INTRODUCTION
Indomethacin is a nonsteroidal anti-inflammatory drug (NSAID) widely used to relieve pain, fever, and inflammation. Its pharmacological actions are exerted mainly via inhibition of cyclooxygenase (COX) activity, leading to lower levels of prostaglandins and other inflammatory mediators [1,2]. However, its major adverse effect is gastrointestinal (GI) ulceration [1], various experimental and clinical models have demonstrated that its deleterious effect on the GI tract may occur through both COX-dependent and COX-independent mechanisms [2–4]. Inhibition of COX causes a reduction of prostaglandin E2 (PGE2) level, leading to the loss of GI mucosal integrity, and decreased cell regeneration [2–4]. The COX-independent mechanisms can involve increased intracellular reactive oxygen species (ROS), excessive misfold proteins in the endoplasmic reticulum (ER), activation of unfolded protein response, and signaling cascade, leading to mitochondrial apoptosis of mucosal cells [3–5].
Mulberry leaves (M. alba L., family Moraceae) have been widely used as herbal tea and health supplements. Its extracts displayed various pharmacological activities such as antioxidant, antidiabetic, hepatoprotective, and antitumor effects [6,7]. They were reported to prevent oxidative stress-mediated cell and tissue damage in cell cultures [e.g., rat insulinoma cell line and hepatoblastoma cell line] and in animal models through antioxidant-related mechanisms [6,8]. There is evidence that they exert their protective action through the increase of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase [6]. In obese mice, they were also capable of suppressing ER stress in high-fat diet-induced tissue damage [9]. Major active ingredients in aqueous and ethanolic mulberry leaf extracts are polyphenolic compounds including chlorogenic acid, gallic acid, naringenin, quercetin, and rutin [10–12]. These polyphenols are known to possess significant antioxidant capacity and can exhibit protective action against cellular stress [13,14]. However, the potential of mulberry leaf extracts as protection against GI toxicity from NSAIDs, particularly indomethacin, and the underlying mechanisms have rarely been explored. Recently, we have demonstrated that the reduction of cellular ROS may be insufficient to prevent indomethacin-mediated apoptosis in the intestinal Caco-2 cells [13]. Several natural polyphenolic antioxidants, including epigallocatechin gallate, caffeic acid, naringenin, quercetin, and rutin, could prevent apoptotic cell death in Caco-2 cells exposed to indomethacin and diclofenac. The underlying protective mechanisms were, in part, related to the capability of these antioxidants to suppress protein misfolding and ER stress [13]. Some phytochemicals, e.g., epigallocatechin gallate, also exerted their protective effect against indomethacin-induced gastric ulceration via upregulation of COX expression and increased PGE2 level in rat models [15]. In this study, the ability of water and ethanol extracts of mulberry leaves to protect against GI toxicity caused by indomethacin was investigated in rats and cell models. This work provides new information on the medicinal benefit of mulberry leaf extracts to prevent indomethacin-induced intestinal ulcers through suppression of ER stress-mediated apoptosis. However, they offer no gastro-protection due to their inability to restore PGE2 levels in the stomach.
MATERIALS AND METHODS
Reagents
Chlorogenic acid (#PHR2002), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH, #D9132), gallic acid (#8.42,649), hydrogen peroxide (H2O2, #H1009), indomethacin (#405,268), naringenin (#W530,098), omeprazole (#19,329), quercetin (#PHL89,262), rutin (#PHL89,270), and tunicamycin (#T7,765) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Mouse monoclonal anti-activating transcription factor-4 (ATF-4, #sc-390,063), anti-Bcl-2-associated X protein (Bax, #sc-7,480), anti-B-cell leukemia/lymphoma 2 protein (Bcl-2, #sc-7,382), anti-C/EBP homologous transcription factor (CHOP, #sc-7,351), anti-eukaryotic initiation factor-2α (eIF-2α, #sc-133,132), and anti-protein kinase R-like ER kinase (PERK, #sc-377,400) were from Santa Cruz Biotechnology (Dallas, TX, USA). Mouse monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, #AP308P) and secondary goat anti-mouse IgG (H&L) horseradish peroxidase (HRP, #AP124P) were from Calbiochem (San Diego, CA, USA). Rabbit monoclonal anti-phosphorylated protein of eIF-2α (p-eIF-2α, #9721), anti-phosphorylated protein of PERK (p-PERK, #3,179), and secondary goat anti-rabbit IgG (H&L) HRP (#7,074) were from Cell Signaling Technology (Beverly, MA, USA).
Preparation of mulberry leaf extracts
Mulberry leaves were collected from Chaiyaphum province, Thailand, in March 2020, and were identified by one of the authors (R. Suttisri). A voucher specimen (No. RS20031) was deposited at the herbarium of the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University. The leaves were dried at 60°C for 4 hours and ground. The powdered leaves (2 kg) were macerated once in 8 l of hot water (60°C) for 30 minutes, or in 8 l of 95% ethanol for 3 days to obtain mulberry leaf water extract (MLWE), and mulberry leaf ethanol extract (MLEE), respectively. The water extract was spray-dried (163 g, 8.15% yield), whereas the ethanol extract was dried under reduced pressure (500 g, 25% yield). Both extracts were kept in an auto-desiccator cabinet at room temperature until use.
Determination of total phenolic contents
The extracts (1 mg/ml) were mixed with Folin–Ciocâlteu phenol reagent (1:4) for 10 minutes, followed by 0.01% sodium carbonate solution for 2 hours [16]. The phenolic content was quantified spectrometrically at 765 nm, and reported as µg gallic acid equivalents per g of extract.
Determination of total flavonoid contents
The extracts (1 mg/ml) were mixed with 10% aluminum chloride in 95% ethanol and 1 M sodium acetate buffer for 2 hours [16]. The flavonoid content was measured spectrometrically at 415 nm and reported as µg quercetin equivalents per g of extract.
High-performance liquid chromatography (HPLC) analysis of MLWE and MLEE
HPLC was performed on a Shimadzu LC-20AD Prominence apparatus equipped with an auto-sampler and a UV detector (280 nm). The extracts were injected into an Eclipse XDB-C18 column (4.6 mm × 250 mm, 5 μm, Agilent, Santa Clara, CA, USA) at 30°C. The mobile phase was 1% formic acid in methanol at a flow rate of 1 ml/minute. Four major phenolic constituents of mulberry leaves including gallic acid, chlorogenic acid, naringenin, and rutin were quantified in both extracts from standard calibration lines [10]. Standard curve equations, with regression values of each phenolic compound, were: gallic acid (Y = 53.58207X—22.63048, R2 = 0.99,952), chlorogenic acid (Y = 22.78086X—29.25108, R2 = 0.99766), naringenin (Y = 61.44265X—17.01460, R2 = 0.99960), and rutin (Y = 16.39271X—4.12033, R2 = 0.99943). Results were reported as µg per g of extract.
Determination of DPPH scavenging activity
Solutions (6.25–8,000 µg/ml) of the extracts were mixed with DPPH solution (200 µM) for 30 minutes. Then, absorbance was read at 517 nm [7].
Animal experiment
Animals
Male Sprague–Dawley rats (200–250 g) were obtained from the National Laboratory Animal Center, Mahidol University, Thailand. All experiments were conducted according to the ethical standards approved by the National Laboratory Animal Center Animal Care and Use Committee (NLAC-ACUC), Mahidol University, Thailand (Approval No. RA2020-27). Rats were acclimatized for 1 week in a temperature and humidity-controlled room with a 12-hour light/dark cycle and allowed free access to standard rodent chow and water.
Dosage information
Rats (6 animals per group) were orally fed once daily with the test materials [i.e., vehicle (5% NaHCO3 solution, pH 7.0), MLWE, MLEE (500, 1,000, and 2,000 mg/kg) or omeprazole (100 mg/kg)] for 5 days. Then, all rats, except those in the vehicle control group, were administered a single oral dose of indomethacin (40 mg/kg) [17]. They were sacrificed 24 hours after indomethacin treatment for tissue (stomach and jejunum) collection.
Macro- and microscopic assessments of GI lesions
Ulceration of stomach and jejunum tissues was assessed at macro- and microscopic levels using the 0–5 scoring system (Table 1) [18,19,20]. For macroscopic evaluation, the ulcerated lesions were photographed, and the areas were measured and scored (Table 1). Results were reported as ulcer index (UI), which was the ulcer score average calculated from the total ulcer scores over the number of animals ulcerated. For microscopic evaluation, the tissues were fixed with 10% formaldehyde and embedded into paraffin blocks. Subsequently, they were cut into 3-µm sections and stained with hematoxylin-eosin dye. Histologic damages were examined under light microscopy (10X) and scored (Table 1) [18,19,20].
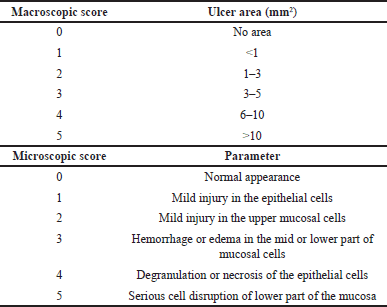 | Table 1. Macro and microscopic score evaluations. [Click here to view] |
Western blot analysis
The 5% (w/v) tissue homogenates were prepared in 50 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl, 1% Tritron-X, 0.1% SDS, 1 mM EDTA, 1 mM PMSF, 1 mM NaF, 1 mM Na3VO4, and protease inhibitor cocktails. After 10-minute centrifugation at 18,000 × g at 4°C, the supernatants were collected for western blot analysis. Protein (40 µg per sample) was electrophoresed on 7%–15% SDS-PAGE gels, electro-transferred to a PVDF membrane, and probed with primary specific antibodies (1:1,000) against ATF-4, Bax, Bcl-2, CHOP, eIF-2α, phosphorylated eIF-2α (p-eIF-2α), PERK, and phosphorylated PERK (p-PERK) and against GAPDH (1:2,000). Subsequently, membranes were incubated with HRP-conjugated secondary antibody (1:2,000) and developed with the Supersignal® West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL, USA). The protein bands were visualized under a luminescence-image analyzer (ImageQuant™ LAS 4,000, GE Healthcare Bio-sciences, Japan) and quantified using ImageJ program (NIH, Bethesda, MD, USA).
Determination of PGE2 production
PGE2 in tissue samples was extracted with ethyl acetate, and measured using a competitive PGE2 ELISA Kit (Boster Bio, Pleasanton, CA, USA).
Cell culture experiment
Cell culture
Human gastric cancer cell line AGS (ATCC® CRL-1739™) was maintained in F12-K media containing 1% penicillin/streptomycin solution and 10% fetal bovine serum (FBS). Caco-2 cells (ATCC® HTB-37™) were cultured in Dulbecco’s modified eagle medium supplemented with 1% nonessential amino acid, 2.5 mM L-glutamine, and 10% FBS. Cells were maintained at 37°C in a humidified air atmosphere of 5% CO2.
Cell viability
Cells were treated with the extracts at different concentrations (0.5, 1, and 5 mg/ml) for 24 hours prior to incubation with a stress inducer (i.e., indomethacin 1,000 µM, tunicamycin 20 µg/ml, or H2O2 500 µM) for 48–72 hours. Dimethylsulfoxide (DMSO) at 0.5% (v/v) was used as the control. At the end of the treatment period, cells were washed with phosphate buffer saline (PBS) 3 times, and then incubated with methylthialazole tetrazolium (MTT) (0.5 mg/ml) for 4 hours. Intracellular MTT-formazan crystals were dissolved with DMSO and quantified spectrometrically at 570 nm.
Determination of intracellular ROS level
Cells were treated with the extracts at different concentrations (0.5, 1, and 5 mg/ml) for 2 hours, followed by the addition of either 10 µM dihydroethidium (DHE) (for superoxide anion) or 100 µM 2’,7’-dichlorofluorescin diacetate (for H2O2) and incubated for 30 minutes. DMSO at 0.5% (v/v) was used as a control. Then, after washing with PBS 3 times, the cells were treated with 1000 µM indomethacin for 2 hours. The fluorescence intensity was determined at excitation/emission wavelengths of 470/590 nm (for DHE) and 485/535 nm (for DCF).
Statistical analysis
Data are presented as the mean ± S.D. for the in vivo studies (n = 6), and as the mean ± S.D. of three independent experiments for the in vitro studies. Statistical differences between groups were analyzed by one-way analysis of variance, followed by the Bonferroni post hoc test. p < 0.05 indicates statistical significance.
RESULTS
Phytochemical analysis of MLWE and MLEE
HPLC chromatograms of MLWE and MLEE showed clearly separated peaks corresponding to those of the polyphenolic standards [i.e., gallic acid (RT 5.84 minute), chlorogenic acid (RT 18.01 minutes), naringenin (RT 31.44 minutes), and rutin (RT 37.87 minute)] (Supplementary Fig. 1). MLEE contained polyphenols 5.87 mg/g, flavonoids 6.14 mg/g, chlorogenic acid 2.58 mg/g, naringenin 1.36 mg/g, and rutin 0.35 mg/g, while MLWE contained polyphenols 3.36 mg/g, flavonoids 3.72 mg/g, chlorogenic acid 1.44 mg/g, and gallic acid 0.45 mg/g (Supplementary Table 1).
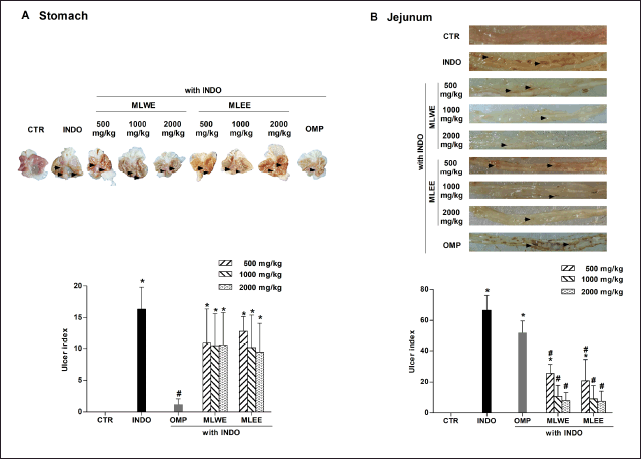 | Figure 1. MLWE and MLEE on ulceration, with UI in the stomach (A) and jejunum (B) of rats previously administered with indomethacin (INDO; 40 mg/kg). Omeprazole (OMP; 100 mg/kg) was used as positive control. Pictures of ulcerated lesions from one representative rat are shown. Each column represents mean ± S.D. obtained from six rats (n = 6). *p < 0.05 compared with control (CTR). #p < 0.05 compared with INDO alone. [Click here to view] |
Effects of MLWE and MLEE on indomethacin-induced GI ulceration
After 24-hour treatment, indomethacin (40 mg/kg) significantly increased the areas of ulceration in rat stomach and jejunum (Figs. 1 and 2). Pretreating the rats with MLWE or MLEE, even at 500 mg/kg, significantly reduced indomethacin-mediated ulceration in the jejunum at both macroscopic and microscopic levels by 62.2% and 52.1%, respectively (Figs. 1B and 2B). Their protective effects were not observed in the stomach tissue (Figs. 1A and 2A), contrary to omeprazole, which significantly reduced the ulceration and mucosal damage in the stomach, but not in the jejunum tissue.
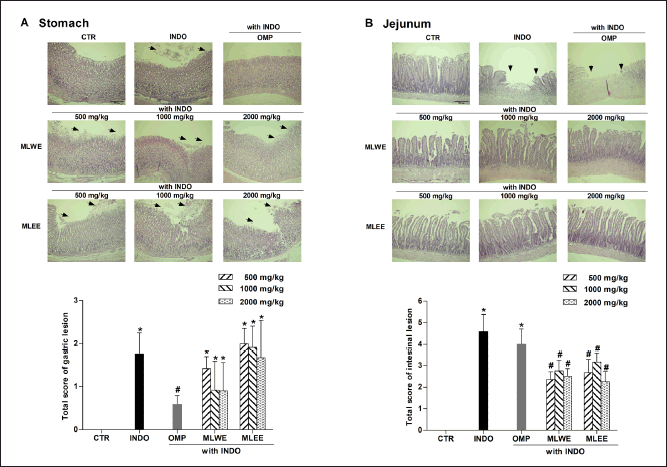 | Figure 2. MLWE and MLEE on histological ulcer score in the stomach (A) and jejunum (B) of rats previously administered with indomethacin (INDO; 40 mg/kg). Omeprazole (OMP; 100 mg/kg) was used as positive control. Images of H&E stained gastric and jejunum tissues from one representative rat are shown. Each column represents mean ± S.D. obtained from six rats (n = 6). *p < 0.05 compared with control (CTR). #p < 0.05 compared with INDO alone. [Click here to view] |
Expression of Bax and Bcl-2 apoptotic proteins
Immunoblot analysis of gastric and jejunum tissues in the indomethacin-treated rats revealed that their Bax/Bcl-2 ratio increased significantly by approximately 4.8 and 6.2 times, respectively (Fig. 3A and 3B). Pretreatment with either extract could effectively prevent the indomethacin-mediated increase of the Bax/Bcl-2 ratio in the jejunum (with ratios reduced to approximately 1.5–3-fold), but not in gastric tissues. The increases of Bax/Bcl-2 ratio reduced to approximately 3 folds in the MLWE and MLEE.
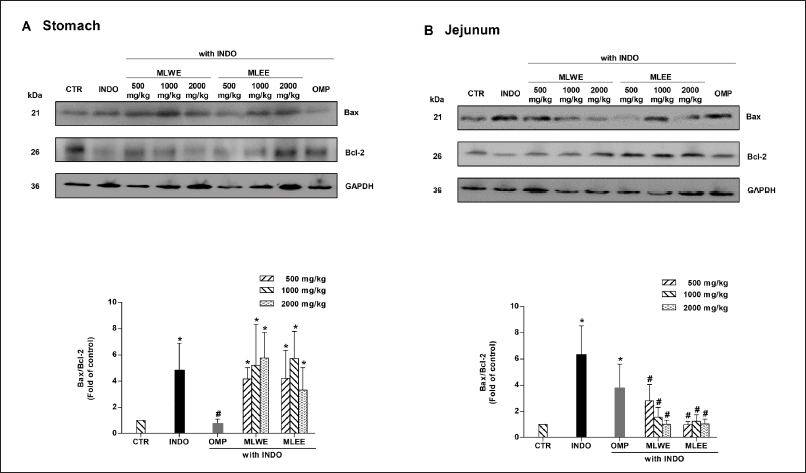 | Figure 3. Expression of Bax and Bcl-2 proteins and their densitometrical analysis in rat stomach (A) and jejunum (B) after 24-hour treatment with indomethacin (INDO; 40 mg/kg) in the absence and presence of MLWE or MLEE or omeprazole (OMP; 100 mg/kg). Each column represents mean ± SD. obtained from six rats (n = 6). *p < 0.05 compared with CTR. #p < 0.05 compared with INDO alone. [Click here to view] |
In contrast, the suppressive effect of omeprazole on the Bax/Bcl-2 ratio was observed only in gastric, but not in jejunum tissues (Fig. 3A and 3B).
Activation of PERK-CHOP signaling in indomethacin-induced GI ulceration
After 24-hour treatment, indomethacin significantly increased expression levels of p-PERK, p-eIF-2α, ATF-4, and CHOP proteins in jejunum tissue by approximately 2.3–5.8 folds, but not in the stomach tissue of rats (Fig. 4A and 4B). Pretreatment with MLWE or MLEE significantly suppressed its effect on the phosphorylation of PERK and eIF-2α proteins, and on the expression of ATF-4 and CHOP proteins, in jejunum tissues. This was evidenced by approximately 40%–74% reductions in p-PERK, p-eIF-2α, ATF-4, and CHOP proteins compared to indomethacin treatment alone (Fig. 4B). Conversely, omeprazole was unable to prevent indomethacin-mediated changes in the expression levels of these proteins (Fig. 4B).
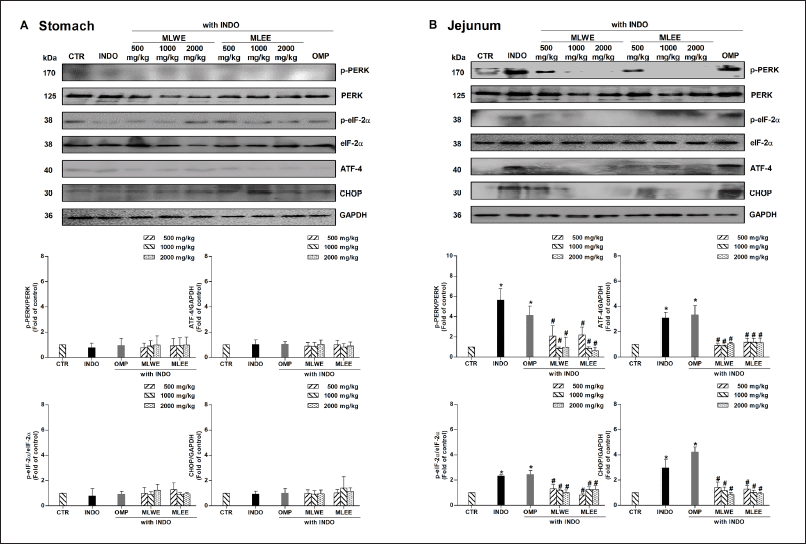 | Figure 4. Immunoblots and their densitometrical analysis of PERK/eIF-2α/ATF-4/CHOP proteins in rat stomach (A) and jejunum (B) after 24-hour treatment with (INDO; 40 mg/kg) in the absence and presence of MLWE or MLEE or OMP; 100 mg/kg. Each column represents mean ± SD. obtained from six rats (n = 6). *p < 0.05 compared with control (CTR). #p < 0.05 compared with INDO alone. [Click here to view] |
PGE2 production
The level of PGE2 in gastric tissue of indomethacin-treated rats was significantly lower (by approximately 98%) than that of the untreated control (Fig. 5A and 5B), whereas its levels in jejunum tissue of both groups were comparable, suggesting that indomethacin did not interfere with jejunal PGE2 production. In contrast to the positive control omeprazole, neither MLWE nor MLEE could attenuate the suppressive effect of indomethacin on gastric PGE2 production.
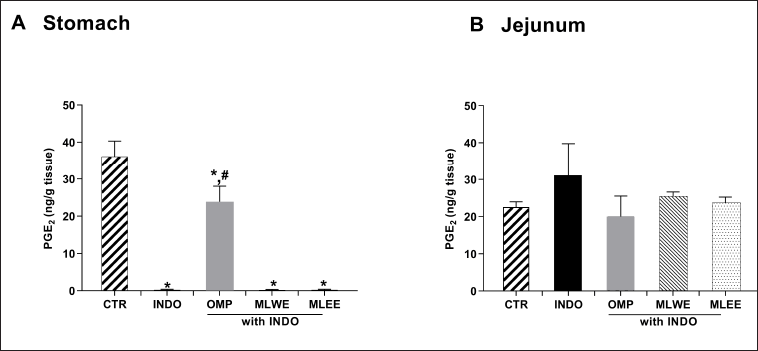 | Figure 5. PGE2 contents in rat stomach (A) and jejunum (B) after 24-hour treatment with (INDO; 40 mg/kg) in the absence and presence of mulberry leaf water (MLWE; 2,000 mg/kg) or ethanol extract (MLEE; 2,000 mg/kg) or omeprazole (OMP; 100 mg/kg). Each column represents mean ± SD. obtained from six rats (n = 6). *p < 0.05 compared with CTR. #p < 0.05 compared with INDO alone. [Click here to view] |
In vitro cell viability
As shown in Figure 6A, both mulberry leaf extracts could not prevent indomethacin-mediated gastric AGS cell death. However, they were able to reduce the cytotoxicity of tunicamycin and H2O2, as indicated by an increase in cell viability to 75%–100%. For the intestinal (Caco-2) cell study (Fig. 6B), both MLWE and MLEE exhibited their cytoprotective effect against all three cell stress inducers, increasing cell viability to 80%–100%. This effect of both extracts, in AGS and Caco-2 cells, was concentration sdependent.
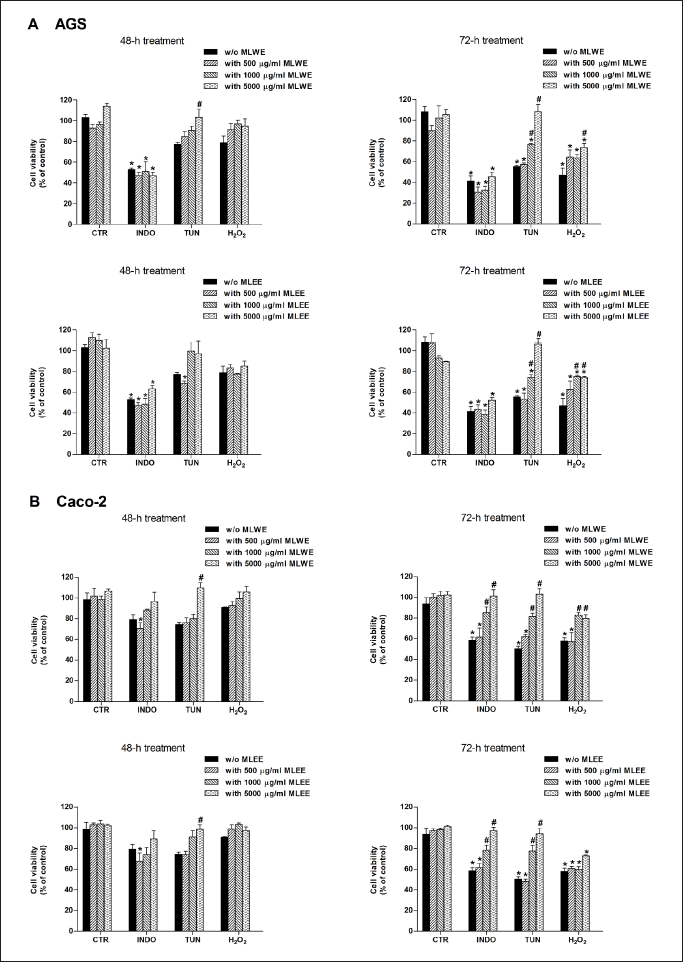 | Figure 6. AGS (A) and Caco-2 (B) cell viability after 48- and 72-hour exposure to stressors [indomethacin (INDO; 1,000 µM), tunicamycin (TUN; 20 µg/ml), or hydrogen peroxide (H2O2; 500 µM)] in the absence and presence of MLWE or MLEE. Each column represents mean ± SD. (n = 3). *p < 0.05 compared with CTR. #p < 0.05 compared with INDO alone. [Click here to view] |
Radical scavenging activity and cellular ROS production
MLWE and MLEE were able to scavenge DPPH radicals with EC50 values of 0.6 and 1 mg/ml, respectively (Supplementary Table 2). As shown in figures 7A and 7B, after 2-hour exposure, indomethacin markedly increased intracellular superoxide anion and H2O2 in AGS and Caco-2 cells. The levels of superoxide anion and H2O2 significantly decreased in the presence of either MLWE or MLEE at 5 mg/ml.
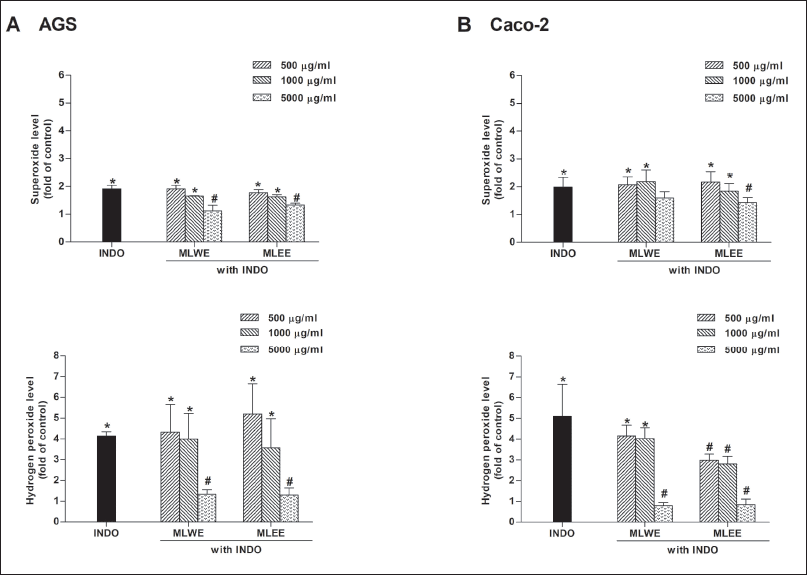 | Figure 7. Effect of MLWE and MLEE on superoxide anion and hydrogen peroxide production in AGS (A) and Caco-2 (B) cells treated with 1,000 µM INDO for 2 hours. Each column represents mean ± S.D. (n = 3). *p < 0.05 compared with control. #p < 0.05 compared with INDO alone. [Click here to view] |
DISCUSSION
Mulberry leaves, in addition to being employed as food for silkworms, have been widely used as health supplements rich with antioxidant polyphenols [6,7]. In this study, we focused on the preventive capability of mulberry leaf extracts against GI ulceration caused by indomethacin. The doses of MLWE and MLEE given orally to rats, at 500–2,000 mg/kg, were estimated to be a human equivalent dose of approximately 7–28 mg/kg, equal to 85–344 mg of dried leaves/kg (for MLWE), and 28–112 mg of dried leaves/kg (for MLEE) [21]. In the Chinese Pharmacopoeia, the suggested human daily dose for mulberry leaves was 167 mg/kg [22]. Thus, it could be presumed that the dose ranges in our study were less than the clinical dose, except when MLWE was used at 2,000 mg/kg. In both extracts used in this study, chlorogenic acid, which is a major bioactive component of mulberry leaves, was found in comparable amounts to previous reports [11,12]. Differences in the pathogenesis of NSAID-induced ulcerative damage in the stomach and small intestine have been demonstrated both clinically and in animal models [1,3]. Indomethacin-induced gastric ulceration has been largely associated with the inhibition of the COX-1 “housekeeper” enzyme in gastric epithelium cells, leading to a reduction of mucosal PGE2 levels [4]. Consequently, multiple events including reduced mucus secretion and gastric cell proliferation, increased acid secretion and apoptotic cell death have been observed in subjects exposed to indomethacin [2,4]. On the contrary, indomethacin can cause intestinal damage mainly through a COX-independent mechanism [3]. It has been demonstrated that indomethacin inhibits mitochondrial complex I activity and increases ROS production, leading to ER stress-mediated apoptosis of intestinal cells [3,5]. In our study, rats exposed to 40 mg/kg indomethacin developed ulceration in the stomach, in correlation to a decreased level of gastric PGE2. Evidently, the gastric cells underwent apoptosis without activating ER stress sensor PERK and eIF-2α/ATF-4/CHOP signaling. We also demonstrated that indomethacin could induce damage to the small intestine without any effect on PGE2 levels. Our results showed that indomethacin increased the expression of p-PERK/p-eIF-2α/ATF-4/CHOP proteins in rat jejunum tissue, in association with a higher Bax/Bcl-2 apoptotic expression ratio and a greater degree of intestinal ulceration.
The GI protection of both MLWE and MLEE against indomethacin toxicity was region-specific. Both extracts were able to significantly reduce mucosal ulcerative lesions caused by indomethacin in rat jejunum. This protective capability, similar to chlorogenic acid and quercetin [20], stemmed from their ability to suppress the activation of PERK/eIF-2α/ATF-4/CHOP ER stress signaling and apoptosis of intestinal cells. In addition to chlorogenic acid and quercetin, other polyphenolic compounds in the extracts such as rutin and naringenin, except gallic acid, might also contribute to their intestinal protection against indomethacin through suppression of CHOP-dependent ER stress [13]. Overall, it was very likely that the intestinal protective effect of both MLWE and MLEE against indomethacin-mediated ER stress ulcers was largely due to their major chemical component, chlorogenic acid.
Similar to previous reports, pretreating the rats with omeprazole (a proton pump inhibitor) successfully prevented gastric apoptotic damages from indomethacin due to preserved level of PGE2 production [23,24]. However, omeprazole was unable to suppress PERK-CHOP signaling of ER stress in jejunum tissues, resulting in apoptotic damage and ulcerative lesions there. Recently, we also demonstrated that quercetin, but not chlorogenic acid, elicited its preventive action against indomethacin-induced gastric injury through PGE2-dependent mechanisms [20]. Although naringenin and rutin have been reported to protect against ethanol-induced gastric lesions, their cytoprotection was not related to PGE2 level [25,26]. Neither MLWE nor MLEE could exert their protective effects against indomethacin in gastric tissues. This may be due to their incapability to preserve normal PGE2 levels during indomethacin exposure. Even though both MLEE and MLWE elicited similar suppressive effects on indomethacin-mediated ER stress in rat jejunum tissues, MLEE showed a greater ability to reduce the apoptotic Bax/Bcl-2 expression. In Caco-2 cells treated with indomethacin, this mulberry extract was also better at reducing the production of H2O2, but not superoxide. These findings could be related to the different amount and composition of phenolic compounds present in each extract. Natural polyphenols including chlorogenic acid and various flavonoids (e.g., catechin, naringenin, quercetin, and rutin) have been shown to be antioxidant with cytoprotective abilities in animals and in various cell culture models [13,14,27]. In addition to suppression of ER stress, we anticipate that MLEE and MLWE would render their protection against indomethacin-induced enteropathy via other apoptosis-related mechanisms such as reduction of oxidative stress or activation of survival signaling pathways such as phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and nuclear factor erythroid-2 related factor 2/heme oxygenase-1 (Nrf-2/HO-1) signaling [28–30]. For example, in order to decrease cell stress, chlorogenic acid reportedly activated the survival Nrf-2/HO-1 signaling pathway via induction of p21Waf1/Cip1 [28]. Naringenin and rutin, which were presented only in MLEE, might upregulate anti-apoptotic Bcl-2 expression through activation of the PI3K/Akt pathway [29,30]. It was worth noting that the free radical scavenging capacity of both mulberry leaf extracts was unable to prevent indomethacin toxicity, and they were less effective in reducing the cytotoxicity to AGS and Caco-2 cells of H2O2 than tunicamycin.
CONCLUSION
Both MLWE and MLEE effectively prevented indomethacin-induced intestinal ulcers, but not gastric ulcers. Their protective mechanisms were associated with the suppression of ER stress-mediated apoptosis in the intestinal tissues and were independent of PGE2 levels. The ineffectiveness of these extracts against gastric ulceration might be related to their inability to maintain PGE2 production in GI tissues during indomethacin exposure. Further clinical studies are needed to investigate the preventive effects of M. alba leaf extracts on indomethacin-induced intestinal ulcers.
ACKNOWLEDGMENT
The authors thank the Second Century Fund, Chulalongkorn University (C2F scholarship to CB), and National Laboratory Animal Centre of Mahidol University for providing animal facilities.
AUTHOR CONTRIBUTIONS
Cherdsak Boonyong: Concept and design, Data acquisition, Data analysis/interpretation, Drafting manuscript, Statistical analysis, Final approval. Wannee Angkhasirisap: Data acquisition, Data analysis/interpretation. Nonthalert Lertnitikul: Material support. Kanchana Kengkoom: Data acquisition, Data analysis/interpretation. Rutt Suttisri: Material support, Critical revision of manuscript. Suree Jianmongkol: Concept and design, Data acquisition, Data analysis/interpretation, Critical revision of manuscript, Statistical analysis, Funding, Supervision, Final approval. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
FINANCIAL SUPPORT
This work was supported by a grant from the Agricultural Research Development Agency (Public Organization), Thailand [Grant CRP6305030450].
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Animal Care and Use Committee of the National Laboratory Animal Center, Mahidol University, Thailand with approval number RA2020-27.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bielsa-Fernández MV, Tamayo-de la Cuesta JL, Lizárraga-López J, Remes-Troche JM, Carmona-Sánchez R, Aldana-Ledesma JM, et al. The Mexican consensus on the diagnosis, treatment, and prevention of NSAID-induced gastropathy and enteropathy. Rev Gastroenterol Mex. (Engl Ed). 2020;85(2):190–206. CrossRef
2. Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. 2018;154(3):500–14. CrossRef
3. Chávez-Piña AE, Abril-Urías RR, Cerecedo D. Mechanisms of damage involved in small intestinal tract caused by nonsteroidal anti-inflammatory drugs. Mex J Med Res ICSA. 2018;6(12):1–5. CrossRef
4. Liu YH, Zhang ZB, Zheng YF, Chen HM, Yu XT, Chen XY, et al. Gastroprotective effect of andrographolide sodium bisulfite against indomethacin-induced gastric ulceration in rats. Int Immunopharmacol. 2015;26(2):384–91. CrossRef
5. Boonyong C, Vardhanabhuti N, Jianmongkol S. Modulation of non-steroidal anti-inflammatory drug-induced, ER stress-mediated apoptosis in Caco-2 cells by different polyphenolic antioxidants: a mechanistic study. J Pharm Pharmacol. 2020;72(11):1574–84. CrossRef
6. Liang HW, Yang TY, Teng CS, Lee YJ, Yu MH, Lee HJ, et al. Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals. Int J Med Sci. 2021;18(1):53–64. CrossRef
7. Ranjan B, Pranab L, Pakrasi PL, Kumar RV. Antioxidant and anti-diabetic potential of MR-1 mulberry variety. Int J Pharm Biol Sci. 2018;8(4):904–14.
8. Wang Y, Li M, Yu X, He S, Wu X, Wang Y. Mulberry leaf flavonoids protect against glucotoxicity-induced INS-1 cell apoptosis. J Tradit Chin Med. 2019;39(2):153–9.
9. Suthamwong P, Minami M, Okada T, Shiwaku N, Uesugi M, Yokode M, et al. Administration of mulberry leaves maintains pancreatic β-cell mass in obese/type 2 diabetes mellitus mouse model. BMC Complement. Med Ther 2020;20(1):136. CrossRef
10. Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A. Mohd Noor NQI. Morus alba L. plant: bioactive compounds and potential as a functional food ingredient. Foods. 2021;10(3):689. CrossRef
11. Ji S, Zhu C, Gao S, Shao X, Chen X, Zhang H, et al. Morus alba leaves ethanol extract protects pancreatic islet cells against dysfunction and death by inducing autophagy in type 2 diabetes. Phytomedicine. 2021;83:153478. CrossRef
12. Kujawska M, Ewertowska M, Adamska T, Ignatowicz E, Flaczyk E, Przeor M, et al. Protective effect of Morus alba leaf extract on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. In vivo. 2016;30(6):807–12. CrossRef
13. Boonyong C, Vardhanabhuti N, Jianmongkol S. Natural polyphenols prevent indomethacin-induced and diclofenac-induced Caco-2 cell death by reducing endoplasmic reticulum stress regardless of their direct reactive oxygen species scavenging capacity. J Pharm Pharmacol. 2020;72(4):583–91. CrossRef
14. Koudoufio M, Desjardins Y, Feldman F, Spahis S, Delvin E, Levy E. Insight into polyphenol and gut microbiota crosstalk: are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants. 2020;9(10):982. CrossRef
15. Farzaei MH, Abdollahi M, Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J Gastroenterol. 2015;21(21):6499–517. CrossRef
16. Sembiring EN, Elya B, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J. 2018;10(1):123–7. CrossRef
17. Carrasco-Pozo C, Castillo RL, Beltrán C, Miranda A, Fuentes J, Gotteland M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-κB and Nrf2. J Nutr Biochem. 2016;27:289–98. CrossRef
18. Mabeku LBK, Nana BN, Bille BE, Tchuenguem RT. Anti-Helicobacter pylori and antiulcerogenic activity of Aframomum pruinosum seeds on indomethacin-induced gastric ulcer in rats. Pharm Biol. 2017;55(1):929–36. CrossRef
19. Sabiu S, Garuba T, Sunmonu T, Ajani E, Sulyman A, Nurain I, et al. Indomethacin-induced gastric ulceration in rats: protective roles of Spondias mombin and Ficus exasperata. Toxicol Rep. 2015;2:261–7. CrossRef
20. Boonyong C, Angkhasirisap W, Kengkoom K, Jianmongkol S. Different protective capability of chlorogenic acid and quercetin against indomethacin-induced gastrointestinal ulceration. J Pharm Pharmacol. 2023;75(3):427–36. CrossRef
21. USFDA. Guidance for industry: estimating the maximum safe starting dose in adult healthy volunteer. Rockville, MA: 2005.
22. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Chin Med Sci Technol Press. 2020;1:310–1.
23. AlKreathy HM, Alghamdi MK, Esmat A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J. 2020;28(8):916–26. CrossRef
24. Liu R, Zhu N, Hao Y, Liu X, Kang J, Mao R, et al. The protective effect of walnut oligopeptides against indomethacin-induced gastric ulcer in rats. Nutrients. 2023;15(7):1675. CrossRef
25. Pérez Guerrero C, Martín MJ, Marhuenda E. Prevention by rutin of gastric lesions induced by ethanol in rats: role of endogenous prostaglandins. Gen Pharmacol. 1994;25(3):575–80. CrossRef
26. Motilva V, Alarcón de la Lastra C, Martín MJ. Ulcer-protecting effects of naringenin on gastric lesions induced by ethanol in rat: role of endogenous prostaglandins. J Pharm Pharmacol. 1994;46(2):91–4. CrossRef
27. Di Sotto A, Locatelli M, Macone A, Toniolo C, Cesa S, Carradori S, et al. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules. 2019;24(17):3103. CrossRef
28. Han D, Gu X, Gao J, Wang Z, Liu G, Barkema HW, et al. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic Biol Med. 2019;137:1–12. CrossRef
29. Hua FZ, Ying J, Zhang J, Wang XF, Hu YH, Liang YP, et al. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int J Mol Med. 2016;38(4):1271–80. CrossRef
30. Li Y, Qin L, Ying L, Dong H, Wang D. Rutin prevents retinal ganglion cell death and exerts protective effects by regulating transforming growth factor-β2/Smad2/3Akt/PTEN signaling in experimental rat glaucoma. Trop J Pharm Res. 2019;18(5):985–93. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website: Link Here [https://japsonline.com/admin/php/uploadss/4465_pdf.pdf]