INTRODUCTION
The availability of biosimilars has the potential to improve patient access to affordable, safe, and effective biological treatments. The introduction of biosimilars frequently results in higher biologic utilization because lower costs provide patients with greater access [1]. Furthermore, as more biosimilars become available, increased competition has been proven to lower the prices of both biosimilars and originator biological products, allowing for significant cost savings [2]. According to a recent IQVIA report, global savings from biosimilars are expected to reach US$383 billion from 2023 to 2027 [3]. Thus, many countries around the world have implemented regulatory measures to facilitate the market entry of biosimilars [4,5]. However, drug lag, defined as the delay between the global first approval of a drug and the approval from the national drug regulatory authority in each country can have implications for timely patient access in a given country [6,7]. Drug lag for biosimilars is particularly of concern for low and middle-income countries, given the unmet need for biologicals in these countries [8]. The introduction of biosimilars in these countries may result in increased affordability, improved access to biological therapies, and a reduction in the financial burden on their healthcare systems. Drug lag comprises submission time lag (the time taken from global first approval to country-specific submission for approval) and regulatory review time (the time taken from country submission to country approval). Although regulatory approval does not always imply market availability, it is a necessary condition for market entry in many countries. Therefore, the absence or delay in biosimilar approval in a country may result in limited access to affordable treatment alternatives to innovative biological products [9,10].
Malaysia, an upper middle-income country in Southeast Asia was one of the first countries in the world to establish a regulatory pathway for biosimilars [4]. Based on the European Medicine Agency (EMA) 2005 guidelines for biosimilars, the guidance document and guidelines for the registration of biosimilars in Malaysia were finalized on 30 July 2008, and considered for adoption on 4 August 2008 [11,12]. Furthermore, the country has implemented additional measures to encourage market entry and utilization of biosimilars [13,14]. However, it is unclear how quickly biosimilar approvals have occurred in Malaysia relative to the global situation and how approvals have evolved over the years. Previous studies have focused on the regulatory framework and processes [11,15,16], and stakeholder perceptions of biosimilars in Malaysia [17,18], with little attention to approval dynamics. Therefore, the objectives of this study were to assess the drug lag for biosimilars approved in Malaysia from their approval in the European Union (EU) and to examine the evolution of biosimilar approvals in Malaysia in terms of the number and approval time lag between successive brands of each biosimilar between 4 August 2008 and 31 August 2023. EU approval was used as the global reference because the Malaysia biosimilar guidelines were based on the EMA biosimilar guidelines. [11], and the EU is the global pioneer in biosimilar approval, with the most approvals and uptake in the world [3,9].
MATERIALS AND METHODS
Study design
This study is a descriptive retrospective study of a cohort of approved biosimilars in the EU and their subsequent approvals in Malaysia between 4 August 2008 and 31 August 2023.
Data sources
The data used in this study was publicly available. Study data was obtained from published sources and from the websites of EMA and the National Pharmaceutical Regulatory Agency (NPRA), Ministry of Health Malaysia. NPRA is responsible for marketing authorization of pharmaceuticals, including biological products in Malaysia [19].
Data collection
Biosimilar approvals in the EU
First, the list of biosimilars approved in Europe as of 12 May 2023, published by the Generics and Biosimilars Initiative (GaBI) [20], was used to identify the product name (brand), international non-proprietary names (INNs), and approval dates of biosimilars in Europe. This list was updated until 31 August 2023 by searching the EMA medicines website [21], with the keyword “biosimilars” and filtering by “human,” “medicines,” “authorised,” and “last updated” (13/05/2023-31/08/2023). Then, for each of the biosimilars on the updated list, a search was conducted on the EMA medicines website again using the product name and applying the filter for European public assessment reports to retrieve marketing authorization details and confirm the exact dates of approval by EMA. If there were any discrepancies between the two sources, the EMA approval dates took precedence over those listed by GaBI.
Biosimilar approvals Malaysia
The list of approved biosimilars in Malaysia as of 31 August 2023 was obtained from the website of NPRA [22]. The list consists of the approved biosimilar active substance INN, product name (brand name), registration number, name of product registration holder in Malaysia, manufacturer name and country of location, and the brand and company name of the reference biological product upon which the approval of the biosimilar was based. The date of approval for each of the listed biosimilars was extracted from the NPRA database of approved new products using the product name of the biosimilar [23]. The database is a register (arranged by year) of approved new products, and it contains the product registration number, product name, name of the product registration holder, name of the manufacturer, product category, date of approval, and date of approval expiration. The product registration number was used to determine which year in the database register to check for the approval date, as each approved product has a unique registration number that includes the year of approval. The first two digits of the registration number refer to the year of approval. The database of approved new products that was publicly available at the time of the current study was from 2013 onward. Therefore, the “list of approved biosimilar products in Malaysia” published in an earlier study [16] was used to identify the approval dates for those approved before 2013 and where the database information is not available Finally, as in an earlier study, when the date information was incomplete and only the year and month were available, the date was assumed to be “15th” [6].
Eligibility criteria
Only biosimilars with an approved reference biological product in Malaysia were included in the study. Additionally, approved biosimilars manufactured in Malaysia but are “For Export Only (FEO)” were not included in the study. In Malaysia, the product registration number for FEO products is differentiated from the product registration number for products registered for the local and export markets with the addition of an “E” suffix [24].
Study population
The study population for drug lag assessment was defined as biosimilars approved in the EU and having the likelihood of experiencing approval in Malaysia between 4 August 2008 and 31 August 2023. The study entry date is the date when a biosimilar approved in the EU enters the study population, and the start of follow-up time began on the study entry date and continued until the approval in Malaysia or the end of the study period (31 August 2023), whichever came first. To ensure that the study data accurately captures the time to approval in Malaysia and that the approval probabilities for all biosimilars are comparable, the study entry date for biosimilars approved in the EU before 4 August 2008 was set at 4 August 2008 [25,26]. This is because biosimilars can only be approved in Malaysia on or after 4 August 2008, when the biosimilar approval pathway was established.
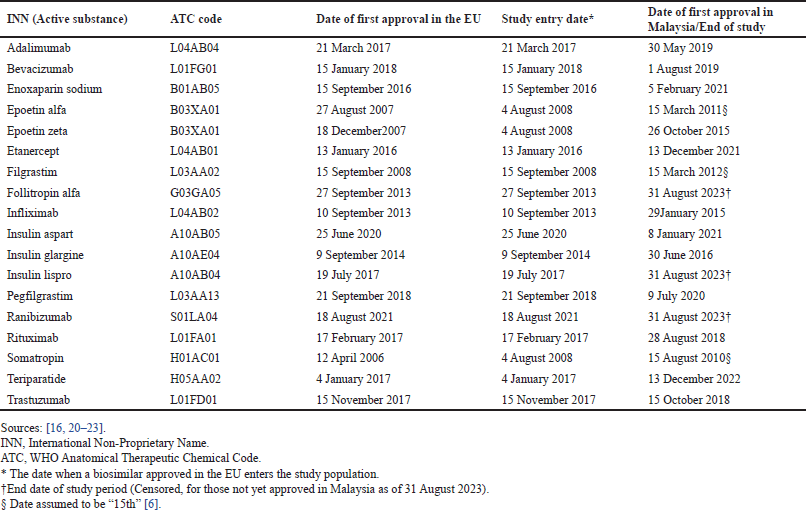 | Table 1. First approved INN biosimilars in the EU with subsequent approval in Malaysia as of 31 august 2023. [Click here to view] |
Measures
Biosimilar drug lag in Malaysia was defined as the time difference (in days) between the study entry date of an INN biosimilar first approved by the EMA and the date of first approval of the same INN biosimilar by the NPRA or the end of the study period (31 August 2023), whichever came first. The time lag (in days) between successive brands of each approved INN biosimilar drug in Malaysia was the difference between the dates of approval of successive brands. In this study, biosimilar brands were identified by their product names. Different dosage strengths, formulations, or presentations of an INN biosimilar with the same brand name were counted only once (using the approval date of the first approved brand name). However, the same brand name INN biosimilars with different countries of manufacture were counted separately.
Statistical analysis
Time-to-event analysis with the Kaplan-Meier method was used to assess biosimilar drug lag in median time (days) [26]. Time-to-event analysis, also known as survival analysis, is a statistical procedure used to analyze the time until an event of interest occurs [26,27]. It is the most appropriate analytical approach when some study participants do not experience the event of interest before the study ends [26]. This approach has been used in previous studies on drug lag [6,28]. In this study, not all biosimilars approved in the EU experienced approval in Malaysia before the end of the study period (31 August 2023). Therefore, for those biosimilars that had not experienced approval in Malaysia by 31 August 2023, the drug lag was censored and computed as the time from the study entry date until 31 August 2023 [6,26]. Due to the small sample size of the study data, the analysis was limited to estimating the overall probabilities of biosimilar approval using the Kaplan-Meier method [27]. The number and time lag between successive brands of each approved biosimilar in Malaysia during the study period were examined using frequencies and percentages. Data analyses were conducted using Microsoft Excel and IBM SPSS version 25.
RESULTS
Biosimilar drug lag
As of 31 August 2023, 19 INN biosimilars were first approved in the EU, 18 of which met the eligibility criteria. Table 1 presents the list of eligible approved INN biosimilars with their first approval date in the EU and their first approval date in Malaysia, or the end date of the study period for those that did not experience approval in Malaysia as of 31 August 2023. Of the 18 eligible INN biosimilars approved in the EU, 15 (83.3%) were subsequently approved in Malaysia.
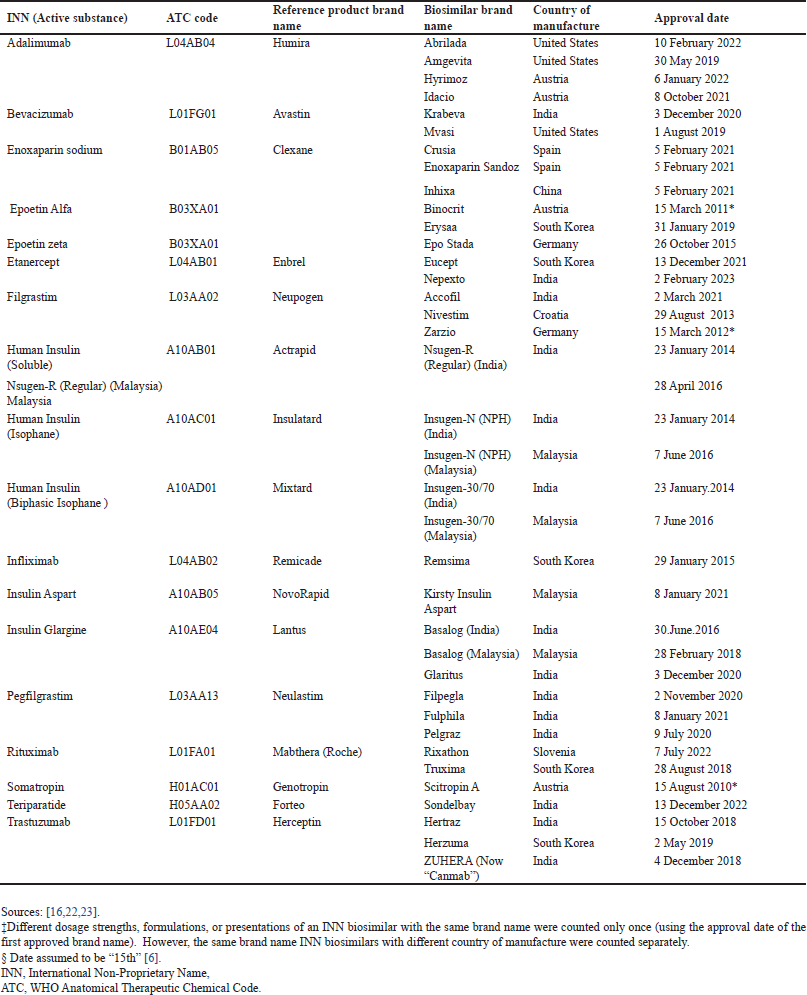 | Table 2. List of approved biosimilar in Malaysia as of 31 August 2023‡. [Click here to view] |
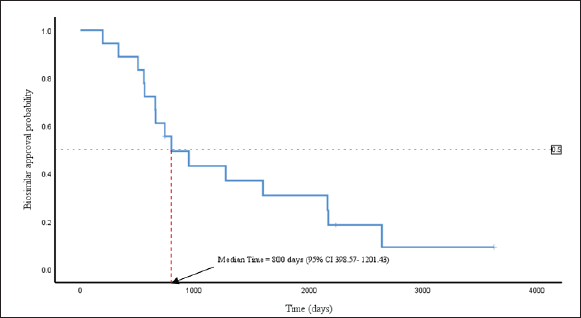 | Figure 1. Kaplan-Meier curve for biosimilar drug lag in Malaysia following approval in the EU. The point on the X-axis where the horizontal dashed line at an approval probability of 0.5 intersects the curve represents the estimated median time lag. CI, confidence interval. [Click here to view] |
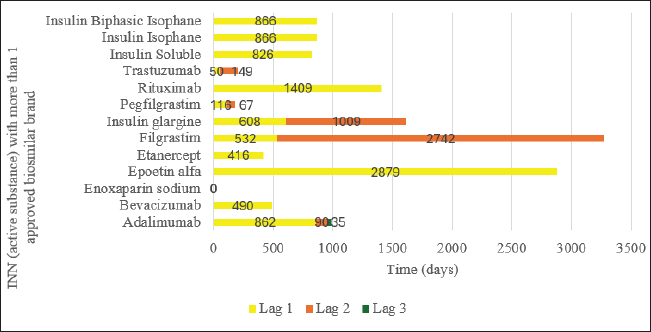 | Figure 2. Time lag between successive brands of approved INN biosimilars in Malaysia with more than one brands. [Click here to view] |
The Kaplan-Meier curve for biosimilar drug lag is presented in Figure 1. It shows the probability of biosimilar approval in Malaysia over time following approval in the EU. The result showed that the overall median approval lag for the first INN biosimilars in our study was 800 days (95% CI 398.57–1201.43) from approval in the EU.
Evolution of biosimilar approvals in Malaysia
During the study period (4 August 2008–31 August 2023), a total of 18 INN biosimilars in 38 different brands were approved in Malaysia (Table 2). A majority (76.3%, n = 29) of the brand approvals occurred in the last half (2016–2023) of the study period compared to the proportion approved (23.7%, n = 9) in the first half (2008–2015). The 18 INN biosimilars fell into four therapeutic categories (WHO ATC 4th level). Antineoplastic and immunomodulating agents account for the highest proportion (44.4%, n = 8 ), followed by alimentary tract and metabolism (27.8%, n = 5).
The number of brand approvals ranged from 1 to 4 per INN biosimilar, with a median of 2 (Interquartile range [IQR] = 1–3). Only adalimumab experienced a fourth brand approval. Figure 2 displays the graphical representation of the time lags between successive brands of approved biosimilars with more than one brand. Lag 1, Lag 2, and Lag 3 represent the time differences between first and second brand approvals, between second and third brand approvals, and between third and fourth brand approvals, respectively.
The longest time lag was between the first and second brands of epoetin alpha (879 days), and the shortest time lag was for enoxaparin sodium. All the enoxaparin sodium brands were approved on the same day. In this study, the median time lag (days) between the first and second biosimilar brand approvals (Median Lag 1) was 608 days (n = 13, IQR = 266–866), while that between the second and third brand approvals (Median Lag 2) was 119.50 days (n = 6, IQR = 50.25–1442.25). Because only one biosimilar (adalimumab) experienced a fourth brand approval, the median time lag between the third and fourth brand approvals was not estimated.
DISCUSSION
This study explored how quickly biosimilar approvals have occurred in Malaysia relative to the global situation and how approvals have evolved over the years since the implementation of the biosimilar regulatory pathway in August 2008 until August 2023.
Biosimilar lag
Using a time-to-event analysis of 18 INN biosimilars approved in the EU, it was found that subsequent approval occurred in Malaysia between August 2008 and August 2023, on average 800 days after EU approval. Previous studies have similarly reported approval time delays for biosimilars between countries and jurisdictions [9,29,30]. Though these studies focused only on approved biosimilars, which may have underestimated the time lag [6], their findings on time lag are generally comparable to the current study. For example, a study of 16 biosimilars of nine originator products that had been approved by both the FDA and the EMA as of February 2019 found that ten biosimilars received EMA approval first, with a median time of 18 months to FDA approval [30]. The result of the median biosimilar approval lag of 800 days in this study is also similar to that of a recent study in Malaysia, which found a median approval lag of 855 days for innovative medicines [31]. While it is recognized that the timing of approval of new drugs, including biosimilars often varies across countries and regions [5,9], any delay could limit timely patient access [7,32]. The observed lag in biosimilar approval in this study therefore suggests that patients may experience delayed access to new biosimilar treatments that have already been approved on the international market. Thus, to mitigate this possibility in countries, there have been calls for streamlined regulatory approval for biosimilars between countries to improve the availability and access to affordable biological therapies around the world [5,10,33]. However, as earlier mentioned, drug lag comprises submission lag time and regulatory review time. The regulatory review time includes both the regulatory authority assessment period and the applicant’s time to respond to enquiries regarding their application [19,32]. In Malaysia, the target regulatory review timeline for new chemical entities (NCEs) and biologics is 245 working days for standard review or 120 working days for priority review [19,24]. Priority review with a target of 120 days may be granted for the first INN biosimilars that have not been previously registered in Malaysia [24]. However, these targets may be shorter or longer in practice depending on various time-dependent factors [15,19]. It can therefore be assumed that the biosimilar time lag observed in this study is attributed to both submission and regulatory review delays. Many factors may contribute to submission and regulatory review delays in the regulatory approval process. These include, including company strategies, the local regulatory and legal environment, specific product-related characteristics, country-specific clinical or regulatory requirements, and regulatory agencies’ resource constraints, such as staffing and expertise [7,32]. Although the specific reasons for the observed biosimilar lag cannot be determined in the current study, the finding suggests that the biosimilar regulatory approval process could be improved. The timely approval of the first biosimilar of a given reference biological product is especially crucial, as the literature indicates that market entry of the first biosimilars results in the greatest cost-savings [2,34]. The date of the first biosimilar market entry date has also been found to be associated with biosimilar uptake [35,36]. For instance, a study of biosimilar competition in Europe found that the average market share captured by the first biosimilar tumor necrosis factor inhibitor more than doubled that for the second biosimilars, and the market share by the first biosimilar erythropoietin similarly almost doubled that for the second biosimilars across all countries [34]. Therefore, identifying and addressing any challenges in the biosimilar approval process can help patients have timely access to life-saving biological therapies. This could include streamlining regulatory approval processes or incentivizing companies to submit their biosimilar registration dossiers on time [5,33,37]. For example, reducing uncertainties over patent or other exclusivity issues and providing clarity on regulatory requirements can potentially foster biosimilar submission and entry. A previous qualitative study conducted in 2017 among Malaysian pharmaceutical manufacturers identified a lack of understanding of regulatory requirements, susceptibility to litigation by originator biological product manufacturers, and a fragmented government support system as some of the challenges in developing biological products in Malaysia [38]. In this regard, the recent revision of Malaysia’s biosimilar guidance is noteworthy [39]. According to NPRA, the revised guidance was initiated to align with the current version of the WHO biosimilar guideline, which was released in 2022, with the goal of making some of the clinical and nonclinical requirements more consistent with current practices and other guidelines, as well as providing clarification and flexibility [39]. In addition to refining regulatory requirements and providing more clarity, adequate resource allocation, training, exploring opportunities for mutual recognition of regulatory assessments, and leveraging existing data from stringent regulatory authorities’ approvals may be needed to expedite the regulatory process in Malaysia [32,33,40].
Biosimilar evolution in Malaysia
Between August 2008 and August 2023, 18 INN biosimilars in 38 different brands were approved in Malaysia, with a median of two biosimilar brands per INN reference biological product. These findings are generally consistent with those of other studies that examined biosimilar approvals around the world [9,41,42]. For example, according to an IQVIA market report [42], as of July 2023, the FDA had approved 40 biosimilar brands across 15 reference biologic products. Depending on factors such as pricing and availability, having multiple biosimilar brands for a given INN can enhance patient access to these biologic treatments [43]. The potential for price-lowering competition from biosimilar entry into the Malaysian market has been reported. For instance, the market entry of biosimilar insulin glargine, first approved in 2016, reportedly caused the price of insulin glargine to fall by 16% in 2019 [44]. Similarly, the introduction of biosimilar trastuzumab in 2018, which costs approximately 50% of the price of the originator trastuzumab, caused the originator to reduce its price of trastuzumab by 52%, following an open tender for supply of trastuzumab by the Ministry of Health Malaysia [45]. Biosimilar competition is especially important in conditions where biological products play a significant role in treatment, such as cancer, inflammatory diseases, and diabetes [46]. Cancer and diabetes pose a significant financial burden in Malaysia. In 2017, diabetes and cancer accounted for about one-third (30.33%) and one-quarter (23.67%) of the RM 1.72 billion spent on drugs for cardiovascular diseases, diabetes, and cancer in Malaysia [47]. For diabetes, different types of insulins accounted for more than a quarter (27.42%) of total expenditures on diabetes medications [47]. Therefore, increasing the availability of cost-saving biosimilars to treat these diseases is essential. However, while competition is beneficial, it is important to ensure that the approved biosimilars maintain high standards of quality and safety. Future research examining the impact of biosimilar competition on healthcare systems, particularly in terms of cost savings and patient access, could complement the findings of the current study by providing a broader understanding of the implications of biosimilar approval policy in Malaysia. This study indicates a notable shift in the approval trends of biosimilar brands in Malaysia over the study period. Specifically, a majority of biosimilar brands were approved during the latter half (2016–2023) compared to the initial half (2008–2015) of the study period. This temporal pattern suggests that biosimilar approval and adoption in the country have accelerated in recent years. This finding is similar to that of a previous study of biosimilar approvals across several countries between 2006 and 2023, which found that most approvals were in the last few years of the study period [9]. The observed increase in biosimilar approvals during the latter half of this study period thus reflects a dynamic evolution in the biosimilar regulatory landscape and healthcare system in Malaysia. This temporal trend may be indicative of regulatory enhancements, increased industry interest, or evolving healthcare policies aimed at promoting the uptake of biosimilars. The pattern observed in this study also aligns with global trends indicating a surge in biosimilar approvals and market penetration in recent years [3,5]. Malaysia’s experience therefore seems to mirror broader international efforts to leverage biosimilars as a means to enhance access to biologic therapies and contain healthcare costs. It is, therefore, imperative that policymakers prioritize measures that promote a supportive environment for biosimilars. One way that could help foster the sustainability of the biosimilar market is to encourage local production [48]. However, strong incentives may be needed to support a cost-efficient domestic manufacturing industry [48]. Furthermore, given the complexity of biologic manufacturing, local manufacturers should adhere to regulatory requirements, and regulators should strengthen monitoring and controls to ensure consistent product quality, safety, and efficacy [5,49]. In sum, the observed temporal shift in biosimilar approvals, with a majority occurring in the latter half of the study period (2016–2023), highlights a dynamic evolution in Malaysia’s biosimilar landscape. This trend has implications for healthcare policy, clinical practice, and future research endeavors, emphasizing the need for continued vigilance and proactive measures to maximize the potential benefits of biosimilar adoption.
Regarding the time lag of biosimilar brand approval after the first approval, this study found that there is a substantial period (608 days) between the introduction of the initial biosimilar into the market and the subsequent approval of the second biosimilar (Lag 1). This prolonged interval could be attributed to potential challenges or complexities in the early stages of biosimilar adoption, including regulatory processes, manufacturing considerations, or market dynamics [50,51]. However, the median time between the approval of the second and the third biosimilar brands (Lag 2) was 119.50 days. This indicates a relatively quicker approval process for the third biosimilar following the approval of the second. The wider interquartile range for lag 2 suggests greater variability in the time taken for subsequent biosimilar approvals during this stage, possibly influenced by factors such as regulatory streamlining, increased industry experience, or market demand. The decreasing median time intervals may indicate an optimization of regulatory processes over time. It is possible that regulatory authorities and industry stakeholders may have become more adept at navigating biosimilar evaluations and approvals, leading to a more streamlined process for subsequent brands [52,53]. In summary, these results underscore the importance of understanding the evolving dynamics of biosimilar approvals, enabling stakeholders to make informed decisions and contribute to a more efficient and competitive market for biosimilars.
STRENGTHS AND LIMITATIONS
This study is the first to examine the dynamics and evolution of biosimilar approvals in Malaysia, filling a crucial gap in understanding regulatory approval trends in this emerging field. Time-to-event analysis, a robust statistical method, was used to comprehensively assess the approval dynamics of both approved and yet-to-be-approved INN biosimilars, providing granularity into understanding biosimilar lag and evolution in Malaysia. Furthermore, both the first approved brands and subsequent brand approvals were considered in this study, offering a holistic view of the biosimilar landscape in Malaysia and allowing for comparisons between pioneering and subsequent products. The long data period (2008–2023) of this study also ensures a thorough understanding of the evolution of biosimilar approvals over time, spanning from the beginning of the biosimilar regulatory pathway to the recent data available. Finally, all available data during the study period was utilized, maximizing the reliability and robustness of our findings and enabling a thorough analysis of biosimilar approval dynamics in Malaysia.
However, this study has some limitations. First, the sample size available for analysis is limited, which may affect the generalizability of the findings and the precision of the estimates. This constraint necessitates caution in extrapolating the results to the broader context of biosimilar approvals in Malaysia. Second, while the study provides a comprehensive description of biosimilar approval dynamics, it is important to note that the data do not permit inferential statistical analysis. As such, while trends and patterns in the approval process can be observed, the study cannot establish causal relationships or make predictions based on statistical inference. Finally, it is crucial to acknowledge that biosimilar approvals do not necessarily equate to product availability in the market. Factors such as pricing, reimbursement policies, and market dynamics can significantly impact the accessibility and uptake of biosimilars, which the current study did not explicitly address. Therefore, while this study offers valuable insights into the regulatory landscape of biosimilar approvals in Malaysia These limitations underscore the need for further research to comprehensively understand the implications of these dynamics on patient access and outcomes.
CONCLUSION
This study sheds light on the dynamics of biosimilar approvals in Malaysia, revealing a lag in approval time relative to the global situation, with an average delay of 800 days following EU approvals. This delay underscores the potential impact on patient access to life-saving treatments. The observed temporal shift in biosimilar approvals, particularly the acceleration in the latter half of the study period, suggests evolving regulatory landscapes and increased industry interest, mirroring global trends. Addressing challenges in the regulatory process and incentivizing timely submissions could enhance patient access to cost-saving biosimilars. Furthermore, understanding the evolving dynamics of biosimilar approvals is crucial for stakeholders to contribute to a more efficient and competitive market for these essential treatments.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors did not receive support from any organization for the submitted work.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS:
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. IQVIA. Biosimilars in the United States 2023-2027: competition, savings, and sustainability. Durham, North Carolina: IQVIA; 2024. [cited 2024 Mar 4]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2023-2027
2. Chen AJ, Kaiser KM, Gascue L, Manetas MA, Van Nuys K. Cancer drug trastuzumab and its biosimilars compete on price for market share. Health Aff. 2023;42(6):779–84. CrossRef
3. IQVIA. The global use of medicines 2023: outlook to 2027. Durham, North Carolina: IQVIA; 2024. [cited 2024 Mar 4]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicines-2023
4. Kang HN, Thorpe R, Knezevic I. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biologicals. 2020;65:1–9. CrossRef
5. Klein K, Gencoglu M, Heisterberg J, Acha V, Stolk P. The global landscape of manufacturers of follow-on biologics: an overview of five major biosimilar markets and 15 countries. BioDrugs. 2023;37(2):235–45. CrossRef
6. Tachibana Y, Narukawa M. Oncology drug lag in Japan: has it improved over the last decade? Int J Clin Oncol. 2023;28(11):1451–60. CrossRef
7. Liberti L, McAuslane N, Patel P. Characterising the influencers of submission lag time for medicines in the emerging markets. Analysis of short and long lag time factors. 2012. [cited 2024 Mar 4]. Available from: https://cirsci.org/wp-content/uploads/2020/05/CIRS_RD_Briefing_51_Lag_Time_in_EM.pdf
8. Calamia M, Abraham I. The economics of biosimilars and challenges to biosimilar adoption in low- and middle-income countries. Expert Opin Biol Ther. 2023;23(8):653–57. CrossRef
9. Machado FLDS, Cañás M, Doubova SV, Urtasun MA, Marín GH, Osorio-de-Castro CGS, et al. Biosimilars approvals by thirteen regulatory authorities: a cross-national comparison. Regul Toxicol Pharmacol. 2023;144:105485. CrossRef
10. Kang HN, Wadhwa M, Knezevic I, Ondari C, Simao M. WHO guidelines on biosimilars: toward improved access to safe and effective products. Ann N Y Acad Sci. 2023;1521(1):96–103. CrossRef
11. Ministry of Health Malaysia. Guidance document and guidelines for registration of biosimilars in Malaysia. Petaling Jaya, Malaysia: Ministry of Health Malaysia; 2008 [cited 2024 Mar 4]. Available from: https://www.npra.gov.my/images/Guidelines_Central/Guidelines_on_Regulatory/GUIDELINES%20FOR%20REGISTRATION%20OF%20BIOSIMILAR%20(1).pdf
12. Generics and Biosimilars Initiative. Biocon wins three-year contract to supply insulin in Malaysia. 2017. [cited 2024 Mar 4]. Available from: https://www.gabionline.net/biosimilars/news/Biocon-wins-three-year-contract-to-supply-insulin-in-Malaysia
13. Pharmaceutical Services Programme, Ministry of Health Malaysia. position statements on the use of biosimilars in the ministry of health, Malaysia healthcare facilities.2022. [cited 2024 Mar 4]. Available from: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/position-statements-use-biosimilars-ministry-health-malaysia-healthcare-facilities.pdf
14. Khoo YSK, Tang TY, Goh PS, Halimi HM, Ab Ghani A. An update on the registration of biosimilars in Malaysia. Ther Innov Regul Sci. 2017;51(1):55–9. CrossRef
15. Mohd Sani N, Aziz Z, Kamarulzaman A. Biosimilars in Malaysia: regulatory framework, approved products, and adverse effects. Ther Innov Regul Sci. 2021;55(3):490–502. CrossRef
16. Chong SC, Rajah R, Chow PL, Tan HC, Chong CM, Khor KY, et al. Perspectives toward biosimilars among oncologists: a Malaysian survey. J Oncol Pharm Pract. 2022;13:10781552221104773. CrossRef
17. Mohd Sani N, Aziz Z, Kamarulzaman A. Malaysian hospital pharmacists’ perspectives and their role in promoting biosimilar prescribing: a nationwide survey. BioDrugs. 2023;37(1):109–20. CrossRef
18. Sani NM, McAuslane N, Kasbon SH, Ahmad R, Yusof FAM, Patel P. An evaluation of Malaysian regulatory process for new active substances approved in 2017 Using the OpERA methodology. Ther Innov Regul Sci. 2020;54(5):1215–24. CrossRef
19. Generics and Biosimilars Initiative. Biosimilars approved in Europe. 2023. [cited 2023 Aug 23]. Available from: https://www.gabionline.net/biosimilars/general/biosimilars-approved-in-europe
20. European Medicines Agency. Medicines. Amsterdam, The Netherlands: European Medicines Agency; 2023. [cited 2023 Nov 28]. Avaiable from: https://www.ema.europa.eu/en/medicines
21. National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. List of approved biosimilars, DCA 388 – 14th September 2023. Petaling Jaya, Malaysia: National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. [cited 2023 Sep 25]. Available from: https://www.npra.gov.my/index.php/en/informationen/new-products-indication/biosimilars-approved.html
22. National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. New products approved. Petaling Jaya, Malaysia: National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. [cited 2023 Sep 25]. Available from: https://www.npra.gov.my/index.php/en/informationen/new-products-indication/new-products-approved-quest3.html
23. National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. Drug registration guidance document (DRGD). 3rd ed. Petaling Jaya, Malaysia: National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia. [cited 2024 Jan 23]. Available from: https://www.npra.gov.my/easyarticles/images/users/1153/DRGD January 2024/Complete-Drug-Registration-Guidance-Document-DRGD-3rd-Edition-7th-Revision-January-2024.pdf
24. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. CrossRef
25. Schober P, Vetter TR. Survival analysis and interpretation of time-to-event data: the tortoise and the hare. Anesth Analg. 2018;127(3):792–98. CrossRef
26. Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 2010;143(3):331–6. CrossRef
27. Son KB. Do free trade agreements matter to drug lag? Recent evidence from Korea after the Korea-U.S. free trade agreement. Int J Health Serv. 2020;50(2):147–55. CrossRef
28. Bird E, Derbyshire M. Biosimilars markets: US and EU compared. GaBI J. 2020; 9(2):90–2. CrossRef
29. Jung EH, Sarpatwari A, Kesselheim AS, Sinha MS. FDA and EMA biosimilar approvals. J Gen Intern Med. 2020;35(6):1908–10. CrossRef
30. Chow WL, Salleh NAM, Kang TS. Access to innovative medicines: regulation change and factors associated with drug approval lag in Malaysia. Ther Innov Regul Sci. 2024;58(3):528–38. CrossRef
31. Patel P, McAuslane N, Liberti L. Trends in the regulatory landscape for the approval of new medicines in Asia. R&D Briefing. 2019;72:1–6. [cited 2024 Mar 4]. Available from: https://cirsci.org/wp-content/uploads/2020/02/CIRS-RD-Briefing-72-Trends-in-the-regulatory-landscape-Asia.pdf
32. Cohen HP, Turner M, McCabe D, Woollett GR. Future evolution of biosimilar development by application of current science and available evidence: the developer’s perspective. BioDrugs. 2023;37(5):583–93. CrossRef
33. QuintilesIMS. The impact of biosimilar competition in Europe. 2017 [cited 2024 Mar 4]. 1–33 pp. Available from: https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf
34. Rémuzat C, Dorey J, Cristeau O, Ionescu D, Radière G, Toumi M. Key drivers for market penetration of biosimilars in Europe. J Mark Access Health Policy. 2017;5(1):1272308. CrossRef
35. Böhm AK, Steiner IM, Stargardt T. Market diffusion of biosimilars in off-patent biologic drug markets across Europe. Health Policy. 2023;132:104818. CrossRef
36. Shajarizadeh A, Hollis A. Delays in the submission of new drugs in Canada. CMAJ. 2015;187(1):E47–E51. CrossRef
37. Khoo YSK, Saleh K. A qualitative study among potential manufacturers on the development of “made in Malaysia” biological products: challenges and proposed solutions. J Commer Biotechnol. 2017;23(4). CrossRef
38. Ministry of Health Malaysia. Guidance document and guidelines for registration of biosimilars in Malaysia. 2nd ed. Petaling Jaya, Malaysia: Ministry of Health Malaysia; 2023 [cited 2024 Feb 21]. Available from: https://www.npra.gov.my/easyarticles/images/users/1047/Direktif/Lampiran-B_GUIDANCE-DOCUMENT-AND-GUIDELINES-FOR-REGISTRATION-OF-BIOSIMILARS-IN-MALAYSIA_Second-Edition-December-2023.pdf
39. Kang HN, Thorpe R, Knezevic I, Casas Levano M, Chilufya MB, Chirachanakul P, et al. Regulatory challenges with biosimilars: an update from 20 countries. Ann N Y Acad Sci. 2021;1491(1):42–59. CrossRef
40. Feldman M, Reilly MS. A white paper: US biosimilars market on pace with Europe. GaBI Journal. 2020;9(4):150–4. CrossRef
41. Yoon E. Now trending: biosimilars. U.S. biosimilar market trends roundup. 2023. [cited 2024 Feb 20]. Available from: https://www.iqvia.com/locations/united-states/blogs/2023/07/now-trending-biosimilars
42. Frank RG, Shahzad M, Kesselheim AS, Feldman W. Biosimilar competition: early learning. Health Econ. 2022;31(4):647–63. CrossRef
43. Malaysian Biotechnology Information Centre. Biosimilars—a case of more for less. The Petri Dish. Petaling Jaya, Malaysian Biotechnology Information Centre; 2019 [cited 2024 Feb 21]. Available from: https://thepetridish.my/2019/05/26/biosimilars-a-case-of-more-for-less/
44. Generics and Biosimilars Initiative. Dramatic price reduction of trastuzumab in Malaysia. 2019. [cited 2024 Feb 23]. Avaialble from: https://www.gabionline.net/biosimilars/general/dramatic-price-reduction-of-trastuzumab-in-malaysia#:~:text=Following this%2C the Ministry of,) (Amendment) Order 2017
45. Chan JCN, Chan ATC. Biologics and biosimilars: what, why and how? ESMO Open. 2017;2(1):e000180. CrossRef
46. Ministry of Health Malaysia, World Health Organization. Direct health-care cost of noncommunicable diseases in Malaysia. Putrajaya, Malaysia: Ministry of Health Malaysia; 2022. [cited 2024 Feb 19]. Available from: https://www.moh.gov.my/moh/resources/Penerbitan/Rujukan/NCD/NCD_Laporan/HEALTH-COST_of_NCDs-7a-WEB.pdf
47. Chhabra H, Mouslim MC, Kashiramka S, Rathore AS. Dynamics of biosimilar uptake in emerging markets. Expert Opin Biol Ther. 2022;22(6):679–88. CrossRef
48. Rathore AS, Chhabra H, Bhargava A. Approval of biosimilars: a review of unsuccessful regulatory filings. Expert Opin Biol Ther. 2021;21(1):19–28. CrossRef
49. Lucio S. The complexities of biosimilars and the regulatory approval process. Am J Manag Care. 2018;24(11 Suppl):S231–6.
50. Wolff-Holz E, Tiitso K, Vleminckx C, Weise M. Evolution of the EU biosimilar framework: past and future. BioDrugs. 2019;33(6):621–34. CrossRef
51. Agrawal G, Dikan J, Lyons M, Poetes R, Prabhakaran M. Getting strategic about new-product submissions in the pharma industry. 2021 [cited 2024 Feb 12]. Available from: https://www.mckinsey.com/industries/life-sciences/our-insights/getting-strategic-about-new-product-submissions-in-the-pharma-industry#/
52. Kurki P, Kang HN, Ekman N, Knezevic I, Weise M, Wolff-Holz E. Regulatory evaluation of biosimilars: refinement of principles based on the scientific evidence and clinical experience. BioDrugs. 2022;36(3):359–71. CrossRef