BC films. The study demonstrated that alterations in the storage times of coconut water in inoculum and fermentation times may affect the crystallinity of BC films. The X-ray diffractograms revealed the strongest primary peaks in BC27 and BC29. This means that the crystallinity increased because there were more crystal components and fewer amorphous components. BC27 and BC29 exhibited the highest CrI values of 0.9205 ± 0.0014 and 0.9232 ± 0.0018 (Table 4). The films also showed the greatest %crystallinity value of 92.6363% ± 0.1171% and 92.8677% ± 0.1556%, respectively (Table 4). Meanwhile, BC15 exhibited the lowest values for CrI (0.9094 ± 0.0010) and %crystallinity (91.6901 ± 0.0804). The study found that increasing the storage times for coconut water and prolonging the fermentation time resulted in a higher level of BC film crystallinity. Overall, BC exhibited a high degree of crystallinity. The BC films produced by A. xylinus had CrI of 0.81 and %crystallinity of 84% in the Hestrin–Schramm medium. In the hot water extract medium, the BC films had CrI of 0.73 and %crystallinity of 79% [50]. Furthermore, another study produced BC film using Acetobacter sp. bacteria, coconut water as the growth medium, and the casting method, resulting in a CrI of 88.58% [74].
Morphological analysis of SEM
The morphological structures of the dry BC film surface were illustrated using SEM analysis at 5,000 magnifications (Fig. 7). BC structure showed irregular fine cellulose fibers arranged in a 3D porous network, which is similar to previous studies [75,76]. The average width of BC fiber using L. parafarraginis was about 0.25–1 µm. It was bigger than BC fiber produced by L. hilgardii IITRKH159 with about 0.039 µm width size [25]. It was suggested that different bacteria, medium, and fermentation conditions affected BC fiber structures.
BC film produced from coconut water with 1 day storage time exhibited a very fine fiber structure with an irregular arrangement and many pores. Whereas BC film with longer fermentation time produced fine fiber structure in smaller quantities and more fiber structure with larger sizes. The result was similar to BC film from coconut water with a storage time of 2 days, but the structure of very fine fiber was less than that of BC film from 1-day storage of coconut water. The BC film with 2 days of storage showed that the fine fiber aggregated to form ribbons. BC25 still had very fine fiber, but it was less than BC27 and BC29. The fiber structure of BC27 and BC29 were larger and the number was more than BC25. Both BC films showed similar structures consisting of overlapping, folding, and random fiber with a slight difference in fiber arrangement and pores. It caused a slight difference in all measured parameters for BC27 and BC29. The larger and more numerous fiber structures in BC27 and BC29 gave better advantages, as mentioned in the previous studies [55,75]. It exhibited better WVTR, moisture content, swelling degree, and mechanical strength properties than other samples. Furthermore, the presence of irregular and folded fiber structures was suggested due to blending and casting processes in the production of dry BC film [26].
Texture analysis of AFM
Figure 8 depicts the micromorphology and microscopic characteristics of the surface of BC29, which is the BC film with the most promising properties. The AFM image exhibited a reticulated structure consisting of an extensive number of randomly orientated and overlapping fibers with irregular ordering. The presence of numerous fibers appeared to aggregate and form a dense bundle and ribbon structure consisting of very fine fibers. Unlike SEM, AFM was able to capture images of fibers that were significantly smaller in width, measuring approximately 0.05 µm. The surface topography of BC29 exhibited a low average roughness (Sa), with a value of 41.83 ± 6.96 nm. A previous study demonstrated that the roughness of BC film produced by using the Hestrin and Schramm medium and molasses medium was 50–90 nm [77]. BC film using the oven-dried method without casting showed a roughness of 21.40 ± 1.80 nm [78]. It seemed that manually preparing BC film with the casting method increased its degree of roughness. In this study, the 3D-AFM image of BC29 confirmed the surface roughness’ homogeneity. The relatively smooth texture of BC29, due to its low degree of roughness, made it suitable for various applications.
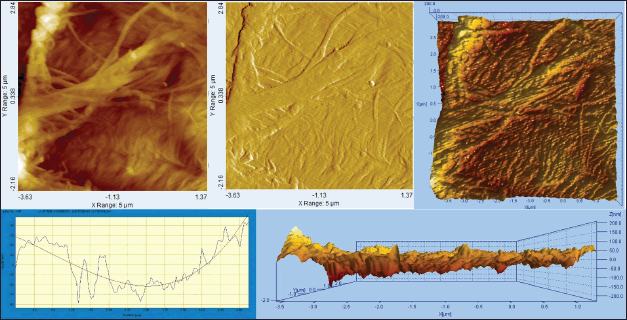 | Figure 8. AFM topography image of BC29. [Click here to view] |
CONCLUSION
This study has successfully developed BC film using L. parafarraginis and coconut water as fermentation medium by casting method. BC film preparation with various storage times of coconut water and fermentation times was studied and concluded that the research design had significant effects on several properties, including its morphological characteristics. Generally, BC film with 2 days storage of coconut water had better characteristics than BC with 1 day storage of coconut water. Furthermore, the longer the fermentation, the better the characteristics. In summary, it was recommended that BC29 had the best conditions to produce the best BC film properties. The FTIR spectra indicated several functional groups of BC film in general. The XRD spectra showed that the crystallinity of all BC films was high. Additionally, the SEM and AFM images of BC29 showed more fiber structure with many pores and its smooth texture giving more advantages, as indicated by its good characteristics.
ACKNOWLEDGMENTS
The authors thank the Faculty of Pharmacy, University of Jember, and Universitas Airlangga for all their support during the research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The research was supported by the Institute for Research and Community Service (LP2M) University of Jember through Hibah Keris Dimas Penelitian under grant project number 3977/UN25.3.1/LT/2022.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hasanin MS, Abdelraof M, Hashem AH, El Saied H. Sustainable bacterial cellulose production by Achromobacter using mango peel waste. Microb Cell Fact. 2023;22(1):1–12. CrossRef
2. Aziz WSWA, Adnan A. Employed bacterial species and bacterial cellulose (BC) applications: the state of play. Squalen Bull Mar Fish Postharvest Biotechnol. 2022;17(3):155–67. CrossRef
3. Liu W, Du H, Zhang M, Liu K, Liu H, Xie H, et al. Bacterial cellulose-based composite scaffolds for biomedical applications: a review. ACS Sustain Chem Eng. 2020;8(20):7536–62. CrossRef
4. Ruka DR, Simon GP, Dean K. Harvesting fibrils from bacterial cellulose pellicles and subsequent formation of biodegradable poly-3-hydroxybutyrate nanocomposites. Cellulose. 2014;21(6):4299–308. CrossRef
5. Bootten TJ, Harris PJ, Melton LD, Newman RH. WAXS and 13C NMR study of Gluconoacetobacter xylinus cellulose in composites with tamarind xyloglucan. Carbohydr Res. 2008;343(2):221–9. CrossRef
6. Ullah H, Santos HA, Khan T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose. 2016;23(4):2291–314. CrossRef
7. Islam SU, Ul-Islam M, Ahsan H, Ahmed MB, Shehzad A, Fatima A, et al. Potential applications of bacterial cellulose and its composites for cancer treatment. Int J Biol. Macromolecules. 2021;168:301–9. CrossRef
8. Dutton JJ. Coralline hydroxyapatite as an ocular implant. Ophthalmology. 1991;98(3):370–7. CrossRef
9. Cavicchioli M, Corso CT, Coelho F, Mendes L, Saska S, Soares CP, et al. Characterization and cytotoxic, genotoxic and mutagenic evaluations of bacterial cellulose membranes incorporated with ciprofloxacin: a potential material for use as therapeutic contact lens. World J Pharm Pharm Sci. 2015;4(7):1626–47.
10. Torgbo S, Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl Mater Today. 2018;11(2):34–49. CrossRef
11. Ko?aczkowska M, Siondalski P, Kowalik MM, P?kas R, D?uga A, Zaj?c W, et al. Assessment of the usefulness of bacterial cellulose produced by Gluconacetobacter xylinus E25 as a new biological implant. Mater Sci Eng C. 2019;97(4):302–12. CrossRef
12. Pacheco G, Nougeira CR, Meneguin AB, Trovatti E, Silva MCC, Machado RTA, et al. Development and characterization of bacterial cellulose produced by cashew tree residuces as alternative carbon source. Ind Crops Prod. 2017;107(15):13–9. CrossRef
13. Pacheco G, De Mello CV, Chiari-Andreo BG, Isaac VLB, Ribeiro SJL, Pecoraro E, et al. Bacterial cellulose skin masksproperties and sensory tests. J Cosmet Dermatol. 2018;17(5):840–7. CrossRef
14. Amorim JDP, Costa AFS, Galdino CJSJ, Vinhas GM, Santos E, Sarubbo LA. Bacterial cellulose production using industrial fruit residues as subtract to industrial application. Chem Eng Trans. 2019;74:1165–70.
15. Amorim JDP, Junior CJGS, Costa AFS, Nascimento HA, Vinhas GM, Sarrubo LA. BioMask, a polymer blend for treatment and healing of skin prone to acne. Chem Eng Trans. 2020;79(1):205–10.
16. Chunshom N, Chuysinuan P, Techasakul S, Ummartyotin S. Dried-state bacterial cellulose (Acetobacter xylinum) and polyvinylalcohol-based hydrogel: an approach to a personal care material. J Sci Adv Mater Devices. 2018;3(3):296–302. CrossRef
17. Lazic T, Falanga V. Bioengineered skin constructs and their use in wound healing. Plast Reconstr Surg. 2011;127 Suppl 1:75S–90S. CrossRef
18. Halib N, Amin MCIM, Ahmad I. Physicochemical properties and characterization of nata de coco from local food industries as a source of cellulose. Sains Malays. 2012;41(2):205–11.
19. Revin VV, Liyas’kina EV, Sapunova NB, Bogatyreva AO. Isolation and characterization of the strains producing bacterial cellulose. Microbiology. 2020;89(1):86–95. CrossRef
20. Adetunji V, Adegoke G. Bacteriocin and cellulose production by lactic acid bacteria isolated from West African soft cheese. Afr J Biotechnol. 2007;6(22):2616–9. CrossRef
21. Sumardee JNS, Mohd-Hairul AR, Mortan SH. Effect of inoculum size and glucose concentration for bacterial cellulose production by Lactobacillus acidophilus. IOP Conf Ser Mater Sci Eng. 2020;991:1–5. CrossRef
22. Belicová A, Mikulášová M, Dušinský R. Probiotic potential and safety properties of Lactobacillus plantarum from Slovak Bryndza cheese. Biomed Res Int. 2013;2013:1–8. CrossRef
23. Seto A, Saito Y, Matsushige M, Kobayashi H, Sasaki Y, Tonouchi N, et al. Effective cellulose production by a coculture of Gluconacetobacter xylinus and Lactobacillus mali. Appl Microbiol Biotechnol. 2006;73(4):915–21. CrossRef
24. Kim KM, Kim J, Yang KW. Effect of acetic acid concentration and mixed culture of lactic acid bacteria on producing bacterial cellulose using Gluconacetobacter sp. gel_SEA623-2. Korean J Microbiol. 2014;50(3):227–32. CrossRef
25. Khan H, Kadam A, Dutt D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr Polym. 2020;229:115513. CrossRef
26. Ningsih IY, Hidayat MA, Kuswandi B, Erawati T. Effect of coconut water storage time and inoculum size of Lentilactobacillus parafarraginis on dried bacterial cellulose properties. Trop J Nat Prod Res. 2024;8(2):6291–99. CrossRef
27. Devanthi PVP, Pratama F, Kho K, Taherzadeh MJ, Aslanzadeh S. The effect of Dekkera bruxellensis concentration and inoculation time on biochemical changes and cellulose biosynthesis by Komagataeibacter intermedius. J Fungi. 2022;8(1206):1–13. CrossRef
28. Hodel KVS, Fonseca LMS, Santos IMS, Cerqueira JC, dos Santos-Junior RE, Nunes SB, et al. Evaluation of different methods for cultivating Gluconacetobacter hansenii for bacterial cellulose and montmorillonite biocomposite production: wound-dressing applications. Polymers (Basel). 2020;12(2):267. CrossRef
29. Wang J, Tavakoli J, Tang Y. Bacterial cellulose production, properties and applications with different culture methods—a review. Carbohydr Polym. 2019;219:63–76. CrossRef
30. Jozala AF, de Lencastre-Novaes LC, Lopes AM, Santos-Ebinuma VC, Mazzola PG, Pessoa Jr A, et al. Bacterial nanocellulose production and application: a 10-year overview. Appl Microbiol Biotechnol. 2016;100(5):2063–72. CrossRef
31. Tan TC, Cheng LH, Bhat R, Rusul G, Easa AM. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem. 2014;142:121–8. CrossRef
32. Rani MU, Udayasankar K, Appaiah KAA. Properties of bacterial cellulose produced in grape medium by native isolate Gluconacetobacter sp. J Appl Polym Sci. 2011;120(5):2835–41. CrossRef
33. Gayathry G. Production of bacterial cellulose from coconut liquid endosperm using Acetobacter xylinum sju-1. J Pure Appl Microbiol. 2013;7(3):1–7.
34. Kamarudin S, Sahaid KM, Sobri TM, Mohtar WY, Radiah ABD, Norhaliza H. Different media formulation on biocellulose production by Acetobacter xylinum (0416). Pertanika J Sci Technol. 2013;21(1):29–36.
35. Badan Pusat Statistik. Produksi perkebunan kelapa sawit dan kelapa menurut Kabupaten/Kota dan jenis tanaman di Provinsi Jawa Timur, 2021-2022. Jakarta, Indonesia: BPS Republik Indonesia; 2023.
36. Badan Pusat Statistik. Produksi tanaman perkebunan, 2023. Jakarta, Indonesia: BPS Republik Indonesia; 2024.
37. Prades A, Dornier M, Diop N, Pain JP. Coconut water preservation and processing: a review. Fruits. 2012;67(3):157–71. CrossRef
38. Nurfajriani N, Pulungan AN, Yusuf M, Bukit N. Preparation and characterization of bacterial cellulose from culturation of Acetobacter xylinum in coconut water media. J Phys Conf Ser. 2021;1811(1):012070. CrossRef
39. Suryani S, Purnawati Y, Putri SG, Rahmawati R, Akbar Y, Yusra Y. Novel probiotic isolation of coconut water’s helpful lactic acid bacteria cure Covid-19 patients. ARRUS J Eng Technol. 2022;2(1):1–11. CrossRef
40. Pramana WA, Septinova D, Riyanti R, Husni A. Pengaruh air kelapa hasil fermentasi terhadap kualitas fisik daging broiler. Jurnal Riset dan Inovasi Peternakan. 2018;2(2):7–13.
41. Santosa B, Wignyanto W, Hidayat N, Sucipto S. The quality of nata de coco from sawarna and mapanget coconut varieties to the time of storing coconut water. Food Res. 2020;4(4):957–63. CrossRef
42. Castro C, Zuluaga R, Putaux JL, Caro G, Mondragon I, Gañán P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym. 2011;84(1):96–102. CrossRef
43. Avirasdya RA, Nursiwi A, Sari AM, Zaman MZ, Sanjaya AP. Kinetics study of bacterial cellulose production by Acetobacter xylinum FNCC 0001 with variation of carbon sources. in E3S Web of Conferences. EDP Sciences. 2022;344:03002. CrossRef
44. Indrianingsih AW, Rosyida VT, Jatmiko TH, Prasetyo DJ, Poeloengasih CD, Apriyana W, et al. Preliminary study on biosynthesis and characterization of bacteria cellulose films from coconut water. in IOP Conf Ser Earth Env. 2017;101:012010. CrossRef
45. Fei S, Fu M, Kang J, Luo J, Wang Y, Jia J, et al. Enhancing bacterial cellulose production of Komagataeibacter nataicola through fermented coconut water by Saccharomyces cerevisiae: a metabonomics approach. Curr Res Food Sci. 2024;8:100761. CrossRef
46. Thongwai N, Futui W, Ladpala N, Sirichai B, Weechan A, Kanklai J, et al. Characterization of bacterial cellulose produced by Komagataeibacter maltaceti P285 isolated from contaminated honey wine. Microorganisms. 2022;10(3):1–18. CrossRef
47. Kuswandi B, Jayus, Oktaviana R, Abdullah A, Heng LY. A novel on-package sticker sensor based on methyl red for real-time monitoring of broiler chicken cut freshness. Packag Technol Sci. 2014;27(1):69–81. CrossRef
48. Sulastri E, Zubair MS, Lesmana R, Mohammed AFA, Wathoni N. Development and characterization of ulvan polysaccharides-based hydrogel films for potential wound dressing applications. Drug Des Devel Ther. 2021;15:4213–26. CrossRef
49. Akintunde MO, Adebayo-Tayo BC, Ishola MM, Zamani A, Horváth IS. Bacterial cellulose production from agricultural Residues by two Komagataeibacter sp. strains. Bioengineered. 2022;13(4):10010–25. CrossRef
50. Kiziltas EE, Kiziltas A, Gardner DJ. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr Polym. 2015;124:131–8. CrossRef
51. Lopes TD, Riegel-Vidotti IC, Grein A, Tischer CA, Farla-Tischer PCS. Bacterial cellulose and hyaluronic acid hybrid membranes: production and characterization. Int J Biol Macromol. 2014;67:401–8. CrossRef
52. Allen-McFarlane R, Allen AD, Bansal G, Eribo B. Isolation and characterization of L. parafarraginis (KU495926) inhibiting multidrug-resistant and extended spectrum β-lactamase gram-negative bacteria. J Microbiol Biotechnol Food Sci. 2019;8(4):1041–53. CrossRef
53. Ramana KV, Tomar A, Singh L. Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J Microbiol Biotechnol. 2000;16:245–8.
54. Bangar SP, Suri S, Trif M, Ozogul F. Organic acids production from lactic acid bacteria: a preservation approach. Food Biosci. 2022;46:101615. CrossRef
55. Supian NNI, Zakaria J, Amin KNM, Mohamad S, Mohamad SFS. Effect of fermentation period on bacterial cellulose production from oil palm frond (OPF) juice. IOP Conf Ser Mater Sci Eng. 2021;1092(1):012048. CrossRef
56. Lathifah AN, Nuryana I. Bacterial cellulose production in the overripe guava juice by Acetobacter xylinum as a solution to reduce organic waste. J Kim Ter Indones. 2021;23(2):41–8.
57. Tranggono RI, Latifah F. Buku pegangan dasar kosmetologi. 1st ed. Jakarta, Indonesia: Sagung Seto; 2014.
58. Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc. 2002;92(1):24–33.
59. Clasen C, Sultanova B, Wilhelms T, Heisig P, Kulicke WM. Effects of different drying processes on the material properties of bacterial cellulose membranes. Macromol Symp. 2006;244:48–58. CrossRef
60. Rivero-Buceta V, Aguilar MR, Hernandez-Arriaga AM, Blanco FG, Rojas A, Tortajada M, et al. Anti-staphylococcal hydrogels based on bacterial cellulose and the antimicrobial biopolyester poly(3-hydroxy-acetylthioalkanoate-co-3-hydroxyalkanoate). Int J Biol Macromol. 2020;162:1869–79. CrossRef
61. Xie F, Pollet E, Halley PJ, Avérous L. Starch-based nano-biocomposites. Prog Polym Sci. 2013;38(10–11):1590–628. CrossRef
62. Surini S, Auliyya A. Formulation of an anti-wrinkle hydrogel face mask containing ethanol extract of noni fruit (Morinda citrifolia L.) for use as a nutracosmeceutical product. Int J Appl Pharm. 2017;9:74–6. CrossRef
63. Ochiai T, Nakayama K, Kiyooka S, Araya N, Moritani N. Liquid-retaining sheet and facial mask. United States patent 2015/0125499 A1. 2015 May 07.
64. Wang SS, Han YH, Chen JL, Zhang DC, Shi XX, Ye YX, et al. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers (Basel). 2018;10(9):963. CrossRef
65. Rusdi RAA, Halim NA, Norizan MN, Abidin ZHZ, Abdullah N, Ros FC, et al. Pre-treatment effect on the structure of bacterial cellulose from Nata de Coco (Acetobacter xylinum). Polimery. 2022;67(3):110–18. CrossRef
66. Saleh AK, El-Gendi H, Soliman NA, El-Zawawy WK, Abdel-Fattah YR. Bioprocess development for bacterial cellulose biosynthesis by novel Lactiplantibacillus plantarum isolate along with characterization and antimicrobial assessment of fabricated membrane. Sci Rep. 2022;12(1):2181. CrossRef
67. Ashori A, Sheykhnazari S, Tabarsa T, Shakeri A, Golalipour M. Bacterial cellulose/silica nanocomposites: preparation and characterization. Carbohydr Polym. 2012;90(1):413–8. CrossRef
68. Li D, Ao K, Wang Q, Lv P, Wei Q. Preparation of Pd/bacterial cellulose hybrid nanofibers for dopamine detection. Molecules. 2016;21(5):618. CrossRef
69. Imai T, Sugiyama J. Nanodomains of Iα and Iβ cellulose in algal microfibrils. Macromolecules. 1998;31(18):6275–9. CrossRef
70. Pogorelova N, Rogachev E, Digel I, Chernigova S, Nardin D, Bacterial cellulose nanocomposites: morphology and mechanical properties. Materials. 2020;13(12):1–16. CrossRef
71. Tsalagkas D, Lagana R, Poljansek I, Oven P, Csoka L. Fabrication of bacterial cellulose thin films self-assembled from sonochemically prepared nanofibrils and its characterization. Ultrason Sonochem. 2016;28:136–43. CrossRef
72. Choudhary P, Jaiswal A, Singh S, Gupta SK. Bacterial cellulose based composites: preparation and characterization. Mater Sci Forum. 2020;978:183–190. CrossRef
73. Feng X, Ullah N, Wang X, Sun X, Li C, Bai Y. Characterization of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917. J Food Sci. 2015;80(10):E2217–27. CrossRef
74. Rahmadiawan D, Shi SC, Abral H, Ilham MK, Sugiarti E, Muslimin AN, et al. Comparative analysis of the influence of different preparation methods on the properties of TEMPO-oxidized bacterial cellulose powder films. J Nat Fibers. 2024;21(1):1–15. CrossRef
75. Pham TT, Tran TTA. Evaluation of the crystallinity of bacterial cellulose produced from pineapple waste solution by using Acetobacter xylinum. ASEAN Eng J. 2023;13(2):81–91. CrossRef
76. Potivara K, Phisalaphong M. Development and characterization of bacterial cellulose reinforced with natural rubber. Materials. 2019;12(14):1–17. CrossRef
77. Revin VV, Dolganov AV, Liyaskina EV, Nazarova NB, Balandina AV, Devyataeva AA. Characterizing bacterial cellulose produced by Komagataeibacter sucrofermentans h-110 on molasses medium and obtaining a biocomposite based on it for the adsorption of fluoride. Polymers. 2021;13:1422. CrossRef
78. He W, Zhang Z, Chen J, Zheng Y, Xie Y, Liu W. Evaluation of the anti-biofilm activities of bacterial cellulose-tannic acid-magnesium chloride composites using an in vitro multispecies biofilm model. Regen Biomat. 2021;8(6):1–17. CrossRef