INTRODUCTION
The use of medicinal and aromatic plants in the agri-food and medical fields has emerged as a sustainable solution permitting to overcoming of adverse effects related to synthetic food additives and chemical medicines [1,2]. Ethnobotanical studies play an important role in the discovery of medicinal and aromatic plants used by different populations for their healing potential [3]. Indeed, these studies open the way for researchers to utilize the documented ethnobotanical data for the study of the biological activities of the mentioned medicinal and aromatic plants [4–7], in order to exploit their activities for sustainable practical applications, including the use of plants extracts, essential oils or isolated molecules, as green compounds in different fields such as the food industry, medical, and agricultural sectors.
Chamaerops humilis, Olea oleaster, and Asparagus albus, are three plant species belonging to Arecaceae, Oleaceae, and Liliaceae families, respectively [8,9]. They are naturally distributed in the Mediterranean basin [10–12], in which they are often occurring associated with different areas [13–15]. According to the International Union for Conservation of Nature, they are classified as least concern species [16].
Chamaerops humilis has been traditionally used to treat various ailments such as diabetes, hepatitis, dyspepsia [17] warts [18], bile stones, liver diseases, and diabetes [19]. Different studies have proved the important bioactive potential of C. humilis. Thus, this plant was found to exert antioxidant [20–23], antimicrobial [24–27], anticholinesterase [22,28], antidiabetic [29,30], and antiviral properties [31]. The phytochemical screening of this plant has been the subject of several works which revealed the richness of C. humilis in bioactive compounds including flavonoids, saponins, esters, terpenoids, quinones, phenolic acids, tannins, alcohols, steroids, and alkaloids [21,32–34].
Similarly, the traditional use of O. oleaster in the treatment of several diseases [35–38] has led to the performance of many scientific works demonstrating its biological properties including antioxidant [39,40], antimicrobial [41,42], antiviral [41], and hepatoprotective activities [43]. These activities are related to the multiple active compounds that have been revealed through different phytochemical screening tests and methods [39,44,45].
As for A. albus, it was also used traditionally in folk medicine against a wide variety of diseases: jaundice, dental ailments, respiratory and rheumatic problems [46], stomach disorders [47], and urinary diseases [48,49]. However, to the best of our knowledge, up to date, only four published works exist regarding its phytochemistry and biological properties [10,50–52].
Thus, the aim of this review is to document and summarize data up-to-date related to C. humilis, O. oleaster, and A. albus ethnomedicinal uses and phytochemical compounds and to highlight their biological activities, in order to encourage their use in different sectors as green products and to determine gaps for further studies. To the best of our knowledge, no review is available in this context.
MATERIALS AND METHODS
In order to gather data concerning the ethnomedicinal uses of C. humilis, A. albus, and O. oleaster, their phytochemistry and biological properties, a comprehensive search was carried out in different electronic databases including Google Scholar, Pub Med, Pub Chem, Science Direct, and Scopus. All published papers until December 2023 that represent the aim of our review were included. In addition to these three plant species names, the keywords used for this research were: pharmacological properties, antioxidant, antimicrobial, ethnomedicinal, ethnobotanical, phytochemistry, bioactive compounds, dwarf palm, and wild olive. For the Google Scholar engine, we have applied exclusion criteria taking in to account the existence of these keywords only in the title so as to limit the number of obtained results.
In order to visualize the dominant words in the reviewed studies, their titles and abstracts were analyzed by Vosviewer software.
RESULTS AND DISCUSSION
Dominance of studies related to the traditional use, phytochemistry, and bioactivity of the studied plants
According to >Figure 1 obtained using Vosviewer software, two important points can be drawn. On the one hand, we note the prevalence of studies concerning the traditional use of these plants in Morocco and Algeria (green circles). On the other hand, studies regarding the bioactivity and phytochemistry of the plants studied are dominant for the two species: C. humilis and O. oleaster (red circles). Moreover, these studies concern different parts of these plant species (leaf, fruit, and olive pits), and reveal the presence of certain predominant bioactive compounds such as rutin and oleuropein (red circles).
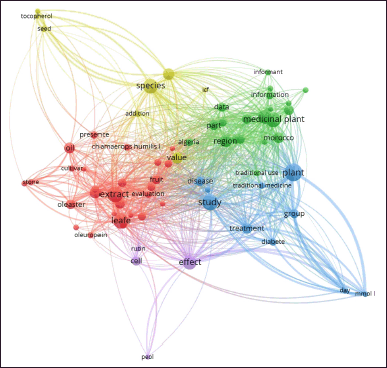 | Figure 1. Visualization of dominant studies regarding traditional use, phytochemistry and bioactivity of the studied plants retrieved from different databases (Google Scholar, Pub Med, Pub Chem, Science Direct and Scopus) using Vosviewer software. [Click here to view] |
Botanical description and geographical distribution
Chamaerops humilis
Chamaerops humilis is naturally distributed in Africa and Europe and constitutes an important floristic element of the western Mediterranean region [53].
This plant is in general in the form of compact and low clumps. It is characterized by:
- Stipe covered with reticulated fibers, which can reach 6–8 m high in protected places.
- Leaves with fan-shaped blades, reaching 70 × 80 cm split up to 2/3 and more.
- Long petiole 20–40 cm, with generally spiny margins (Fig. 2).
Moreover, male flowers are yellow, females are green, and hermaphrodites are greenish yellow. Etamines have fused nets at the base, free carpels, sessile stigma, ovules erect with basal placentation, and monosperm bay [9].
Olea oleaster
Geographically, O. oleaster is distributed in the whole Mediterranean Basin [54].
This plant represents the wild olive tree or shrub, having young, subquadrangular shoots. The leaves are briefly petiolate, simple, opposite, lanceolate, entire, leathery with discolored faces, silvery below and green above. Flowers are hermaphroditic or unisexual, and drupes have thick and bony cores [8] (Fig. 2).
Asparagus albus
Asparagus albus thrives in the Mediterranean region on rocky soils. Precisely, it’s distributed naturally in Northwestern Africa and Southwestern Europe [55]. This plant is characterized by white, fragrant stems and branches, straight or evenly inclined, broad cladodes, usually arched-decurved, slightly thickened claviform, obtusiuscule apex, slightly mucronate. In addition to a curved leaf spur, covered when young with short, dense papillae [9] (Fig. 2).
 | Figure 2. (A) Chamaerops humilis ; (B) Olea oleaster; (C) Asparagus albus. [Click here to view] |
Ethnomedicinal uses
Chamaerops humilis
Despite the occurrence of C. humilis in the Mediterranean region, ethnobotanical documentation of its uses exists only for the two following countries: Algeria and Morocco. Thus, in Algeria, leaf decoction and macerated extract are used against diabetes, hepatitis, and dyspepsia [17,56,57]. Fruits (Powder, decoction, macerated extract, and salad) are taken to fight gingiva, influenza, coughing, asthma, infection of the digestive tract, dyspepsia, breast, brain, and blood cancer [17]. Roots (decoction, powder, and macerated extract) are exploited to treat hepatitis, anemia, intestinal worms, rheumatism, and diabetes, and also for cleaning the uterus after childbirth [17,58]. The palm heart (salad) is eaten against dyspepsia, hypertension, cardiovascular diseases, diabetes [17], bloating, gastric pain, and constipation [56]. The spadicea (salad) is consumed against gastric pain and toning [56]. While the decoction and macerated extract of the heart of palm and spadicea are taken as carminative, and stimulant, against gastritis, gastro-enteritis, gastralgia, diarrhoea, wound of stomach, and constipation [57]. The aerial part infusion is administrated against urinary diseases [59]. In Morocco, leaf decoction is used to heal problems of bile stones, liver diseases, diabetes [19], and respiratory ailments [60]. Fruits (powder, decoction, and raw) are taken against liver diseases, diabetes, bile stones, digestive and urinary disorders, diarrhea, and gingivites [19,60–62]. Roots (decoction, cooked, powder, and infusion) are considered efficient against diabetes [62,63] and neurologic diseases [64]. Heart of palm (raw, salad) is administrated to heal gastrointestinal diseases [60], digestive disorders, cardiovascular system disorders, respiratory system disorders, diabetes, hepatitis, hair loss, and weakening of the immune system [26]. In Eastern Mallorca (Balearic Islands, Mediterranean Sea), leaf lotion is used against warts [18], whereas raw fruits are taken directly as antidiarrheal [18].
Olea oleaster
Olea Oleaster is used in folk medicine by several populations of the Mediterranean region. Thus, in Algeria, leaf decoction or infusion is used to adjust blood pressure [37,38]. In Morocco, different leaf preparations (infusion, decoction, and poultice) are used to treat many ailments, namely diabetes, hypertension, and urinary problems [65], in addition to burns [66], mouth diseases [67], nervousness, and parasitic infections [19]. In Libya, this plant species is used to treat gingivitis, Dyspepsia, Eczema, Constipation, and Earache [68]. In the Iberian Peninsula, leaves infusion or decoction are used to treat hypo or hypertension [35,36], whereas their raw form or decoction is considered as hypotensive, hypoglycemic, and anti-acid medicines [69]. In Turkey, leaves or branches decoction is used as a natural remedy against diabetes or hypertension [70,71].
Asparagus albus
Many ethnobotanical studies have highlighted the traditional use of A. albus in different countries of the Mediterranean region for medicinal purposes. Thus, in western Algeria, A. albus is used as an appetizer and against jaundice, rheumatism, and stomach disorders [72]. In Morocco, young sprouts are used to treat diabetes [36]. Aerial parts and roots are used to get rid of jaundice, dental ailments, respiratory and rheumatic problems [46]. Leaves infusion is consumed against stomach disorders [47], whereas leaves, stems and roots decoction is used for the treatment of several diseases including kidneys, rheumatism, diabetes, anemia, liver, and urinary ailments [73,74]. In Spain, especially in Granada, tender shoots are used as diuretic [35,75]. In Italy, young shoots decoction is also used as a diuretic [48,49].
Phytochemistry
Several studies have been dedicated to the analysis of chemical compounds present in the extracts of these three Mediterranean plant species.
Tables 1–3 show the chemical compounds identified in different plant parts extracts of C. humilis, O. oleaster, and A. albus, respectively. Moreover, they provide also the collection origin of the plant and the method used for their phytochemistry identification.
Chamaerops humilis has been demonstrated to contain a variety of chemical compounds (Table 1). Thus, flavonoids were revealed to be present in the methanolic, ethanolic, and aqueous extracts of its leaves, drupes, and rachis collected from different areas of the Mediterranean basin [21,34,76–79]. Saponins were found to be present in the methanolic extract of its leaves, stems, and underground parts collected from Tokyo [80]. Esters, namely methyl benzoate were identified in the dichloromethane extract of its leaves collected from France [33]. Terpenoids were demonstrated to exist in different extracts of its plant parts [33,57,81,82]. Quinones, phenolic acids, and tannins were exhibited in organic and aqueous extracts of different parts of C. humilis collected from Algeria [21,32,76]. Alcohols and alkaloids were found in the aqueous and dichloromethane extracts of its leaves [32,83].
As for O. oleaster, it is also rich in several bioactive compounds (Table 2), namely secoiridoid glycosides including oleuropein and oleoside which are characteristics of oleaceae family [40,41,39,44,45,84–86], secoiridiods aglycon such as oleuropein aglycon and ligstroside aglycon [45,85,87], glycosides [39–41,85,86], phenolic acids [40,41,45,87,88], flavonoids [39–41,44,45,85–87], and polyphenols [39,45,85,86]. In addition to these compounds which were found in different extracts of O. oleaster collected from several countries of the Mediterranean basin, terpenes and esters were revealed in extracts of this plant collected from Portugual and Tunisia [40,41,85] whereas aldehydes and ketones were revealed in the extracts of O. oleaster leaves and fruits collected from Portugual [40,45].
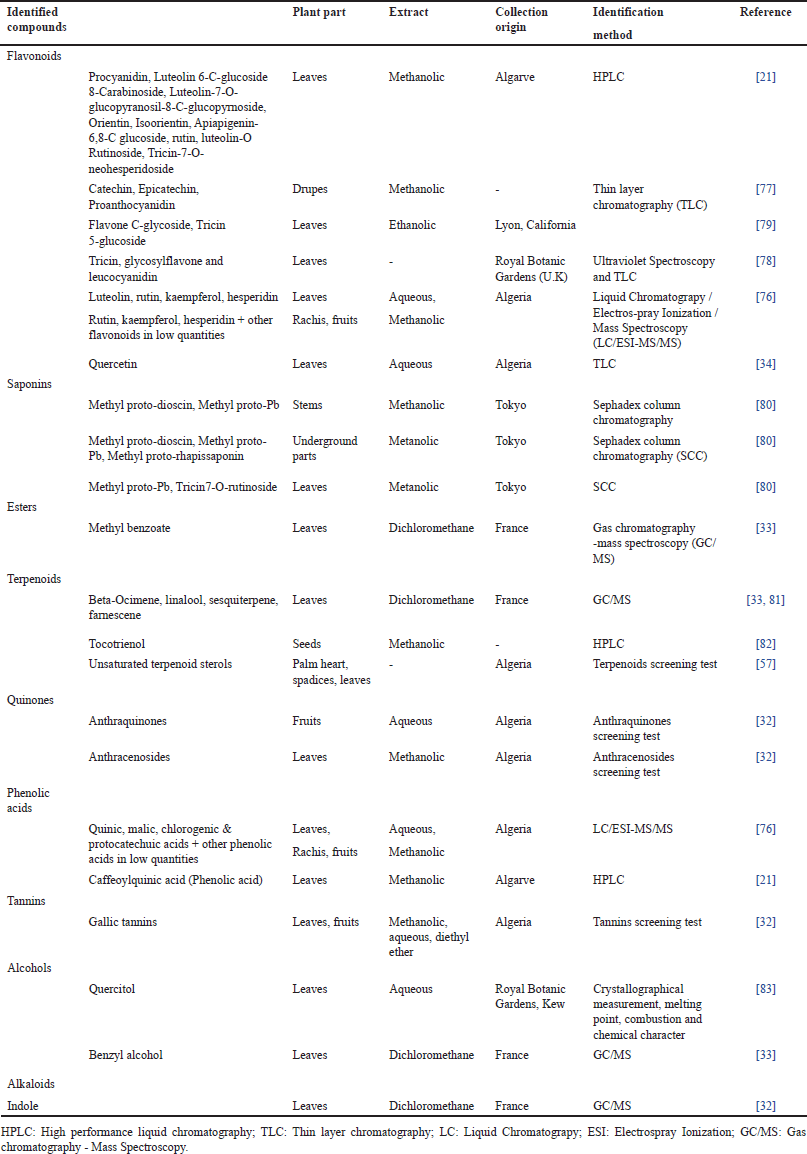 | Table 1. Chemical compounds of C. humilis according to the reported studies. [Click here to view] |
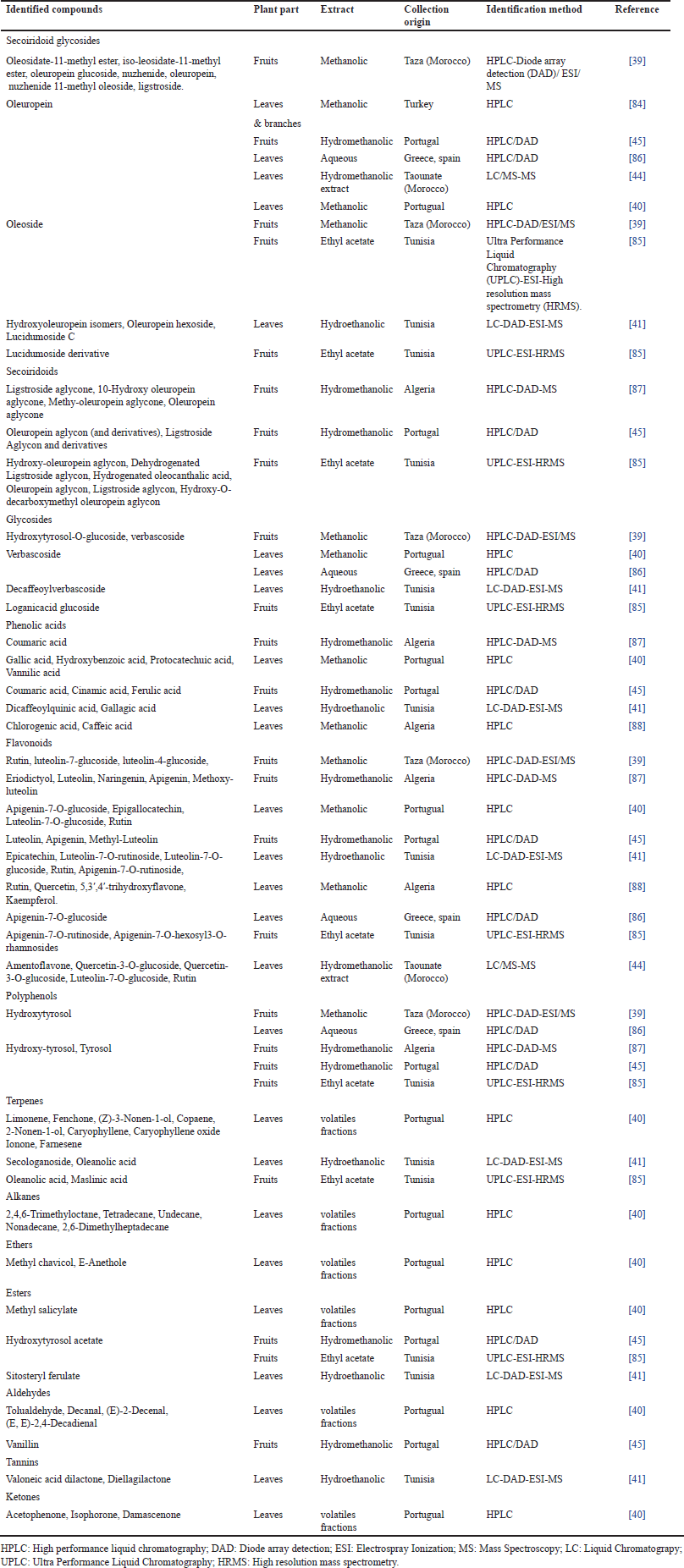 | Table 2. Chemical compounds of O. oleaster according to the reported studies. [Click here to view] |
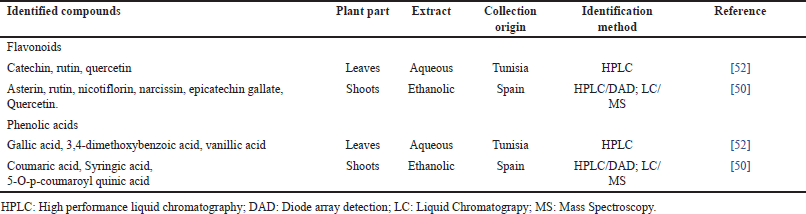 | Table 3. Chemical compounds of A. albus. [Click here to view] |
Regarding A. albus, only a few works in Tunisia and Spain have been dedicated to the determination of its phytochemistry. Indeed, three flavonoids have been found in its leave’s aqueous extracts, namely catechin, rutin, and quercetin [52], whereas for the same plant part, in addition to quercetin and rutin, ethanolic extract was revealed to contain other flavonoids: Asterin, nicotiflorin, narcissin and epicatechin gallate [50]. Moreover, this extract contained also several phenolic acids: Coumaric acid, Syringic acid, and 5-O-p-coumaroyl quinic acid [50]. A. albus leaves aqueous extract was found to include other phenolic acids: Gallic acid, 3,4-dimethoxybenzoic acid, and vanillic acid [52].
Overall, these plant species are rich in a wide variety of bioactive compounds, moreover, as it was noted for other plants, their chemical composition varies according to different factors such as the solvent of extraction, the collection origin, and the plant part [5].
Biological activities
Different biological activities were revealed to be exerted by the studied plant species (Fig. 3). Thus, C. humilis was found to exhibit antioxidant, antimicrobial, antilithiasic, antidiabetic, neuroprotective, and antiviral activities. Olea oleaster was demonstrated to have antioxidant, antimicrobial, antidiabetic, antiviral, hepatoprotective, antihyperlipidemic, and cytotoxic activities. As for A. albus, to the best of our knowledge, only three biological activities were proved scientifically, namely, antioxidant, antimicrobial, and hepatoprotective properties.
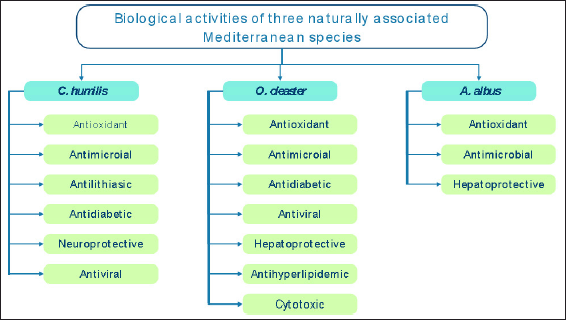 | Figure 3. Biological activities of C. humilis, O. oleaster and A. albus. [Click here to view] |
Antioxidant activity
Concerning the antioxidant activity of extracts of the three medicinal plant species: C. humilis, O. oleaster, and A. albus, several studies have shown the antioxidant activity of extracts obtained from C. humilis and O. oleaster different parts, whereas only very few studies have been published for A. albus (Table 4).
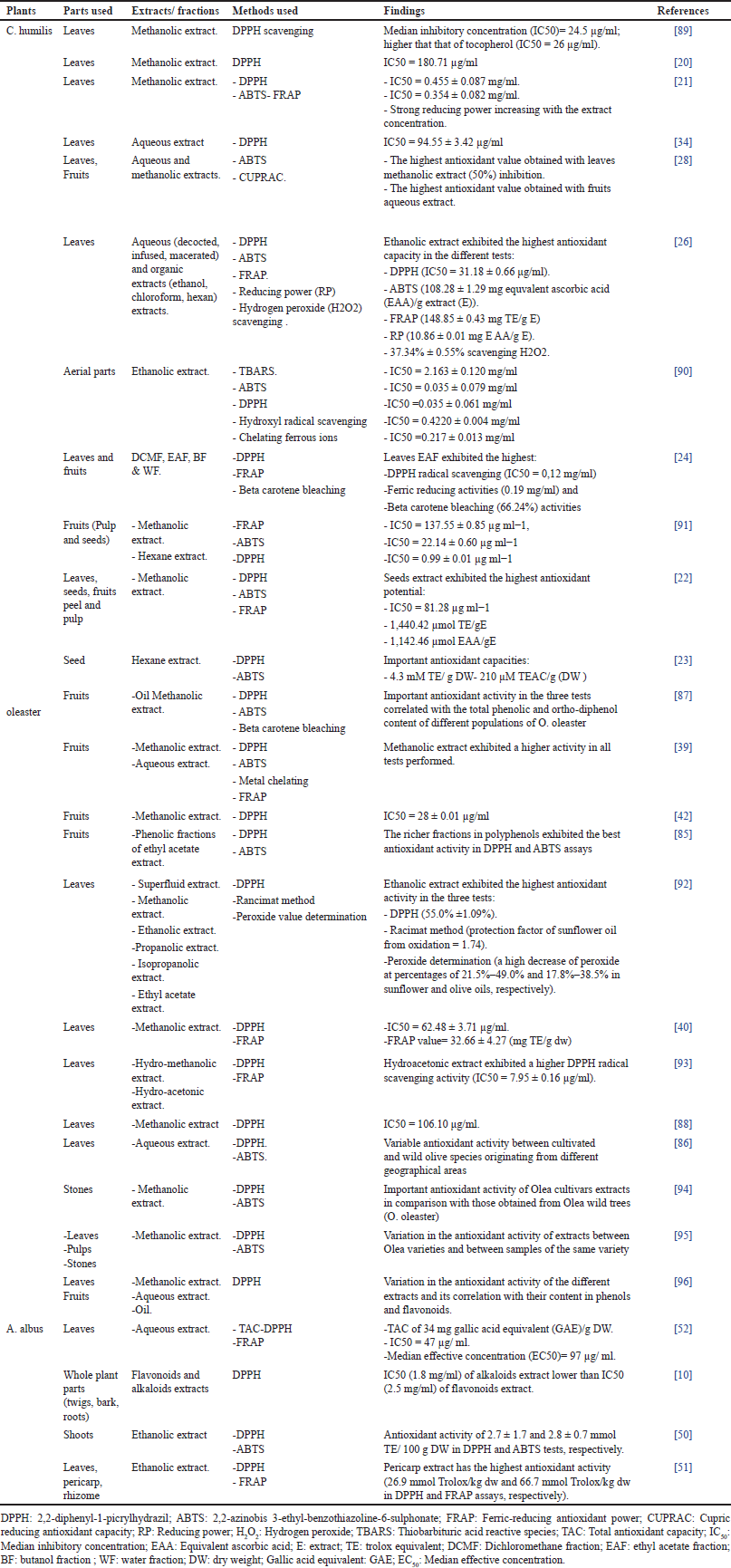 | Table 4. Antioxidant activity of C. humilis, O. oleaster and A. albus according to reported work [Click here to view] |
Thus, concerning C. humilis, Khoudali et al. [89] investigated the antioxidant capacity of C. humilis leaves methanolic extract using 2,2-diphenyl-1-picrylhydrazil (DPPH) scavenging test. The obtained results indicated a high ability of the tested extract to scavenge DPPH radicals (median inhibitory concentration IC50 = 24.5 µg/ml). Although Benahmed-Bouhafsoun et al. [20] tested the antioxidant activity of C. humilis using the same extract and method, they obtained an IC50 = 180.71 µg/ml. The difference noted in the antioxidant activity between these studies can be attributed to the chemical composition of each studied sample which varies with several factors such as the the geographical origin of the plant, the harvest period, environmental conditions, genotype, and so on.
Coelho et al. [21] have also investigated the antioxidant capacity of C. humilis leaves methanolic extract using different methods: DPPH (IC50 = 0.455 ± 0.087 mg/ml), 2,2-azinobis 3-ethyl-benzothiazoline-6-sulphonate (ABTS): IC50 = 0.354 ± 0.082 mg/ml and ferric-reducing antioxidant power (FRAP) assay; and thereby demonstrated a strong reducing power increasing with the extract concentration. The analysis of the chemical composition of this extract using high-performance liquid chromatography (HPLC) has revealed the presence of C- and O- flavones as well as its O-methylated derivatives [21]. These phenolic compounds may be responsible for the antioxidant activity of C. humilis leaves methanolic extract.
As regards C. humilis leaves aqueous extract, it has been shown [34] to exhibit also an ability of DPPH free radicals scavenging (IC50 = 94.55 ± 3.42 µg/ml), probably thanks to its polyphenols (25.20 ± 0.46 mg gallic acid equivalent (GAE)/ g of dried extract (DE)) and flavonoids (17.26 ± 0.61 mg catechin equivalent (CE)/g DE) content. Bouhafsoun et al. [28] investigated the antioxidant activity of leaves and fruits methanolic and aqueous extracts using two methods namely ABTS and cupric reducing antioxidant capacity (CUPRAC). The results showed that C. humilis leaves methanolic extract exhibited the highest ABTS scavenging (50.11%), whereas fruits induced the strongest antioxidant activity in CUPRAC assay. Thus, it can be concluded that the antioxidant activity of plant extracts depends on many factors involving the solvent used, plant organ, and the test performed. In another study, Miguel et al. [90] investigated the antioxidant activity of C. humilis aerial parts ethanolic extract and found an IC50 = 2.163 ± 0.120 mg/ml, IC50 = 0.035 ± 0.079 mg/ml, IC50 = 0.035 ± 0.061 mg/ml, IC50 = 0.4220 ± 0.004 mg/ml, and an IC50 = 0.217 ± 0.013 mg/ml for thiobarbituric acid reactive species (TBARS), ABTS, DPPH, hydroxyl radical scavenging and chelating ferrous ions tests, respectively. The antioxidant capacity of C. humilis leaves ethanolic, chloroformic, and aqueous (decocted, infused, and macerated) extracts has been studied by Lachkar et al. [26]. The results showed that the ethanolic extract exhibited the highest antioxidant capacity in the different tests performed: DPPH (IC50 = 31.18 ± 0.66 μg/ml), ABTS (108.28 ± 1.29 mg equivalent ascorbic acid (EAA)/g extract (E)), FRAP (148.85 ± 0.43 mg, trolox equivalent (TE)/g E), reducing power (10.86 ± 0.01 mg E AA/g E) and hydrogen peroxide (H2O2) scavenging (37.34% ± 0.55%) assays. The antioxidant potential of this extract was revealed to be correlated to its high polyphenols (100.27 ± 0.66 μg GAE/mg E) and catechic tannins content (52.11 ± 0.24 μg CE/mg E). More interestingly, Belhaoues et al. [24] studied the antioxidant activity of C. humilis in different fractions obtained from leaves and fruits: dichloromethane fraction (DCMF), ethyl acetate fraction (EAF), n-butanol fraction (BF), and water fraction (WF). Chamaerops humilis leaves EAF exhibited the highest DPPH radical scavenging (IC50 = 0.12 mg/ml), ferric reducing activities (0.19 mg/ml), and beta carotene bleaching (66.24%) activities. This important antioxidant ability of C. humilis leaves EAF could be related to its richness in phenolic compounds (214.80 ± 3.26 mg GAE/g fraction) in comparison with the other fractions [24]. Moreover, all the obtained fractions from C. humilis leaves exhibited a higher antioxidant activity than those obtained from its fruits. These results may be explained by the abundance of phenolic compounds in leaves [24]. This result may be explained by the abundance of phenolic compounds in leaves [24]. The study of the effect of the extraction technique (soxhlet and cold maceration using methanol) was carried out by Eddahhaoui et al. [91]. The parts tested were fruits (pulp and seeds). Seed extract obtained by cold maceration exhibited the highest antioxidant activity: IC50 = 137.55 ± 0.85 µg ml−1, IC50 = 22.14 ± 0.60 µg ml−1, and IC50 = 0.99 ± 0.01 µg m−1, for FRAP, ABTS, and DPPH assays, respectively. In fact, it was reported that cold maceration allows a good solubility of active compounds and prevents them from deterioration [91]. Moreover, seed extract obtained by maceration was richer in secondary metabolites [total phenolic compounds (TPC)= 281.64 ± 0.23 mg GAE/gE, total flavonoid content (TFC)= 74.08 ± 0.71 mg quercetin equivalent (EQ)/gE, total tannic compounds (TTC) (333.83 ± 0.96 mg CE/g E)] than pulp extract obtained by the same method (TPC = 55.81 ± 0.12 GAE/gE, TFC = 15.24 ± 0.53 mg EQ/gE, TTC = 0 mg CE/g E). Gonçalves et al. [22] studied the antioxidant activity of C. humilis methanolic extract obtained from different parts (Leaves, seeds, fruits peel, and pulp). Seeds extract exhibited the highest antioxidant potential in the different tests used: DPPH (IC50 = 81.28 µg ml−1), ABTS (1,440.42 µmol TE/gE), and FRAP (1,142.46 µmol EAA/gE). This result could be explained by the richness of this extract in condensed tannins and phenolics (170.00 µmol CE/gE and 1,564.88 µmol GAE/gE, respectively) [22]. Furthermore, Mokbli et al. [23] investigated the antioxidant ability of C. humilis seed oil using the ABTS and DPPH methods. The measured antioxidant capacities were 210 µM TE/g dry weight (DW) for the ABTS test and 4.3 mM TE/g DW for the DPPH assay. These findings revealed the important antioxidant potential of C. humilis seed oil wich could be attributed to its high TPC = 91 μg/g oil and TFC = 18 μg/g oil [23].
As for O. oleaster, Theodora et al. [92] studied the antioxidant potential of different solvents extracts (superfluid, methanolic, ethanolic, propanolic, isopropanolic, and ethyl acetate) of O. oleaster leaves using DPPH radical scavenging assay, rancimat method and peroxide value determination. The results revealed that ethanolic extract was the most efficient in inhibiting DPPH radical. Moreover, this extract exhibited a good capacity in preventing sunflower oil from oxidation in rancimat method and showed a higher ability to protect virgin olive oil and sunflower oil from oxidation in peroxide value determination assay. The high antioxidant power of ethanolic extract could be related to its content in several bioactive compounds including caffeic acid, luteolin, catechin, and oleuropein as it was reported in this study. Moreover, Makowska-W?s et al. [40] and Zighed et al. [88] have also demonstrated the antioxidant activity of O. oleaster leaves methanolic extract using different assays (DPPH, FRAP) assays, and they have attributed its activity to its content in several phenolic compounds such as rutin, oleuropein, chlorogenic, and caffeic acids. Mezouar et al. [93] investigated the antioxidant activity of O. oleaster leaves hydromethanolic and hydroacetonic extracts using DPPH and FRAP assays, and found a higher ability of DPPH radicals scavenging for the hydroacetonic extract. Besides they revealed that this extract was richer in polyphenols and flavonoids compared to hydromethanolic extract. In another study, Kabach et al. [39] found that methanolic extracts of O. oleaster fruits exhibited a higher antioxidant activity than their aqueous extracts in different tests performed (DPPH, ABTS, metal chelating and FRAP assays). This activity probably resulted from the richness of fruits methanolic extract in different phenolic compounds including rutin, verbascoside, oleuropein, and oleoside [39]. Ghazghazi et al. [41] also proved the antioxidant capacity of O. oleaster fruits methanolic extract which was rich in polyphenols and exerted a DPPH radicals scavenging activity at a low median inhibitory concentration (IC50 = 28 ± 0.01 µg/ml). Bouarroudj et al. [87] studied the antioxidant ability of methanolic extract obtained from the oil of four populations of O. oleaster originating from Algeria. The methods used in their investigation were DPPH, ABTS, and B-carotene bleaching assays. The results obtained showed that methanolic extracts of the four studied populations exhibited an important antioxidant activity in the different used tests. Furthermore, this activity was correlated with total phenolic and ortho-diphenol content of the extracts of the different O. oleaster populations. Moreover, a variation in the antioxidant activity of methanolic, aqueous and oil extracts of wild and cultivated Olea trees was oserved by Al-Owamri et al. [96], with a correlation between the antioxidant activity of these extracts revealed by DPPH assay and their content in phenols and flavonoids. Besides, Hannachi et al. [94] investigated the antioxidant activity of different O. oleaster and Olea cultivars stones methanolic extracts using DPPH and ABTS assays. In their study, all extracts exerted an important antioxidant effect, with mean values of 6.88 ± 0.88 and 0.48 ± 0.15 mM TEAC; 12.14 ± 1.72 and 0.61 ± 0.001 mM TEAC for O. oleaster and olea cultivars extracts, respectively. These results demonstrate that extracts obtained from Olea cultivars have a higher antioxidant activity in comparison to those obtained from wild Olive trees. This activity could be related to the richness of Olea cultivars extracts in polyphenols and flavonoids [94, 96]. Similarly, Hannachi et al. [95] showed the antioxidant activity of leaf, pulp, and stone of two Olea cultivars and two O. oleaster trees methanolic extracts using DPPH and ABTS tests. In this study, significant differences in the antioxidant activity and in total polyphenols and flavonoids contents were observed, not only between Olea wild and cultivars trees extracts, but also between extracts of the same variety. These differences could be attributed to the intra-varietal variations. Recently, Martínez-Navarro et al. [86] revealed that leaves aqueous extracts of cultivars and Olea wild trees originating from Spain and Greece, exhibited a different antioxidant capacity in DPPH and ABTS tests. These findings allow to suggest that in addition to the intra-varietal variations, the geographical distribution of species also has an influence on their antioxidant activity [5]. More interestingly, Ghorbel et al. [85] assessed the antioxidant activity of two phenolic fractions of O. oleaster fruits ethyl acetate extract. They found that the richer fraction in polyphenols gived the best antioxidant activity in DPPH and ABTS assays. Moreover, they have attributed the antioxidant activity of this fraction to the presence of the phenolic compound ‘’oleuropein aglycon ‘’ which was absent in the other fraction.
As regards A. albus, Hamdi et al. [51] assessed the antioxidant activity of A. albus leaves, pericarp, and rhizome ethanolic extracts using DPPH and FRAP assays. The results revealed that pericarp extract has a stronger antioxidant activity than the other extracts (26.9 mmol Trolox/kg dw and 66.7 mmol Trolox/kg dw in DPPH and FRAP assays, respectively). Moreover, Serairi-Beji et al. [52] assessed the antioxidant activity of A. albus leaves aqueous extract using three assays : total antioxidant capacity (TAC), DPPH, and FRAP tests. The results showed that this extract exhibited a good antioxidant effect in all tests performed (TAC = 34 mg GAE/g DW), IC5O of 47 µg/ml and median effective (EC50) of 97 µg/ml in DPPH and FRAP assays, respectively. This activity is probably related to the high content of the tested extract in phenolic compounds including gallic acid, catechin and rutin [52]. In another study, Alami et al. [10] investigated the antioxidant activity of alkaloids and flavonoids extracts of A. albus using DPPH assay. They found that alkaloids extract exerted a higher antioxidant activity (IC50 = 1.8 mg/ml) than flavonoids extract (IC50 = 2.5 mg/ml). More recently, Chileh Chelh et al. [50] investigated the antioxidant activity of A. albus shoots ethanolic extract using DPPH and ABTS assays. Indeed, the tested extract exhibited an important antioxidant activity of 2.7 ± 1.7 and 2.8 ± 0.7 mmol TE/100 g DW in DPPH and ABTS tests, respectively.
Antimicrobial activity
Different studies have demonstrated the antimicrobial potential of these three Mediterranean medicinal plant species.
Concerning C. humilis, Lachkar et al. [26] investigated the activity of C. humilis leaves ethanolic, chloroformic and hexanic extracts obtained by two techniques (maceration and soxhlet extraction) against several bacteria: Staphylococcus aureus (S. aureus) (CECT976), Bacillus subtilis (B. subtilis) (DSM6633), Listeria innocua (CECT4030), Escherichia coli (E. coli) K12, Proteus mirabilis and Pseudomonas aeruginosa (P. aeruginosa) (CECT118). The results of this study showed that soxhlet extracts induced a better antibacterial activity, with the highest effect obtained against L. innocua using chloroformic extract with a minimal inhibitory concentration “MIC” = 1.25 mg/ml. Furthermore, Belhaoues et al. [24] demonstrated the susceptibility of various bacteria namely S. aureus, E. coli, P. aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis (E. faecalis), and Salmonella typhimurium to different solvents fractions obtained from leaves and fruits of C. humilis: DCMF, EAF, BF, and WF. The lowest MIC was obtained with leaves EAF against E. faecalis (MIC = 0.25 mg/ml).
Hasnaoui et al. [25] studied the antibacterial effect of C. humilis essential oil against S. aureus (SP2VSS2LG), P. aeruginosa (7853), E. coli (27922), Listeria monocytogenes (L. monocytogenes) (15313), and B. subtilis (6633). The most sensitive bacteria were P. aeruginosa, S. aureus, and E. coli (MIC = 250 mg/ml). Besides, C. humilis was shown to exhibit also an antifungal activity. Thus, in the study conducted by Okkacha et al. [27], C. humilis essential oil, and hexanic, chloroformic and aqueous extracts were tested against a panel of mold strains: Aspergillus ?avus (A. flavus), Aspergillus niger (A. niger), Aspergillus ochraceus, Alternaria. Spp, Rhizopus stolonifera, and Penicilium viridicatum and yeasts: Candida albicans (C. albicans) (ATCC 10231), C. albicans (2679), and C. albicans (IPP444). A total inhibition of mycelial growth was obtained at the concentration of 2.5 μl for A. niger, and at the concentration of 12.5 μl for A. ochraceus and Rhizopus stolonifer. As for yeasts, the obtained MIC were: MIC = 250 mg/ml for C. albicans (ATCC 10231) and C. albicans (2679) and MIC = 500 mg/ml for C. albicans (IPP444).
As regards O. oleaster, Bouchoucha et al. [97], tested for the first time the antimicrobial activity of O. oleaster aerial parts essential oil. The bacteria tested were S. aureus ATCC25923 and ATCC 43300, E. coli ATCC 25922, Klebsiella pneumonia ATCC700603, E. faecalis ATCC 51299, P. aeruginosa ATCC27853, B. subtilis ATCC6633. All these bacteria presented almost the same susceptibility to the essential oil, with an average inhibition zone of 11 mm. The chemical analysis of the essential oil revealed the presence of several compounds including Nonanal, theaspiranea A and 3-hexen-1-ol, benzoate. These compounds could be responsible for the antimicrobial activity. In another study, Ben-Amor et al. [41] assessed the antibacterial and antifungal activity of O. oleaster leaves hydro-ethanolic extract using micro-dilution method. The results showed that the tested extract exhibited an important antibacterial activity against S. aureus strains (ATCC 6538 and ATCC 43300), whereas it did not exert any effect against gram-negative bacteria as well as against the yeast C. albicans ATCC 1023. The resistance of Gram-negative bacteria to this extract could be explained by their cell wall structure (rich in Lipopolysaccharides) preventing them from antimicrobial agents.
Djenane et al. [98] studied the antibacterial effect of O. oleaster leaves hydromethanolic extract on minced beef contaminated by food-borne bacteria: Salmonella enterica ser. Enteritidis and Shiga toxin-producing E. coli O157:H7. They obtained a significant reduction of pathogenes level after treatment with this extract which was revealed rich in oleuropein. This compound was demonstrated to exert an important antimicrobial activity [99]. Furthermore, Ghazghazi et al. [42] studied the antimicrobial activity of fruit pulp methanolic extract of O. oleaster against a panel of microorganisms, namely the bacteria: E. coli ATCC 8739, Salmonella typhimurium NCTC 6017, S. aureus ATCC 29213, P. aeruginosa ATCC 27853, Aeromonas hydrophila EI, L. monocytogenes ATCC 7644, and Bacillus cereus ATCC 1247, the fungi (A. niger and A. flavus) and the yeast C. albicans ATCC 2091. The results showed that this extract was efficient against all tested microorganisms. Moreover, the minimal MIC value (3.125 mg/ml) was obtained against the two fungi: A. flavus and A. niger.
Concerning A. albus, to the best of our knowledge, up-to date, only two studies have been performed regarding its antimicrobial activity. Thus, Hamdi et al. [51] studied the antimicrobial activity of A. albus leaves, pericarp, and rhizome antimicrobial activity against several human bacteria, fungi, and resistant bacteria. The results showed that leaves extract was powerful against several bacteria (L. monocytogenes, Enterococcus faecium, E. faecalis, Streptococcus agalactiae, and S. aureus) and against all fungi: C. albicans, Candida glabrata, Candida parapsilosis, and Candida tropicalis (MIC< 1 mg/ml). This antimicrobial activity could be related to the richness of this extract in rutin [51]. More recently, Alami et al. [10] tested the antifungal activity of flavonoids and alkaloids of A. albus whole plant extracts against fusarium oxysporum using poisoned food techniques. The results showed that both flavonoids and alkaloids extracts induced the highest mycellium growth inhibition of 85% which reveals the strong antifungal activity of these extracts.
Antilithiasic activity
Beghalia et al. [100] investigated in vitro the inhibitory effect of C. humilis aqueous extract obtained by infusion against calcium oxalate crystallisation. Chamaerops humilis extract used at concentrations of 25% and 50% induced the best inhibition of calcium oxalate crystallization with respective concentrations of 94.86% and 93.07%. The identification of the inhibitory effect was achieved using polarised light photography, in fact, C. humilis has affected the aggregation phase and crystals growth. It was suggested that calcium oxalate cristallisation inhibition by plants extracts may be due to their active compounds [101] that affect the potential energy of atoms and prevent them from bonding to each other, inhibiting, therefore, crystals formation [100]. Similarly, Beghalia et al. [102] found that C. humilis aqueous extract obtained by decoction, exhibited in vitro the same inhibition effects of calcium oxalate crystallization: 94.86% and 93.07% at the concentration of 25% and 50%, respectively. These findings suggest that aqueous extracts of C. humilis (obtained by infusion and decoction) could be exploited in the development of natural drugs effective against urinary stones problems.
Antidiabetic activity
The antidiabetic activity of C. humilis and O. oleaster extracts has been proven through different in vitro and in vivo studies, whereas no published study has been dedicated to demonstrating the antidiabetic activity of A. albus although its ethnomedicinal use in the treatment of diabetes has been documented.
Thus, concerning C. humilis, Attaallah et al. [29] tested the antidiabetic effect of C. humilis extract obtained by infusion using a new electrochemical sensing system (glucometer). This system, a blood glucose reading device, allows the measurement of the enzyme alpha glucosidase (AG) inhibition by MAPs. The results of this study showed that the aqueous extract of C. humilis induced a significant inhibition of AG with a median inhibitory concentration IC50 = 5.90 mg/ml. In another study [30], the hypoglycemic and the hypolipidemic effect of C. humilis aqueous extract was tested on Meriones shawi rats. In the acute study, the oral administration of a single dose of this extract by experimentally induced hyperglycemia, hyperlipidemia, obese (HHO) rats has led to a significant decrease of their plasma glucose levels (From 12.04 ± 0.94 mmol/l to 6.88 ± 1.38 mmol/l), whereas in the sub-chronic study, the oral administration of C. humilis aqueous extract during 30 days by HHO rats resulted in a significant decrease of their weight (from 241 ± 8 to 165 ± 11 g) and plasma glucose levels (from 12.04 ± 0.94 to 4.84 ± 0.22 mmol/l), as well as in total cholesterol (from 3.46 ± 0.21 to 0.62 ± 0.02 mmol/l) and triglycerides (from 1.15 ± 0.17 to 0.37 ± 0.03 mmol/l). These findings support the traditional use of C. humilis in the treatment of diabetes and its complications [17,26,63].
As regards O. oleaster, Bechiri et al. [103] found that aqueous extract of O. oleaster leaves exhibited an inhibitory effect on α-amylase with an IC50 = 1.23 ± 0.08 mg/ml. Moreover, this extract showed also an in vivo antidiabetic effect in starch loading induced diabetic rats, by reducing their postprandial blood glucose level after 180 minutes of starch administration. Similarly, Mezouar et al. [93] examined the in vitro antidiabetic effect of O. oleaster hydromethanolic and hydroacetonic extracts on the inhibition of α-amylase enzyme, and obtained an important antidiabetic effect of the hydromethanolic extract (0.91 ± 0.02 mg/ml) which was higher than that obtained with the hydroacetonic extract (0.54 ± 0.02 mg/ml). In another study, Mechchate et al. [44], demonstrated the antidiabetic effect of flavonoids extract of O. oleaster leaves. In fact, the studied extract at concentrations of 25 and 50 mg/ml induced an important antidiabetic effect in mice with severe diabetes (<450 mg/dl) resulting from alloxan injection. The investigation of flavonoids chemical profile of the tested extract revealed the presence of different flavonoids responsible for the antidiabetic effect namely amentoflavone, quercetin-3-O-glucoside, quercetin-3-O-hexose-deoxyhexose, luteolin-7-O-glucoside, oleuropein, and rutin. More recently, Kabach et al. [39] revealed the antidiabetic effect of O. oleaster fruits methanolic extract using in vitro assays. Indeed, this extract induced the inhibition of α-amylase and AG enzymes at low concentrations (IC50 = 2.367 and 1.272 mg/ml, respectively). This activity could be attributed to the richness of the tested extract in phenolic compounds such as oleoside, ligstroside, and oleuropein glucoside [39].
Neuroprotective activity
Bouhafsoun et al. [28] tested using the Ellman method, the effect of methanolic and aqueous extract of C. humilis leaves and fruits against two enzymes linked with neurodegenerative diseases, namely acetylcholinesterase (AChe) and butyrylcholinesterase (BChe). The obtained results revealed that all fruits extracts were moderately active against BChe at 200 μg/ml, whereas, they were not active against AChe. Also, no leaves extract was active against the two targeted enzymes. These findings could be explained by the presence of certain active compounds in C. humilis fruits extracts that are able to exert an inhibitory effect on BChe enzyme. Thus, further studies are required in order to investigate the different chemical compounds of C. humilis methanolic extract and their possible mechanism on BChe enzyme. Similarly, Gonçalves et al. [22] studied the inhibitory activity of C. humilis methanolic extract obtained from different parts (leaves, seeds, fruit peel, and pulp) against three enzymes: AChe, BChe, and tyrosinase (Tyr) associated with neurodegenerative diseases. The strong inhibitory activity was obtained with seeds and pulp extracts against Tyr (IC50 = 268.97 and 279.99 µg ml−1, respectively), as well as with seeds and peel extracts against AChe (IC50 = 660.16 and 653.68 µg ml−1, respectively) and against BChe (IC50 = 304.86 and 701.54 µg ml−1, respectively). In this study, the enzymes inhibition activity was highly correlated with the total condensed tannins content of the studied extracts. These results suggest that these compounds may be responsible for: Tyr; AChe and BChe inhibitory activity of the extracts which can be used as natural alternative sources of neurodegenerative diseases medical agents.
Antiviral activity
The viral replication and transcription of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (the new strain of Coronavirus) is mediated by a key enzyme: the main protease (MPRO) of SARS-CoV-2. Thus, this enzyme constitutes the main target of SARS-CoV-2 drugs. The inhibitory potential of natural molecules against SARS-CoV-2 MPRO was investigated by Ouassaf et al. [31] by molecular and dynamics simulations studies using different softwares (Autodock Vina software and Web server SwissADME). Among 50 molecules of several medicinal plants; kaempferol and Tricin-7-O-neohesperidoside which are secondary metabolites of C. humilis [21,76] displayed suitable molecular properties for SARS-CoV-2 MPRO inhibition. These results are promising for the development of natural drugs against SARS-CoV-2 from C. humilis chemical compounds.
In another study, Ben-Amor et al. [41] demonstrated the antiviral activity of O. oleaster leaves hydroethanolic extract against herpes simplex virus type 1). In fact, this extract induced an important selective effect against pre-infection and post-infection of Vero cells without inducing a cytotoxic impact on these cells. This effect could be related to the presence of several phenolic compounds such as Oleuropein hexoside, Oleanolic acid, and Epicatechin [41].
Hepatoprotective
The hepatoprotective activity of some extracts of O. oleaster and A. albus has been demonstrated. Thus, Al-Attar et al. [43] assessed the hepatoprotective effect of O. oleaster leaves aqueous extract in albino mice. They found that this extract reduced significantly hepatocirrosis resulting from thioacetamide administration by the tested animals. Indeed, histopathological observations revealed the attenuation of structural damage in mice liver after their treatment with the extract. Serairi-Beji et al. [52] studied the hepatoprotective activity of A. albus leaves aqueous extract in male waster rats intoxicated with Tetrachloromethane. The results showed that this extract exerted a high protective activity by reducing levels of enzymes hepatic markers (lactate and aspartate transaminases), and by preventing the histological structure of rats liver from damage. The hepatoprotective effect of this extract could be related to its richness in phenolic compounds such as gallic acid, catechin, rutin, and quercetin [52].
Antihyperlipidemic
Olea oleaster oil has been revealed to play an important role in the modulation of plasma lipids. Thus, Belarbi et al. [11] demonstrated the effect of O. oleaster oil on the modulation of plasma lipîds in human. Indeed, after 1 month of O. oleaster oil administration by 40 healthy Algerian human subjects, a significant attenuation of their total cholesterol, low-density lipoprotein cholesterol, and plasma triglyceride concentration was recorded, in comparison with non-consuming people originating from the same area.
Cytotoxic activity
The cytotoxic activity of different O. oleaster extracts has been proven. Thus, Makowska-W?s et al. [40] studied the cytotoxic activity of O. oleaster leaves methanolic extract against human normal (prostate epithelial (PNT2), skin fibroblasts (BJ)) and cancer cells (hepatocellular carcinoma (Hep G2)), prostate cancer (DU-145 and PC-3), and melanoma (HTB-140 and WM793). For this study, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed. The results showed that the tested extract exhibited an important cytotoxic activity against prostate cancer cells and had less effect on the viability of liver cancer cells, whereas it did not impact the viability of melanoma and normal cells. These results may be exploited in the development of new drugs from O. oleaster leaves extracts against prostate cancer. However, further in vivo studies are definitely needed. Furthermore, it is important to mention that the cytotoxic activity of O. oleaster leaves methanolic extract is probably related to its richness in bioactive compounds, including phenolic acids and the major polyphenol compound (Oleuropein) [40]. In another study, Zeriouh et al. [104] demonstrated that O. oleaster aqueous extract limited human colon cancer (HCT116) xenograft growth in mice, and induced cellular apoptosis of two colorectal cell lines (HCT116 and HCT8) through the activation of caspase enzymes. Furthermore, Ghorbel et al. [85] revealed the autophagic activity of O. oleaster fruits EAFs in human foreskin fibroblasts. This activity of the tested extract could be exploited in cancer treatment, since autophagy is becoming nowadays an essential approach in tumors suppression and in cancer management. Recently, Kabach et al. [39] investigated the cytotoxic effect of O. oleaster fruits methanolic extract against two cell lines of human breast cancer (MCF-7 and MDA-MB-468) using the same assay. They obtained an important cytotoxic activity for the studied extract with a median anti-proliferative inhibitory concentration of 46.75 and 35.16 µg/ml against MCF-7 and MDA-MB-468, respectively. The demonstrated cytotoxic activity could be attributed to the presence of several bioactive compounds in the extract such as rutin, oleuropein, and verbascoside [39], which were demonstrated to possess a high cytotoxic effect [103,105-107].
CONCLUSION
This review shows that C. humilis, O. oleaster, and A. albus are interesting medicinal plant species that can be used not only in the medical field for human therapy, but also in the food industry and agricultural sectors. Indeed, different ethnobotanical studies have revealed the traditional use of these Mediterranean plants for the treatment of several ailments. Moreover, numerous scientific works conducted either in vitro or in vivo on animal models have shown the multiple biological activities of C. humilis and O. oleaster, including their antioxidant, antimicrobial, antidiabetic, and antiviral properties. However, in the case of A. albus, there is a lack of experimental studies on its biological activities. Thus, further studies are required in this context. Furthermore, the bioactive potential of these three Mediterranean plant species was attributed in several studies to their richness in bioactive compounds belonging to different chemical classes, such as flavonoids and phenolic acids which are abundant in the three plant species, and other compounds like oleuropein; a secoiridoid present only in O. oleaster. In addition, it is important to mention that it is extremely interesting to exploit the therapeutical potential of the studied Mediterranean plants in the development of natural drugs. Moreover, the antimicrobial and antioxidant properties of these plant species are encouraging their use in the food industry and agricultural sector as green additives and bio-pesticides replacing the chemical products used in these fields.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by CNRST (National center for scientific and technical research, Morocco) by a fellowship grant (5 USMBA2022).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Benkhaira N, El Hachlafi N, Jeddi M, Abdnim R, Bnouham M, Koraichi SI, et al. Unveiling the phytochemical profile, in vitro bioactivities evaluation, in silico molecular docking and ADMET study of essential oil from Clinopodium nepeta grown in Middle Atlas of Morocco. Biocatal Agric Biotechnol. 2023;54 (2023):102923–38. CrossRef
2. Guía-García JL, Charles-Rodríguez AV, Reyes-Valdés MH, Ramírez-Godina F, Robledo-Olivo A, García-Osuna HT, et al. Micro and nanoencapsulation of bioactive compounds for agri-food applications: a review. Ind Crop Prod. 2022;186(2022):115198–211. CrossRef
3. Benamar K, Koraichi SI, Benamar S, Fikri-Benbrahim K. Manuscript ethnomedicinal use of plants by Ain Chkef (North Central Morocco) community to boost immunity and overcome SARS COV-2 infection. Ethnobot Res Appl. 2024;29(2024):1–17. CrossRef
4. Balkrishna A, Sharma H, Kukreti A, Kumari A, Saini P, Arya V, et al. Traditional uses and phytopharmacology of Cirsium arvense: Bioprospecting potential of a weed from temperate biome. J Appl Pharm Sci. 2024;14(5):030–7.
5. Benamar K, Koraichi SI, Fikri-Benbrahim K. Ethnobotany, phytochemistry and pharmacological activities of Celtis australis: a review. J Herbmed Pharmacol. 2023;12(1):54–72. CrossRef
6. Chraibi M, Farah A, Balouiri M, Barkai H, Sadiki M, Benbrahim KF. Chemical characterization and antimicrobial activity of Moroccan Pelargonium asperum essential oil. J Appl Pharm Sci. 2016;6(12):042–6. CrossRef
7. Rastogi V, Porwal M, Sikarwar MS, Singh B, Choudhary P, Mohanta BC. A review on phytochemical and pharmacological potential of Bhut Jolokia (a cultivar of Capsicum chinense Jacq.). J Appl Pharm Sci. 2024;14(5):079–90. CrossRef
8. Fennane M, Tattou MI, Mathez J, Ouyahya A, El Oualidi J. Flore pratique du Maroc: manuel de détermination des plantes vasculaires. Angiospermae (Leguminosae-Lentibulariceae). 1st ed. Rabat, Morocco: Institut scientifique Ed; 2007.
9. Fennane M, IBN Tattou M, Mathez J, Ouyahya A, El Oualidi J. Flore pratique du Maroc: manuel de détermination des plantes vasculaires, Dicotylédones, Monocotylédones. 1st ed. Rabat, Morocco: Institut Scientifique Ed; 2014.
10. Alami O, Dif MM, Berrichi A. Antifungal studies of alkaloids and flavonoids of Asparagus albus from the Arid Region of Algeria. J Biol Sci C Physiol Mol Biol. 2022;14(1):13–20. CrossRef
11. Belarbi M, Bendimerad S, Sour S, Soualem Z, Baghdad C, Hmimed S, et al. Oleaster oil positively modulates plasma lipids in humans. J Agric Food Chem. 2011;59(16):8667–9. CrossRef
12. Garcia-Castano JL, Terrab A, Ortiz MA, Stuessy TF, Talavera S. Patterns of phylogeography and vicariance of Chamaerops humilis L.(Palmae). Turk J Bot. 2014;38(6):1132–46. CrossRef
13. Challi D, Dahmani J, Belahbib N. Phytosociological analysis of the Sidi Boughaba Biological Reserve, Kénitra, Morocco. Hacquetia. 2023;23(1):35–68. CrossRef
14. Gianguzzi L, Bazan G. The Olea europaea L. var. sylvestris (Mill.) Lehr. forests in the Mediterranean area. Plant Sociol. 2019;56(2):3–34. CrossRef
15. Gianguzzi L, Bazan G. A phytosociological analysis of the Olea europaea L. var. sylvestris (Mill.) Lehr. forests in Sicily. Plant Biosyst. 2020;154(5):705–25. CrossRef
16. Fennane M. Eléments pour un Livre rouge de la flore vasculaire du Maroc. 10 ed. Montpellier, France: Tela-Botanica; 2018.
17. Medjati N, Hasnaoui O, Babali B, Hachemi N. Ethnobotanical investigation of Chamaerops humilis in the area of Beni Snous (Western of Algeria). Mediterr Bot. 2019;40(2):177–84. CrossRef
18. Carrió E, Vallès J. Ethnobotany of medicinal plants used in eastern Mallorca (Balearic Islands, Mediterranean Sea). J Ethnopharmacol. 2012;141(3):1021–40. CrossRef
19. El-Hilaly J, Hmammouchi M, Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J Ethnopharmacol. 2003;86(2-3):149–58. CrossRef
20. Benahmed-Bouhafsoun A, Djied S, Mouzaz F, Kaid-Harche M. Phytochemical composition and in vitro antioxidant activity of Chamaerops humilis L. extracts. Int J Pharm Pharm Sci. 2013;5(3):741–4.
21. Coelho JP, Veiga JG, Elvas-Leitão R, Brigas AF, Dias AM, Oliveira MC. Composition and in vitro antioxidants activity of Chamaerops humilis L. 2017 IEEE 5th Portuguese Meeting on Bioengineering (ENBENG); Coimbra, Portugal: IEEE. CrossRef
22. Gonçalves S, Medronho J, Moreira E, Grosso C, Andrade PB, Valentão P, et al. Bioactive properties of Chamaerops humilis L.: antioxidant and enzyme inhibiting activities of extracts from leaves, seeds, pulp and peel. 3 Biotech. 2018;8(2):1–8. CrossRef
23. Mokbli S, Sbihi HM, Nehdi IA, Romdhani-Younes M, Tan CP, Al-Resayes SI. Characteristics of Chamaerops humilis L. var. humilis seed oil and study of the oxidative stability by blending with soybean oil. J Food Sci Technol. 2018;55(6):2170–9. CrossRef
24. Belhaoues S, Amri S, Bensouilah M, Seridi R. Antioxidant, antibacterial activities and phenolic content of organic frac-tions obtained from Chamaerops humilis L. leaf and fruit. Int J Biosci. 2017;11(2017):284–97. CrossRef
25. Hasnaoui BO, Adli DE, Sennour R. Antibacterial activity of essential oils of Chamaerops humilis (Arecaceae) on some pathogenic bacteria. Res J Pharm Biol Chem Sci. 2013;4(4):626–33.
26. Lachkar N, Lamchouri F, Toufik H. Ethnopharmacological survey, mineral and chemical content, in vitro antioxidant, and antibacterial activities of aqueous and organic extracts of Chamaerops humilis L. var. argentea Andre leaves. BioMed Res Int. 2022;2022(1):1–17. CrossRef
27. Okkacha H, Houari ADE, Noureddine H. Effect of Chamaerops humilis L.(Arecaceae) essential oils on the inhibition of some fungal strains isolated from wheat silos. Indian J Appl Res. 2014;4(11):49–52.
28. Bouhafsoun A, Boga M, Boukeloua A, Temel H, Kaid-Harche M. Determination of anticholinesterase and antioxidant activities of methanol and water extracts of leaves and fruits of Chamaerops humilis L. J Appl Nat Sci. 2019;11(1):144–8. CrossRef
29. Attaallah R, Elfadil D, Amine A. Screening study of enzymatic inhibition of medicinal plants for the treatment of diabetes using a glucometer biosensor approach and optical method. J Herb Med. 2021;28(2021):100441–9. CrossRef
30. Gaamoussi F, Israili ZH, Lyoussi B. Hypoglycemic and hypolipidemic effects of an aqueous extract of Chamaerops humilis leaves in obese, hyperglycemic and hyperlipidemic Meriones shawi rats. Pak J Pharm Sci. 2010;23(2):212–21.
31. Ouassaf M, Belaidi S, Chtita S, Lanez T, Abul Qais F, Md Amiruddin H. Combined molecular docking and dynamics simulations studies of natural compounds as potent inhibitors against SARS-CoV-2 main protease. J Biomol Struct Dyn. 2022;40(21):11264–73. CrossRef
32. Benmehdi H, Hasnaoui O, Benali O, Salhi F. Phytochemical investigation of leaves and fruits extracts of Chamaerops humilis L. J Mater Environ Sci. 2012;3(2012):320–37.
33. Caissard JC, Meekijjironenroj A, Baudino S. Anstett MC. Localization of production and emission of pollinator attractant on whole leaves of Chamaerops humilis (Arecaceae). Am J Bot. 2004;91(8):1190–9. CrossRef
34. Marouf A, Bouguedoura N, Bennaceur M, Bengag A. Phytochemical profile and antioxidant activity of Phoenix dactylifera L., Phoenix canariensis L. and Chamaerops humilis L. IV Int Date Palm Conf. 2010;882(2010):1099–108. CrossRef
35. Benítez G, González-Tejero MR, Molero-Mesa J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J. Ethnopharmacol. 2010;129(1):87–105. CrossRef
36. Hachi M, Ouafae B, Hachi T, Mohamed EB, Imane B, Atmane R, et al. Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco). Lazaroa. 2016;37(2016):1–11. CrossRef
37. Hassaïne S, Saïdi A, Belhadj OA. Ethnobotanical study of medicinal plants used in the treatment of high blood pressure in the region of Tlemcen (Northwestern Algeria). J Pharm Pharmacogn Res. 2019;7(1):1–11.
38. Meddour R, Sahar O, Ouyessad M. Ethnobotanical survey on medicinal plants in the Djurdjura National Park and its influence area, Algeria. Ethnobot Res Appl. 2020;20(2020):1–25. CrossRef
39. Kabach I, Bouchmaa N, Ben Mrid R, Zouaoui Z, Maadoudi ME, Kounnoun A, et al. Olea europaea var. Oleaster a promising nutritional food with in vitro antioxidant, antiglycation, antidiabetic and antiproliferative effects. J Food Meas Charact. 2023;17(1):882–94. CrossRef
40. Makowska-W?s J, Galanty A, Gdula-Argasi?ska J, Tyszka-Czochara M, Szewczyk A, Nunes R, et al. Identification of predominant phytochemical compounds and cytotoxic activity of wild olive leaves (Olea europaea L. ssp. sylvestris) harvested in south Portugal. Chem Biodivers. 2017;14(3):1–10. CrossRef
41. Ben-Amor I, Musarra-Pizzo M, Smeriglio A, D’Arrigo M, Pennisi R, Attia H, et al. Phytochemical characterization of Olea europea leaf extracts and assessment of their anti-microbial and anti-HSV-1 activity. Viruses. 2021;13(6):1085–100. CrossRef
42. Ghazghazi H, Aouadhi C, Hamrouni S, Mnif W. Antibacterial, antifungal and antioxidant activities of Tunisian Olea europea ssp. Oleaster fruit pulp and its essential fatty acids. Int J Pharm Pharm Sci. 2015;7(1):52–5.
43. Al-Attar AM, Alrobai AA, Almalki DA. Effect of Olea oleaster and Juniperus procera leaves extracts on thioacetamide induced hepatic cirrhosis in male albino mice. Saudi J Biol Sci. 2016;23(3):363–71. CrossRef
44. Mechchate H, Es-Safi I, Bourhia M, Kyrylchuk A, El Moussaoui A, Conte R, et al. In-vivo antidiabetic activity and in-silico mode of action of LC/MS-MS identified flavonoids in oleaster leaves. Mol. 2020;25(21):5073–86. CrossRef
45. Rodrigues N, Pinho T, Casal S, Peres AM, Baptista P, Pereira JA. Chemical characterization of Oleaster, Olea europaea var. sylvestris (Mill.) Lehr., oils from different locations of Northeast Portugal. Appl Sci. 2020;10(18):6414–28. CrossRef
46. Naciri AA, Adil-Kalili SM, Saloua-Essaih MT, Abdelmounaim-Belahyane RB. Ethnobotanical knowledge of wild food plants in Khenifra, a province in the Middle Atlas region of Morocco. GSC Adv Res Rev. 2022;13(2):180–200. CrossRef
47. Benamar K, Koraichi SI, Benamar S, Fikri-Benbrahim K. Ethnobotanical study of medicinal plants used by the population of Ain Chkef (North central Morocco). Ethnobot Res Appl. 2023;26:1–23. CrossRef
48. Guarrera PM, SavoV. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: a review. J. Ethnopharmacol. 2013;146(3):659–80. CrossRef
49. Tuttolomondo T, Licata M, Leto C, Savo V, Bonsangue G, Gargano ML. Ethnobotanical investigation on wild medicinal plants in the Monti Sicani Regional Park (Sicily, Italy). J Ethnopharmacol. 2014;153(3):568–86. CrossRef
50. Chileh Chelh T, Rincon-Cervera MA, Gomez-Mercado F, Lopez-Ruiz R, Gallon-Bedoya M, Ezzaitouni M, et al. Wild Asparagus shoots constitute a healthy source of bioactive compounds. Molecules. 2023;28(15):5786–803. CrossRef
51. Hamdi A, Jaramillo-Carmona S, Beji R, Tej R, Zaoui S, Rodríguez-Arcos R, et al. The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res Int. 2017;99(2017):720–9. CrossRef
52. Serairi-Beji R, Aidi Wannes W, Hamdi A, Tej R, Ksouri R, Saidani-Tounsi M, et al. Antioxidant and hepatoprotective effects of Asparagus albus leaves in carbon tetrachloride-induced liver injury rats. J Food Biochem. 2018;42(1):12433–44. CrossRef
53. Guzmán B, Fedriani JM, Delibes M, Vargas P. The colonization history of the Mediterranean dwarf palm (Chamaerops humilis L., Palmae). Tree. Genet Gen. 2017;13(1):1–10. CrossRef
54. Fanelli V, Mascio I, Falek W, Miazzi MM, Montemurro C. Current status of biodiversity assessment and conservation of wild olive (Olea europaea L. subsp. europaea var. sylvestris). Plants. 2022;11(4):1–15. CrossRef
55. Llorens L, Tomàs J, Ferriol P, García MT, Gil L. Floral aroma and pollinator relationships in two sympatric late-summer-flowering Mediterranean Asparagus species. Plants. 2023;12(18):3219–35. CrossRef
56. Hasnaoui O, Bouazza M, Benali O, Thinon M. Ethno botanic study of Chamaerops humilis L. var. argentea Andre (Arecaceae) in Western Algeria. Agric J. 2011;6(1):1–6. CrossRef
57. Hasnaoui O, Benali O, Bouazza M, Benmehdi H. Ethnobotanical approaches and phytochemical analysis of Chamaerops humilis L.(Arecaceae) in the area of Tlemcen (western Algeria). Res J Pharm Biol Chem Sci. 2013;4(2):910–8.
58. Allali H, Benmehdi H, Dib MA, Tabti B, Ghalem S, Benabadji N. Phytotherapy of diabetes in west Algeria. Asian J Chem. 2008;20(4):2701–11.
59. Taibi K, Abderrahim LA, Boussaid M, Taibi F, Achir M, Souana K, et al. Unraveling the ethnopharmacological potential of medicinal plants used in Algerian traditional medicine for urinary diseases. Eur J Integr Med. 2021;44:101339–57. CrossRef
60. Nekhla H, El Ghadraoui L, Ousaaid D, Harrach A, Tarmoun K, Squalli W, et al. Ethnobotanical survey of Chamaerops humilis L. in the rural Commune of Sidi Youssef Ben Ahmed, Sefrou Province, Morocco. Trop J Nat Prod Res. 2021;5(9):1586–90. CrossRef
61. Bellakhdar J. Pharmacopée marocaine traditionnelle. Paris, France: Ibis Press; 1997.
62. Chetoui A, Kaoutar K, Boutahar K, El Kardoudi A, BenChaoucha-Chekir R, Chigr F, et al. Herbal medicine use among Moroccan type 2 diabetes patients in the Beni Mellal-Khenifra region. J Herb Med. 2021;29(2021):100480–9. doi: htpps://doi.org/10.1016/j.hermed.2021.100480
63. Belhaj S, Chaachouay N, Zidane L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J Pharm Pharmacogn Res. 2021;9(5):619–62. CrossRef
64. Bouyahya A, Abrini J, Et-Touys A, Bakri Y, Dakka N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur J Integr Med. 2017;13(2017):9–25. doi: htpps://doi.org/10.1016/j.eujim.2017.06.004
65. El Abbouyi PA, Ansari NF, Khyari PSE, Loukili H. Inventory of medicinal plants prescribed by traditional healers in El Jadida city and suburbs (Morocco). Int J Green Pharm. 2014;8(4):242–51. CrossRef
66. Mrabti HN, Doudach L, Mekkaoui M, Khalil Z, Harraqui K, Fozia F. Profile of medicinal plants traditionally used for the treatment of skin burns. Evid-Based Complement Altern Med. 2022;2022(1):1–10. CrossRef
67. El Brahimi R, El Barnossi A, Amrani M, Bari A. Ethnobotanical study and biodiversity of medicinal plants used in the Province of Taza North-Eastern Morocco. Trop J Nat Prod Res. 2022;6(11):1814–31. CrossRef
68. El-Mokasabi FM, Al-Sanousi MF, El-Mabrouk RM. Taxonomy and ethnobotany of medicinal plants in eastern region of Libya. J Environ Sci Toxicol Food Technol. 2018;12(8):14–23. CrossRef
69. Gaspar N, Godinho J, Vasconcelos T, Caldas D, Mendes P, Barros O. Ethnobotany in the Center of Portugal (Santarém), natural products in the new Millennium: prospects and industrial application. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. pp 271–84. CrossRef
70. Akbulut SEFA, Karakose M, Özkan ZC.Traditional uses of some wild plants in Kale and Ac?payam Provinces in Denizli. Kastamonu Univ J For Fac. 2019;19(1):72–81. CrossRef
71. Gürdal B, Kültür ?. An ethnobotanical study of medicinal plants in Marmaris (Mu?la, Turkey). J. Ethnopharmacol. 2013;146(1):113–26. CrossRef
72. Belgacem N, Okkacha H, Benchohra M, Elhadj T. Inventory, diversity and therapeutic uses of medicinal plants in the tiaret mountains (western algeria). Biodivers J. 2021;12(1):129–38.
73. Belhaj S, Dahmani J, Belahbib N, Zidane L. Ethnopharmacological and ethnobotanical study of medicinal plants in the Central High Atlas, Morocco. Ethnobot Res Appl. 2020;20(2020):1–40. CrossRef
74. Merrouni IA, Kharchoufa L, Bencheikh N, Elachouri M. Ethnobotanical profile of medicinal plants used by people of North-eastern Morocco: cross-cultural and historical approach (Part I). Ethnobot Res Appl. 2021;21(2021):1–45. CrossRef
75. Benítez G, Molero-Mesa J, González-Tejero MR. Wild edible plants of Andalusia: traditional uses and potential of eating wild in a highly diverse region. Plants. 2023;12(6):1218–48. CrossRef
76. Bouhafsoun A, Yilmaz MA, Boukeloua A, Temel H, Harche MK. Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci Technol. 2018;38(2018):242–7. CrossRef
77. Delle Monache F, Ferrari F, Poce-Tucci A, Marini-Bettolo GB. Catechins with (+)-epi-configuration in nature. Phytochemistry. 1972;11(7):2333–5. CrossRef
78. Williams CA, Harborne JB, Clifford HT. Flavonoid patterns in the monocotyledons. Flavonols and flavones in some families associated with the Poaceae. Phytochemistry. 1971;10(5):1059–63. CrossRef
79. Williams CA, Harborne JB, Clifford HT. Negatively charged flavones and tricin as chemosystematic markers in the Palmae. Phytochemistry. 1973;12(10):2417–30. CrossRef
80. Hirai Y, Sanada S, Ida Y, Shoji J. Studies on the constituents of Palmae plants. III.: the constituents of Chamaerops humilis L. and Trachycarpus wagnerianus BECC. Chem Pharm Bull. 1986;34(1):82–7. CrossRef
81. Dufaÿ M, Hossaert-McKey M, Anstett MC. Temporal and sexual variation of leaf-produced pollinator-attracting odours in the dwarf palm. Oecologia. 2004;139(2004):392–8.
82. Siles L, Cela J, Munné-Bosch S. Vitamin E analyses in seeds reveal a dominant presence of tocotrienols over tocopherols in the Arecaceae family. Phytochemistry. 2013;95(2013):207–14. CrossRef
83. Müller H. CLXV.—the occurrence of quercitol (quercite) in the leaves of Chamaerops humilis. J Chem Soc Transactions. 1907;91(1907):1766–67. CrossRef
84. Altinyay Ç, Levent A. HPLC analysis of oleuropein in olea europaea l. J Fac Pharm. 2006;35(1):1–11.
85. Ghorbel A, Wedel S, Kallel I, Cavinato M, Sakavitsi ME, Fakhfakh J. Extraction yield optimization of oleaster (Olea europaea var. sylvestris) fruits using response surface methodology, LC/MS profiling and evaluation of its effects on antioxidant activity and autophagy in HFF cells. J Food Meas Charact. 2021;15(2021):4946–59. CrossRef
86. Martínez-Navarro ME, Kaparakou EH, Kanakis CD, Cebrián-Tarancón C, Alonso GL, Salinas MR. Quantitative determination of the main phenolic compounds, antioxidant activity, and toxicity of aqueous extracts of olive leaves of Greek and Spanish genotypes. Horticulturae. 2023;9(1):55–64. CrossRef
87. Bouarroudj K, Tamendjari A, Larbat R. Quality, composition and antioxidant activity of Algerian wild olive (Olea europaea L. subsp. Oleaster) oil. Ind Crops Prod. 2016;83(2016):484–91. CrossRef
88. Zighed A, Derradji L, Hadef Y. Determination of polyphenolic components by high performance liquid chromatography (HPLC) and evaluation of the antioxidant activity of leaves of Olea europaea L. var. sylvestris (Miller) Lehr. GSC Biol Pharm Sci. 2022;20(1):95–9. CrossRef
89. Khoudali S, Essaqui A, Zertoubi M, Azzi M, Benaissa M. Study of antioxidant activity and anticorrosion action of the methanol extract of dwarf palm leaves (Chamaerops humilis l.) from morocco. J Mater Environ Sci. 2014;5(3):887–98.
90. Miguel M, Bouchmaaa N, Aazza S, Gaamoussi F, Lyoussi B. Antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of eleven extracts of Moroccan plants. Fresenius Environ Bull. 2014;23(6):1–14.
91. Eddahhaoui FZ, Boudalia M, Harhar H, Chahboun N, Tabyaoui M, Guenbour A. Effect of the extraction technique on the bioactive compounds and the antioxidant capacity of the Chamaerops humilis L. fruit (pulp and seeds). Chem Data Collect. 2022;40(2022):1–14. CrossRef
92. Theodora L, Andriana E, Lazou V, Sinanoglou J, Evangelos SL. Phenolic extracts from wild olive leaves and their potential as edible oils. Antioxid Foods. 2013;2(2013):18–31. CrossRef
93. Mezouar D, Azzi R, Abbou F, Mouderas F, Aissaoui M, Boucif LF. Alpha-amylase inhibitory activity and antioxidant effect of Olea var. europaea sylvestris leaves extracts. Genet Biodivers J. 2021;5(2):146–58. CrossRef
94. Hannachi H, Elfalleh W, Marzouk S. Oil, protein, antioxidants and free radical scavenging activity of stone from wild olive trees (Olea europaea L.). Pak J Pharm Sci. 2013;26(3):503–10.
95. Hannachi H, Elfalleh W, Laajel M, Ennajeh I, Mechlouch RF, Nagaz K. Chemical profiles and antioxidant activities of leaf, pulp, and stone of cultivated and wild olive trees (Olea europaea L.). Int J Fruit Sci. 2020;20(3):350370–92. CrossRef
96. Al-Owamri FSN, Al Sibay LSA, Reddy SH, Hussain SA, Gangireddygari VSR. Phytochemical, antioxidant, hair growth and wound healing property of Juniperus excelsa, Olea oleaster and Olea europaea. J King Saud Univ Sci. 2023;35(2):102446–52. CrossRef
97. Bouchoucha S, Boukhebti H, Oulmi A, Mouhamadi Y, Chaker AN. Chemical composition and antimicrobial activity of essential oils of two wild olive subspecies Oleaeuropaea L. var. sylvestris and the endemic olive Olea europaea subsp. lapperinie from Algeria. Nat Prod Res. 2023;1:1–13. CrossRef
98. Djenane D, Gómez D, Yangüela J, Roncalés P, Ariño A. Olive leaves extract from Algerian oleaster (Olea europaea var. Sylvestris) on microbiological safety and shelf-life stability of raw halal minced beef during display. Foods. 2018;8(1):1–17. CrossRef
99. Topuz S, Bayram M. Oleuropein extraction from leaves of three olive varieties (Olea europaea L.): antioxidant and antimicrobial properties of purified oleuropein and oleuropein extracts. J Food Process Preserv. 2022;46(6):15697–728. CrossRef
100. Beghalia M, Ghalem S, Allali H, Belouatek A. Marouf A. Effect of herbal extracts of Tetraclinis articulata and Chamaerops humilis on calcium oxalate crystals in vitro. Gomal J Med Sci. 2007;5(2):1–4.
101. Atmani F, Khan SR. Effect of an extract from Herniaria hirsute on calcium oxalate crystallisation in vitro. B J U Int. 2000;85(6):621–5. CrossRef
102. Beghalia M, Ghalem S, Allali H, Belouatek A, Marouf A. Inhibition of calcium oxalate monohydrate crystal growth using Algerian medicinal plants. J Med Plants Res. 2008;2(3):66–70.
103. Ruzzolini J, Peppicelli S, Andreucci E, Bianchini F, Scardigli A, Romani A, et al. Oleuropein, the main polyphenol of Olea europaea leaf extract, has an anti-cancer effect on human BRAF melanoma cells and potentiates the cytotoxicity of current chemotherapies. Nutrients. 2018;10(12):1950–67. CrossRef
104. Bechiri A, Chekroun E, Rachid A, Djaziri R. In vitro evaluation of α-amylase inhibitory activity of some medicinal plants used in treatment of diabetes mellitus in Algeria and their effect on postprandial hyperglycemia in normal rats. Int J Phytomed. 2015;7(2):171–5.
105. Zeriouh W, Nani A, Belarbi M, Dumont A, de Rosny C, Aboura I, et al . Phenolic extract from oleaster (Olea europaea var. Sylvestris) leaves reduces colon cancer growth and induces caspase-dependent apoptosis in colon cancer cells via the mitochondrial apoptotic pathway. PLoS One. 2017;12(2):170823–42. CrossRef
106. Caparica R, Júlio A, Araújo MEM, Baby AR, Fonte P, Costa JG, et al. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules. 2020;10(2):233–48. CrossRef
107. ?enol H, Tulay P, Ergören MÇ, Hano?lu A, Çali? ?, Mocan G. Cytotoxic effects of verbascoside on MCF-7 and MDA-MB-231. Turk J Pharm Sci. 2021;18(5):637–51. CrossRef