INTRODUCTION
Depression is a highly prevalent neuropsychiatric condition characterized by multifaceted origins. Prior studies have identified disturbances in the hypothalamic-pituitary-adrenal axis, increased inflammation, glutamatergic and monoaminergic system dysfunctions, and reduced neurogenesis and neuroplasticity [1]. However, these results were not observed in all patients, indicating diversity of pathophysiology associated with depression necessitates further study. Studies show that neuroinflammation plays a significant role in the pathophysiology of depression [2,3]. Pro-inflammatory factor activation, including IL-1, IL-6, and TNF-α, has been implicated in major depressive disorder-related neuronal injury [4–6]. Contrarily, antidepressants, selective serotonin reuptake inhibitor fluoxetine, which inhibits serotonin reuptake, reduce inflammation by inhibiting microglial activation [7–9].
Due to their neuroprotective qualities and low incidence of side effects, herbal treatments have become the preferred pharmacological agents for treating various neuropsychiatric disorders [10,11]. In Ayurveda, Celastrus paniculatus (CP), Celastraceae, also known as Jyotishmati, Malkangni, and Kangani, is used as a nervine tonic. In several animal models, CP has already demonstrated neuroprotective qualities [12,13].
Practitioners of ayurvedic medicine extensively used Tribulus terrestris L. (TT) as a therapeutic herb. Phytochemical investigations indicated that cinnamic acid, phytosterol, alkaloids, flavonoids, steroidal glycosides, and steroidal saponins provided a significant therapeutic benefit against neurodegeneration [14]. Recent studies demonstrated that flavonoids and saponins had outstanding neuroprotective effects in neurodegenerative disorders [15–17].
The precise neural processes responsible for TT and CP’s antidepressant-like effects are still unknown. Specifically, it is unknown whether these herbs protect neurons in depression by inhibiting inflammatory processes. Thus, this study attempted to determine if TT and CP could aid in reducing neuroinflammation and cognitive deficiencies caused by chronic immobilization stress (CIS).
MATERIALS AND METHODS
Animals
Male Wistar rats (200–220 g) aged 1.5 months were procured from the commercial vendor. The rats were housed in a typical animal housing with a temperature control system (27°C ± 3°C), humidity, and a 12-hour light/dark cycle. The animals also had free access to food and water, except during stress. The study protocol was approved by the Institutional Animal Ethics Committee, KLE College of Pharmacy, KLE Academy of Higher Education and Research, India (Approval No. 02/BVR/2021, Dated: 12/10/21).
Experimental design
The rats were randomly divided as follows: (1) the control group, which was not exposed to any stress and was orally administered 0.5 ml vehicle (5% DMSO and 1% Tween-20); (2) the CIS + VEH group, rats were exposed to CIS protocol 2 hour/day (10.00 am–12.00 noon) for 10 days duration, followed by 14 days of vehicle injection (i.p); (3) the CIS + FLU group, which received fluoxetine (10 mg/kg, p.o.) treatment for 14 days after being exposed to the CIS; (4) the CIS + CP group, which was exposed to the CIS and then received 14 days of therapy with CP oil (400 mg/kg, p.o.); (5) the CIS + TT group, which was exposed to the CIS followed by TT (250 mg/kg, p.o) treatment for 14 days; (6) the CIS + COMB group, which was exposed to the CIS followed by the combination treatment (CP 400 mg/kg and TT 250 mg/kg p.o) for 14 days. The doses of CP and TT have been selected based on previous studies [15,16]. The number of animals in each group was determined based on earlier studies [17–19].
Drugs and chemicals
The fruit of the plant TT [20–22] was collected from Siliguri, West Bengal, Maity, and this material was authenticated by Botanist Dr. N. Dhatchanamoorthy, Assistant Professor, Plant Systematic and Nomenclature, Foundation for Revitalisation of Local Health Traditions (FRLHT), Bengaluru, India, and the voucher specimen was kept in FRLHT Coll. No. 4720. The ethanolic extraction was done by using a Soxhlet extractor with the condenser method [18]. The powder was kept out of direct sunlight and stored in an airtight container. CP seed oil was procured from the Sadvaidyasala Pvt. Ltd. M.G.S. Road, analytical laboratory, Nanjangud, India, and batch No. 003S. The oil was dissolved in 5% DMSO and 1% Tween 20.
CIS
As previously mentioned, the rats were subjected to immobilization stress in immobilization bags for 2 hours each day, from 10:00 am to 12:00 pm, for 10 days [17–18].
Sucrose preference test (SPT)
Rats’ anhedonia was assessed using the SPT described earlier [17,18,23–26]. During the first 48 hours of adaptation, each rat was kept individually and given tap water and a 1% w/v sucrose solution. Rats had no access to food or water for 18 hours before the test phase. Then, they were given two bottles: one containing 100 ml of 1% sucrose solution and the other 100 ml of drinking water for 2 hours. The amount of sucrose preferred was estimated by applying: Sucrose preference = (quantity of sucrose solution consumed/total amount of liquid consumed) X 100).
Forced swim test (FST)
To assess rats’ “behavioural despair,” the FST was conducted the day following the sucrose preference test, as previously reported [17,18,23–26]. During the training session (15 minutes), each rat was put into a plastic container (25 ± 2t C, 60 cm high, 45 cm in diameter, 40 cm deep) to swim. The test trial was done 24 hours later, with each rat being kept in the container for 5 minutes. The behavior was video-recorded for offline analysis.
Open field test (OFT)
Anxiety, exploration, and locomotor activity were assessed using the OFT [17,18,26]. The rat was placed in an open field arena (100 × 100 × 40 cm) made of wood, painted black with white lines, which had 25 squares. The rat was given 5 minutes to move around freely, and its behavior was recorded, coded, and subjected to offline analysis. Time spent in the center and periphery and the number of squares crossed in the center and periphery were measured. After removing every rat, the arena was thoroughly cleaned using 70% ethanol.
Elevated plus maze (EPM)
The EPM consists of two closed and open arms shaped like a “+” sign, measuring 50 × 10 × 40 cm and placed approximately 50 cm above the ground. A rat was allowed 5 minutes to freely explore after being placed in the middle of the maze, facing the open arm. Time spent in both open and closed arms, the total number of head dips and open-arm entrances, and the duration of each activity were noted [17,18,26,27].
Novel object recognition test (NORT)
The NORT was performed with an open-field apparatus and consisted of three parts: acclimatization, training, and testing. Rats were allowed to acclimate to the apparatus for 10 minutes in the center of the open field during the habituation phase. The next day, rats were given 10 minutes to explore two identical objects that had been put in opposite corners of the open field during the training phase. After 24 hours of training, the rats were given 10 minutes to explore one familiar and novel object. Both training and test sessions were video recorded, coded, and analyzed offline. The duration of time spent examining both familiar and unfamiliar objects was noted. Analyses have been done for the discrimination and recognition indices [28,29].
T-maze spontaneous alteration task
Spatial working memory was measured using the T-maze-rewarded alternation task [27,30]. The rats were introduced to the T-maze after an acclimatization session. The rats were administered a restricted diet before the experiment, which kept their body weight at 85% of what it would have been at rest when fed freely. They also had constant access to water. The rats were given 15 minutes to explore the T maze and pick up food pellets dispersed throughout the maze. Following 2 days of acclimatization, the rats underwent training for the rewarded alternation. Each rat received ten trials per day, and the rat had to switch arms to receive the reward during the acquisition as only one arm was baited in each trial. Using 70% alcohol, the maze was cleaned during the 30-second inter-trial break to remove olfactory cues. A memory retention test was performed 2 days following the final training session. 10 trials were continually given to rats, with a 30-second inter-trial interval. In 10 trials, the number of correct entries into the baited arm was recorded.
Enzyme-Linked Immunosorbent Assay (ELISA)
Following the manufacturer’s directions, ELISA kits were utilized to measure the amounts of TNF-α, IL-6, and brain-derived neurotrophic factor (BDNF) (BD OptEIATM, Biosciences). After stress and treatment protocol, the animals were sacrificed, the brain was isolated, and tissues were washed with ice-cold phosphate-buffered saline (0.01 M, pH = 7.4). The prefrontal cortex and the hippocampus have been separated, weighed, minced, and homogenized in PBS (9:1, volume: weight). The homogenates were centrifuged for 5 minutes at 5,000 g to separate the supernatant. Using bovine serum albumin as a reference protein, the total protein content was determined using the Lowry et al. [31] method, and samples were stored at −80°C until estimation. The standard curve for each biomarker was done, and the O.D. absorbance was recorded in the microplate reader at 450 nm [32].
Data analysis
GraphPad Prism 5 was used to carry out the statistical analyses. The data were analyzed using a one-way ANOVA followed by Tukey’s multiple comparisons test or a repeated measures two-way ANOVA followed by the Bonferroni multiple comparisons test. The data are provided as mean ± SEM. p < 0.05 is considered statistically significant at the probability level.
RESULTS
CP and TT treatments prevented depressive behaviors caused by CIS
Figure 1 displays the results of the SPT, and a one-way ANOVA revealed a statistically significant difference in the percentage of sucrose consumed by each group [F(5,42) = 35.77, p < 0.001] (Fig. 1A). CIS exposure decreased sucrose consumption compared to normal control (p < 0.001). Administration of 400 mg/kg of CP and 250 mg/kg of TT daily, both alone and in combination, for 14 days enhanced preference for sucrose water in stressed animals (p < 0.001).
The one-way ANOVA findings showed significant differences in immobility time across the groups in FST [Fig. 1B; F(5,42) = 59.65, p < 0.001]. Stress caused a longer period of immobility than normal control (p < 0.001). Remarkably, the immobility was decreased in the CIS + CP, CIS + TT, and CIS + TT + CP groups.
 | Figure 1. CP and TT showed antidepressant activity in an animal model of CIS. (A) Two weeks of treatment with CP (400 mg/kg) and TT (250 mg/kg) reversed the sucrose preference in stressed rats. (B) CP and TT treatment decreased immobility in stressed rats in FST. All data are presented as mean ± SEM (n = 8/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) vs. CIS + VEH. ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
 | Figure 2. CP and TT exhibited anxiolytic effects in the OFT. (A) Chronic treatment of CP (400 mg/kg) and TT (250 mg/kg) increased the time spent in centre of the arena open arms. (B) CP and TT treatment decreased the time spent in the periphery. (C) Treatment increased the number of squares crossed in the centre. (D) The number of squares in the periphery. All data are presented as mean ± SEM (n = 8/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. #p < 0.05, ##p < 0.01, ###p < 0.001 Control group (non-stressed) versus CIS + VEH. *p < 0.05, **p < 0.01, ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
CP and TT treatments restored anxiety-like behavior in the chronically stressed animals
In the OFT, chronic immobilization reduced the time spent in the center [Fig. 2A, F(5,42) = 8.474, p < 0.001], increased time spent in the periphery [Fig. 2B, F(5,42) = 6.406, p < 0.001], decreased the number of squares crossed in the center [Fig. 2C, F(5,42) = 5.242, p < 0.001], and increased the number of squares crossed in the periphery [Fig. 2D, F(5,42) = 19.74, p < 0.001]. Interestingly, this anxiety phenotype was completely reversed by chronic CP and TT treatment (p < 0.001). CP and TT-treated rats spent more time in the center [Fig. 2A, F(5,42) = 8.474, p < 0.001] and in the periphery [Fig. 2B, F(5,42) = 6.406, p < 0.001], and crossed more squares in the center [Fig. 2C, F(5,42) = 5.242, p < 0.001], and in the periphery [Fig. 2D, F(5,42) = 19.74, p < 0.001]. These data suggest that chronic treatment with CP and TT for 2 weeks reversed anxiety-like behavior in chronically stressed rats.
Rats in each group spent significantly different amounts of time in the open arms of the EPM (p < 0.001). In contrast to the control group, the CIS rats spent less time in open arms. The treated rats (CP combined with 250 mg/kg of TT) remained longer in the open arms [Fig. 3A, F(5,42) = 24.55, p < 0.001]. In addition, time spent in closed arms was reduced in the treatment groups [Fig. 3B, F(5,42) = 19.62, p < 0.001]. Compared to the individual treatment, the combination treatment increased open-arm entries. The number of head dips was significantly reduced in the CIS rats compared to the control group. Remarkably, in stressed rats, the number of head dips in the open arms increased with the treatment [Fig. 3D, F(5,42) = 11.80, p < 0.001].
 | Figure 3. CP and TT displayed anti-anxiety effects in the EPM. (A) Chronic treatment of CP (400 mg/kg) and TT (250 mg/kg) increased the time spent in the open arms. (B) CP and TT treatments decreased the time spent in the closed arms. (C) Treatment increased the number of open-arm entries. (D) The number of head dips in the open arms. All data are presented as mean ± SEM (n = 8/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) versus CIS + VEH. **p < 0.01, ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
CP and TT ameliorated object recognition memory in stressed rats
In the training part of the NORT, all groups spent the same time examining the two familiar things. Between the groups, there was no noticeable statistical difference (data not presented). During the test, a one-way ANOVA and a Tukey’s post-hoc test revealed significant differences between the groups in the amount of time spent exploring familiar [F(5,42) = 58.57, p < 0.001; Fig. 4A] and new objects [F(5,42) = 32.83, p < 0.001; Fig. 4B]. In the CIS group, there was a substantial difference in the amount of time spent examining novel objects compared to those in the control group. Besides, the duration increased in the CIS + CP, CIS + TT, and combination groups. CIS rats exhibited a significantly lower recognition index [F(5,42) = 110.4, p < 0.001; Fig. 4C] and discrimination index [F(5,42) = 145.8, p < 0.001; Fig. 4D], and both recognition and discrimination ratios were increased after CP and TT treatment.
CP and TT improved impaired memory in T-maze alteration task
The learning curve of rats in each group is displayed in Fig. 5A. Data shows that, compared to the control animals, the rats in the CIS group had a significantly lower ability for spatial working memory. In contrast, the CP, TT, and their combination groups showed significant memory restoration. Interestingly, the combination group had significantly better spatial memory than individual drugs. Stressed rats exhibited a significantly lower number of correct choices than in the control group across all time points [p < 0.001; Fig. 5A], signifying compromised working memory in chronic stress conditions. Remarkably, the results of the two-way ANOVA with the Bonferroni post-hoc test showed that CP, TT, and the combination treatment significantly increased the number of correct choices across all days [Fig. 5B, F(5,378) = 24.23, p < 0.001].
 | Figure 4. CP and TT treatments resulted in better recognition memory in the NORT. (A) Time spent exploring familiar objects. (B) Time spent exploring novel objects. (C) Recognition index. (D) Discrimination index. All data are presented as mean ± SEM (n = 8/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) versus CIS + VEH. ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
When compared to the control group, CIS rats demonstrated fewer correct responses in the retention test [p < 0.001; F(5,42) = 22.51, Fig. 5C]. CP, TT, and the combination groups performed better than in the CIS group. The number of correct choices made by these groups is higher than that of the stressed group.
Potential effect of CP and TT on the BDNF, IL-6, and tumor necrosis factor-α (TNF-α) levels in the prefrontal cortex and hippocampal regions
Significantly lower BDNF levels are revealed by a one-way ANOVA and Tukey’s test in the hippocampal tissue of the CIS group [Fig. 6A; p < 0.001; F(5,29) = 55.37]. Further investigation revealed that a 10-day immobilization stress dramatically raised the proinflammatory marker TNF-α [Fig. 6B; F(5,29) = 265.1, p < 0.001] and IL-6 [Fig. 6C; p < 0.001; F(5,29) = 226.0] levels in the hippocampal region. Interestingly, CIS rats were treated with CP and TT, and the combination caused a significant decrease in hippocampal TNF-α and IL-6 but increased BDNF concentrations in the chronically stressed group.
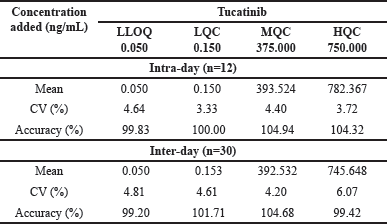 | Figure 5. CP and TT treatments ameliorated working memory deficits in the T-maze alteration task. (A) Learning curve during 10 days of training in the T-maze task. (B) The number of correct choices on 9th and 10th days of training. (C) The number of correct choices during the retention test. All data are presented as mean ± SEM (n = 8/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) versus CIS + VEH. ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
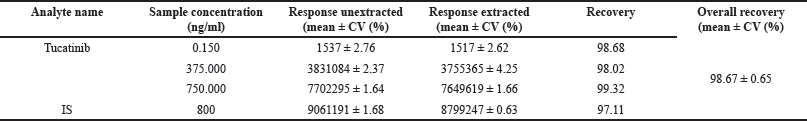 | Figure 6. Two weeks of treatment with C. paniculatus and TT completely restored molecular markers in the hippocampal tissue, estimated by the ELISA method. (A) BDNF; (B) TNF-α; (C) IL-6. All data are presented as mean ± SEM (n = 6/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) versus CIS + VEH. ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
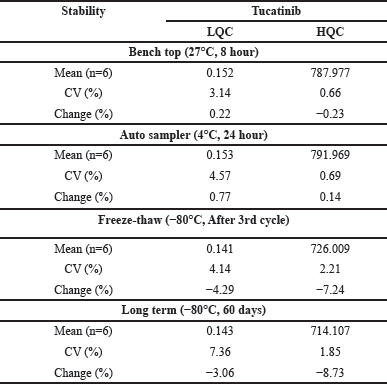 | Figure 7. Chronic treatment with C. paniculatus and TT effectively improved molecular markers in the prefrontal cortical tissue, as estimated by the ELISA method. (A) BDNF; (B) TNF-α; (C) IL-6. All data are presented as mean ± SEM (N = 6/group). A one-way ANOVA followed by Tukey’s post-hoc test was done for analysis. ###p < 0.001 Control group (non-stressed) versus CIS + VEH. ***p < 0.001 CIS + VEH group versus CIS + FLU, CIS + TT, CIS + CP, CIS + TT + CP groups. [Click here to view] |
Statistical analysis revealed that chronic stress significantly reduced BDNF levels in the prefrontal cortical tissue compared to normal animals [Fig. 7A; F(5,29) = 88.49, p < 0.001]. Also, CIS significantly increased TNF-α [Fig. 7B; p < 0.001; F(5,29) = 349.1] and IL-6 [Fig. 7C; p < 0.001; F(5,29) = 276.8 in the cortical tissue relative to the control. It is interesting to note that following CP and TT treatment, BDNF levels increased, and the overall effect was better than in the individual treatment groups. Additionally, treatment restored proinflammatory markers TNF-α and IL-6 levels to normal values.
DISCUSSION
The current study highlights the beneficial effects of TT fruit ethanolic extract and CP seed oil in restoring memory deficits, anxiety, and depression caused by CIS. Behavioral recovery is associated with the restoration of BDNF and the neuroinflammatory markers TNF-α and IL-6. In our study, we found that stressed rats had higher levels of anxiety and depression than the control rats after being exposed to stress for 10 days. In the novel object recognition and T-maze tests, CIS animals demonstrated diminished memory. Also, prolonged immobilization stress caused reduced hippocampal and prefrontal cortical BDNF levels with increased neuroinflammation (IL-6 and TNF-α).
CIS is a widely used rodent version of depression. More and more evidence suggests that continuous and persistent stress can cause rats to exhibit anxiety and depressive-like behaviors [17,18,26,27,29,33]. Earlier studies on CIS revealed cognitive impairment, hippocampal atrophy, and lower BDNF in the prefrontal cortex and hippocampal regions [17,18]. The survival, differentiation, and growth of neurons depend on BDNF, which plays an important role in memory [34]. There is evidence that the etiology of anxiety and depression is significantly influenced by the elevated brain cytokines brought on by prolonged stress. Previous work has revealed that hippocampus inflammation and oxidative stress may be associated with chronic stress-induced abnormalities [35–37]. Our results demonstrated that repeated stress exposure elevated inflammatory markers. This finding suggests that neuroinflammation plays a part in the emergence of behaviors resembling anxiety, and additionally, it replicates the findings of a few prior studies [35,38].
Interestingly, we found that CP and TT administration decreased anxiety-like behavior and had a noticeable impact on depressive-like behavior. Previous studies showed that both CP and TT have anxiolytic effects on rodents [39–42]. We observed that CP and TT restored hippocampal and prefrontal cortical BDNF levels and reduced neuroinflammation. Our findings thus suggest a possible role for CP and TT treatment in ameliorating stress-related neuroinflammation.
The findings of this study supported the generally accepted opinion that CP may be a useful memory enhancer and possess qualities that improve memory. Agarofuran derivatives, fatty acids such as oleic, linoleic, linolenic, palmitic, stearic, and lignoceric acid, phenolic triterpenoids such as celastrol and paniculatadiol, and alkaloids such as paniculatine and celastrine have all been identified in the seeds and seed oil of CP [13,43–46]. Several studies have shown that CP seeds and seed oil can protect neurons and enhance memory [47–54]. Additionally, CP seed and its aqueous extract have been shown to have neuroprotective properties against H2O2 and glutamate-associated neuronal toxicity [48,49]. By inhibiting NMDA receptors and lowering Ca2+ influx in neurons, CP has been shown to reduce glutamate toxicity in neurons [49,54].
In a model of Huntington’s illness caused by 3-nitro propionic acid, CP seed ethanolic extract showed significant therapeutic potential. The prevention of oxidative damage in animals was related to enhanced locomotor activity, motor coordination, and memory retention following CP therapy [55]. Mice treated with CP seed oil demonstrated strong antidepressant-like effects when given for 14 consecutive days [41], with anti-anxiety effects [40]. Previously, CP seed oil prevented kainic acid-induced hippocampus neuronal death and reversed memory loss [56,57]. Significant antioxidant activity has been suggested as the main mechanism underlying the plant’s neuroprotective and memory-improving properties [52–58]. According to a recent study, CP oil reduced inflammation, corticosteroid levels, and neurotransmitter levels while exhibiting antidepressant effects in a chronic unpredictable stress paradigm [59].
A previous investigation from our laboratory demonstrated that a 2-week duration of CP oil treatment reduced the cognitive deficits caused by the chronic restraint stress paradigm. In the T-maze and 8-arm radial arm maze tests, stressed mice treated with CP oil fared better. Moreover, CP therapy has shown anxiolytic effects under stressful circumstances [27]. CP oil enhanced glutathione, catalase, and superoxide dismutase activities in addition to norepinephrine, dopamine, and serotonin. It also reduced behavioral abnormalities in various memory tasks. In rats suffering from cognitive impairment caused by scopolamine, it decreased neuroinflammatory markers such as NGF, NF-?B, IL-6, and TNF-α [60].
TT, containing cinnamic acid, alkaloids, furanostol steroids, and spirostanol, has shown beneficial effects on the brain [15,61–64]. An additional investigation revealed that the neuroprotective properties of TT can mitigate the neurotoxicity produced by monosodium glutamate [65], and TT ethanolic extract had anxiolytic effects in rats [39].
TT demonstrated antidepressant activity, restored serotonin levels, elevated the production of the interleukin 10 gene, and downregulated the expressions of the IL-1 and indoleamine 2,3-dioxygenase genes in the hippocampus area in a chronic, mild, unexpected stress situation [42]. The active components in TT have anti-inflammatory activity and decrease inflammatory mediators and cytokines TNF-α, IL-6, and IL-10 [21,66,67]. According to earlier studies, TT lowers the concentrations of NF-B, interleukin-1β, TNF-α, nuclear factor kappa beta, and malondialdehyde. Moreover, Bcl-2 anti-apoptotic protein, superoxide dismutase, and peroxisome proliferator-activated receptor-gamma (PPARγ) levels were increased by treatment [68–70].
CONCLUSION
This study examined the synergistic effects of TT and CP on chronic immobilization stress-induced deficits in cognition, neuroinflammation, BDNF, heightened anxiety, and depressed behavior. Future studies are required to understand the active components responsible for the restoration of chronic stress-induced cognitive deficits and neuroinflammation. Previous reports state that both CP and TT are safe to use as medications since they are non-toxic and have no side effects. These findings may lead to the development of novel therapy to treat stress-related cognitive impairments in the future.
The current investigation has certain shortcomings. Notably, the study did not estimate the chemical components of the CP and TT herbs responsible for cognitive benefits in chronic immobilization stress. Further investigations are warranted to analyze the active constituents of CP and TT. Additionally, neurotransmitter estimations in various brain regions further validate the neuroprotective effect of CP and TT.
AUTHORS CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors would like to thank KLE Academy of Higher Education and Research (KAHER), Belagavi, India, for providing financial support.
CONFLICTS OF INTEREST STATEMENT
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee, KLE College of Pharmacy, KLE Academy of Higher Education and Research, India (Approval No. 02/BVR/2021, Dated: 12/10/21).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr. 2017;27:101–11. CrossRef
2. Alcocer-Gómez E, Casas-Barquero N, Marín-Aguilar F, Bullón P, Carrión AM, Sanchez-Alcazar JA, et al. P. 1. f. 022 NLRP3-inflammasome complex is implicated in depressive behaviour induced by stress. Eur Neuropsychopharmacol. 2015;25:S236–37. CrossRef
3. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. CrossRef
4. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56. CrossRef
5. Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–50. CrossRef
6. Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2013;31:143–52. CrossRef
7. Mariani N, Everson J, Pariante CM, Borsini A. Modulation of microglial activation by antidepressants. J Psychopharmacol. 2022;36:131–50. CrossRef
8. Obuchowicz E, Bielecka AM, Paul-Samojedny M, Pude?ko A, Kowalski J. Imipramine and fluoxetine inhibit LPS-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol Rep. 2014;66:34–43. CrossRef
9. Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, et al. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation. 2022;19:132. CrossRef
10. Maiti P, Dunbar GL. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci. 2018;19:1637. CrossRef
11. W?sik A, Antkiewicz-Michaluk L. The mechanism of neuroprotective action of natural compounds. Pharmacol Rep. 2017;69:851–60. CrossRef
12. Nadkarni KM. Indian Materia Medica. New Delhi, India: Popular Prakashan Pvt. Ltd; 2002.
13. Bhanumathy M, Chandrasekar SB, Chandur U, Somasundaram T. Phyto-pharmacology of Celastrus paniculatus: an overview. Int J Pharm Sci Drug Res. 2010a;2:176–81.
14. Hammoda HM, Ghazy NM, Harraz FM, Radwan MM, ElSohly MA, Abdallah II. Chemical constituents from Tribulus terrestris and screening of their antioxidant activity. Phytochemistry. 2013;92:153–9. CrossRef
15. Chauhdary Z, Saleem U, Ahmad B, Shah S, Shah MA. Neuroprotective evaluation of Tribulus terrestris L. in aluminum chloride induced Alzheimer’s disease. Pak J Pharm Sci. 2019;32:805–16.
16. Gattu M, Boss KL, Terry AV Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997;57:793–9. CrossRef
17. Shilpa BM, Bhagya V, Harish G, Bharath MS, Shankaranarayana Rao BS. Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Biol Psychiatry. 2017;76:88–100. CrossRef
18. Shilpa BM, Bhagya V, Shankaranarayana Rao BS. Escitalopram treatment ameliorates chronic immobilization stress induced depressive behavior and cognitive deficits by modulating BDNF expression in the hippocampus. J Appl Pharm Sci. 2024;14:170–82. CrossRef
19. Son H, Yang JH, Kim HJ, Lee DK. A chronic immobilization stress protocol for inducing depression-like behavior in mice. JoVE. 2019;15:e59546. CrossRef
20. Wang Y, Zhao H, Liu Y, Guo W, Bao Y, Zhang M, et al. GC-MS-based metabolomics to reveal the protective effect of gross saponins of Tribulus terrestris fruit against ischemic stroke in rat. Molecules. 2019;24:793. CrossRef
21. Saleem U, Chauhdary Z, Raza Z, Shah S, Rahman MU, Zaib P, et al. Anti-Parkinson’s activity of Tribulus terrestris via modulation of AChE, α-Synuclein, TNF-α, and IL-1β. ACS omega. 2020;5:25216–27. CrossRef
22. Majumdar A, Saraf M, Andrades N, Kamble R. Preliminary studies on the antioxidant activity of Tribulus terrestris and Eclipta alba. Pharmacogn Mag. 2008, 4:102.
23. Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Neonatal clomipramine induced endogenous depression in rats is associated with learning impairment in adulthood. Behav Brain Res. 2008;187:190–4. CrossRef
24. Bhagya V, Srikumar BN, Raju TR, Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology (Berl). 2011;214:477–94. CrossRef
25. Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. The selective noradrenergic reuptake inhibitor reboxetine restores spatial learning deficits, biochemical changes, and hippocampal synaptic plasticity in an animal model of depression. J Neurosci Res. 2015;93:104–20. CrossRef
26. Mahati K, Bhagya V, Christofer T, Sneha A, Shankaranarayana Rao BS. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol Learn Mem. 2016;134:379–91. CrossRef
27. Bhagya V, Christofer T, Shankaranarayana Rao BS. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian J Pharmacol. 2016;48:687–93. CrossRef
28. Fidilio A, Grasso M, Caruso G, Musso N, Begni V, Privitera A, et al. Prenatal stress induces a depressive-like phenotype in adolescent rats: The key role of TGF-β1 pathway. Front Pharmacol. 2022;13:1075746. CrossRef
29. Romero-Miguel D, Casquero-Veiga M, Fernández J, Lamanna-Rama N, Gómez-Rangel V, Gálvez-Robleño C. Maternal supplementation with N-Acetylcysteine modulates the microbiota-gut-brain axis in offspring of the poly I:C rat model of schizophrenia. Antioxidants (Basel). 2023;12:970. CrossRef
30. Bhagya VR, Srikumar BN, Veena J, Shankaranarayana Rao BS. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res. 2017;95:1602–10. CrossRef
31. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–75.
32. Tripathi SJ, Chakraborty S, Srikumar BN, Raju TR, Shankaranarayana Rao BS. Inactivation of basolateral amygdala prevents chronic immobilization stress-induced memory impairment and associated changes in corticosterone levels. Neurobiol Learn Mem. 2017;142:218–29. CrossRef
33. Joo Y, Choi KM, Lee YH, Kim G, Lee DH, Roh GS, et al. Chronic immobilization stress induces anxiety-and depression-like behaviors and decreases transthyretin in the mouse cortex. Neurosci Lett. 2009;461:121–5. CrossRef
34. Miao Z, Wang Y, Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int J Mol Sci. 2020;21:1375. CrossRef
35. McWhirt J, Sathyanesan M, Sampath D, Newton SS. Effects of restraint stress on the regulation of hippocampal glutamate receptor and inflammation genes in female C57BL/6 and BALB/c mice. Neurobiol Stress. 2019;10:100169. CrossRef
36. Pezeshki-Nia S, Asle-Rousta M, Mahmazi S. Spinacia oleracea L. extract attenuates hippocampal expression of TNF-α and IL-1β in rats exposed to chronic restraint stress. Med J Islam Repub Iran. 2020;34:10. CrossRef
37. Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, et al. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation. 2020;17:178. CrossRef
38. Johnson JD, Barnard DF, Kulp AC, Mehta DM. Neuroendocrine regulation of brain cytokines after psychological stress. J Endocr Soc. 2019;3:1302–20. CrossRef
39. Kafeel H, Rukh R. Anxiolytic activity of ethanolic extract of aerial parts of Tribulus terrestris in mice. J Phytopharmacol. 2015;4:17–21. CrossRef
40. Rajkumar R, Kumar EP, Sudha S, Suresh B. Evaluation of anxiolytic potential of Celastrus oil in rat models of behaviour. Fitoterapia. 2007;78:120–4. CrossRef
41. Valecha R, Dhingra D. Behavioral and biochemical evidences for antidepressant-like activity of Celastrus paniculatus seed oil in mice. Basic Clin Neurosci. 2016;7:49–56.
42. Zhang W, YU Z, Mei T, HU K, Liu M. Antidepressant effect and mechanism of gross saponins of Tribulus terrestris. Chinese Pharmacol Bull. 2017;33:343–8. CrossRef
43. den Hertog Jr HJ, Hackmann JT, Nanavati DD, Dev S. Malkanguniol, one of the polyalcohols from Celastrus paniculatus Willd. Tetrahedron Lett. 1973;11:845–8. CrossRef
44. Tu YQ, Wu TX, Li ZZ, Zhen T, Chen YZ. Sesquiterpene polyol esters from Celastrus paniculatus. J Nat Prod. 1991;54:1383–6. CrossRef
45. Tu YQ, Chen YZ, Wu DG, Zhang XM, Hao XJ. Sesquiterpenoids from Celastrus paniculatus. J Nat Prod. 1993;56:122–5. CrossRef
46. Wagner H, Heckkel E, Sonnenbichler J. Isolation and structures of a new sesquiterpene (malkangunin) and two new sesquiterpene alkaloids (celapanin, celapanigin) from Celastrus paniculatus Willd. Tetrahedron Lett. 1974;2:213–6.
47. Bhanumathy M, Harish MS, Shivaprasad HN, Sushma G. Nootropic activity of Celastrus paniculatus seed. Pharm Biol. 2010b;48:324–7. CrossRef
48. Godkar P, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed water soluble extracts protect cultured rat forebrain neuronal cells from hydrogen peroxide-induced oxidative injury. Fitoterapia. 2003;74:658–69. CrossRef
49. Godkar P, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed water soluble extracts protect against glutamate toxicity in neuronal cultures from rat forebrain. J Ethnopharmacol. 2004;93:213–9. CrossRef
50. Godkar P, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate induced injury in embryonic rat forebrain neuronal cells. Phytomedicine. 2006;3:29–36. CrossRef
51. Lekha G, Kumar BP, Rao SN, Arockiasamy I, Mohan K. Cognitive enhancement and neuroprotective effect of Celastrus paniculatus Willd. seed oil (Jyothismati oil) on male Wistar rats. J Pharm Sci Technol. 2010a;2:130–8.
52. Lekha G, Mohan K, Samy IA. Effect of Celastrus paniculatus seed oil (Jyothismati oil) on acute and chronic immobilization stress induced in swiss albino mice. Pharmacognosy Res. 2010b;2:169–74. CrossRef
53. Nalini K, Karanth KS, Rao A, Aroor AR. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J Ethnopharmacol. 1995;47:101–8. CrossRef
54. John E, Mishra B, Tripathi AS, Aleesha R. Protective effect of Celastrus paniculatus on cognitive function in glutamate-induced brain-injured mice by reducing the intracellular influx of Ca. Int J Dev Neurosci. 2022;82:331–8. CrossRef
55. Malik J, Karan M, Dogra R. Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: possible antioxidant mechanism. Pharm Biol. 2017;55:980–90. doi: 10.1080/13880209.2017.1285945
56. Pujari GRS, Subramanian V, Rao SR. Effects of Celastrus paniculatus Willd. and Sida cordifolia Linn. in kainic acid induced hippocampus damage in rats. Indian J Pharm Educ Res. 2019;53:537–44. CrossRef
57. Pujari GRS, Subramanian V, Rao SR. Neuroprotective role of Celastrus paniculatus willd and Sida cordifolia Linn on kainic acid induced neuronal damage in neurodegenerative diseases. Pharm Sci Asia. 2022;49:282–91. CrossRef
58. Kumar MHV, Gupta YK. Antioxidant property of Celastrus paniculatus willd.: a possible mechanism of enhancing cognition. Phytomedicine. 2002;9:302–11. CrossRef
59. Chahuan S, Grover S, Singh S. Amelioration of modified chronic unpredictable stress using Celastrus paniculatus seed oil alone and in combination with fluoxetine. Drug Chem Toxicol. 2023;46:879–94. CrossRef
60. Faldu KG, Patel SS, Shah JS. Celastrus paniculatus oil ameliorates NF-KB mediated neuroinflammation and synaptic plasticity in the scopolamine-induced cognitive impairment rat model. Metab Brain Dis. 2023;38:1405–19. CrossRef
61. Prabhu N, Hadigal S, Sheetal U, Shenoy A. Effect of Tribulus terrestris on learning and memory in Wistar rats. Pharmacogn J. 2014;6:68–71. CrossRef
62. Ranjithkumar R, Alhadidi Q, Shah ZA, Ramanathan M. Tribulusterine containing Tribulus terrestris extract exhibited neuroprotection through attenuating stress kinases mediated inflammatory mechanism: in vitro and in vivo studies. Neurochem Res. 2019;44:1228–42. CrossRef
63. Vangalapati B, Manjrekar PA, Hegde A, Ullal S. Land caltrop (Tribulus terrestris) fruit extract improves learning, memory and cognitive flexibility in streptozotocin-nicotinamide induced diabetes animal model. Int J Pharm Sci Rev Res. 2016;38:243–7.
64. Zhu W, Du Y, Meng H, Dong Y, Li L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem Cent J. 2017;11:60. CrossRef
65. Swaminathan G, Jupudi S, Sathish R, Roshan NS. Tribulus terrestris Linn attenuates neurotoxicity induced by monosodium-glutamate: an in vivo evidence. Int J Pharm Res. 2021;13:2065–75. CrossRef
66. Lee HH, Ahn EK, Hong SS, Oh JS. Anti-inflammatory effect of tribulusamide D isolated from Tribulus terrestris in lipopolysaccharide-stimulated RAW2647 macrophages. Mol Med Rep. 2017;16:4421–8. CrossRef
67. Zhao WR, Shi WT, Zhang J, Zhang KY, Qing Y, Tang JY, et al. Tribulus terrestris L. extract protects against lipopolysaccharide induced inflammation in RAW 264.7 macrophage and zebrafish via inhibition of Akt/MAPKs and NF-κB/iNOS-NO signaling pathways. Evid Based Complement Alternat Med. 2021;2021:6628561. CrossRef
68. Alzahrani S, Ezzat W, Elshaer RE, Abd El-Lateef AS, Mohammad HMF, Elkazaz AY, et al. Standarized tribulus terrestris extract protects against rotenone-induced oxidative damage and nigral dopamine neuronal loss in mice. J Physiol Pharmacol. 2018;69:979–94. CrossRef
69. Roghani M, Omid Malayeri S, Malayeri S. Effect of Tribulus terrestris oral feeding on learning and memory in streptozotocin-diabetic rats: investigating the role of lipid peroxidation. J Guilan Univ Med Sci. 2013;22;88–95.
70. Shamsi B, Abedi B, Hosseini SA. Effect of resistance training and Tribulus terrestris consumption on avoidance and working memory in rats exposed to stanozolol. Avicenna J Neuro Psycho Physiology. 2021;8:84–9. CrossRef