INTRODUCTION
In recent years, the prevalence of colorectal cancer (CRC) has increased significantly at a global rate. In 2020, CRC had been the third-most diagnosed cancer and the second-leading cause of cancer death. There are an estimated 1.93 million new instances of CRC diagnosed worldwide and 0.94 million deaths from the disease. These data points represent 10% of the total 19.29 million new cases of cancer globally and 9.4% of all cancer-related fatalities (total of 9.96 million deaths) [1,2]. Between 2020 and 2050, the estimated economic cost of various cancers totals $25.2 trillion when adjusted to constant 2017 prices, which translates to an annual levy amounting to 0.55% of the global gross domestic product. Among these cancers, CRC stands out as one of the top five contributors to the overall economic costs, representing 10.9% of the total economic burden [3].
Considering the huge amount of CRC financial burden, preventive actions are crucial to be taken. CRC screening is thought to be the most efficient way to prevent the development of CRC through the elimination of precancerous lesions and improving early diagnosis [2]. There are currently several screening methods in use, with varying degrees of sensitivity and specificity. These methods include stool-based tests [fecal occult blood test (FOBT), fecal immunochemical test (FIT), and FIT-DNA test]; radiologic tests computed tomographic colonography (CTC), double contrast barium enema (DCBE); and endoscopic examinations [flexible sigmoidoscopy (FS), colonoscopy, and colon capsule endoscopy] [4].
Early CRC screening enhanced life-year gain and decreased CRC mortality and incidence as compared to starting CRC screening at age 45 versus 50. Furthermore, Austrian National Cancer Screening Committee recommends the implementation of CRC screening program for all adults aged 45–47. The stool-based test reduces the incidence and mortality up to 45%–50% respectively [5,6]. Another important factor in benefit estimation is the degree of engagement in screening programs. People’s willingness to engage may also result in increased funding for out-of-pocket expenses associated with screening, which lowers the costs of screening programs for health policymakers. Assessing willingness to pay (WTP) for CRC screening reveals the perceived value of screening, guiding efficient resource allocation and policy development [7]. Higher WTP correlates with increased screening participation and early detection, reducing treatment costs and the economic burden of CRC. Several studies measuring the WTP for CRC screening have been conducted, providing valuable insights yet revealing considerable variability in methodologies, populations, and findings [8–17]. To gain a deeper understanding, a comprehensive analysis and evaluation of the factors determining WTP for CRC screening is necessary. This would enhance economic considerations in designing and implementing more effective and targeted public health strategies. Thus, this study aims to analyze existing research on WTP for CRC screening and examine every feasible factor influencing WTP.
MATERIALS AND METHODS
A systematic review of WTP for CRC screening was conducted in December 2023. To ensure a transparent, comprehensive, and standardized approach, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to guide this review [18]. Initial searches were performed using three electronic databases (PubMed, Science Direct, and Scopus). To find relevant studies in the field, a thorough literature search was done through the title and abstract. The keywords utilized in the search were “willingness-to-pay” AND “colorectal cancer” AND “screening”.
The writers independently selected and reviewed all English-language articles that described WTP for CRC screening. Mendeley reference manager program was used to manage the reference and eliminate duplicates. After the screening procedure, the writers evaluated each included study's acceptability by reading it through in its entirety. After removing irrelevant publications, full-text papers were obtained and their eligibility assessed. Studies were considered qualified if they met the following criteria: (1) were fully written in English; (2) investigated the WTP for CRC screening; (3) accessible full-text publication; and (4) quantitatively analyze determinant factors regarding WTP. This study rejected data from (1) Clinician respondents, (2) irrelevant study aims, (3) a review study, and (4) a qualitative study.
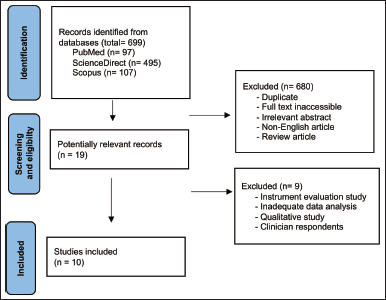 | Figure 1. PRISMA diagram of retrieved studies. [Click here to view] |
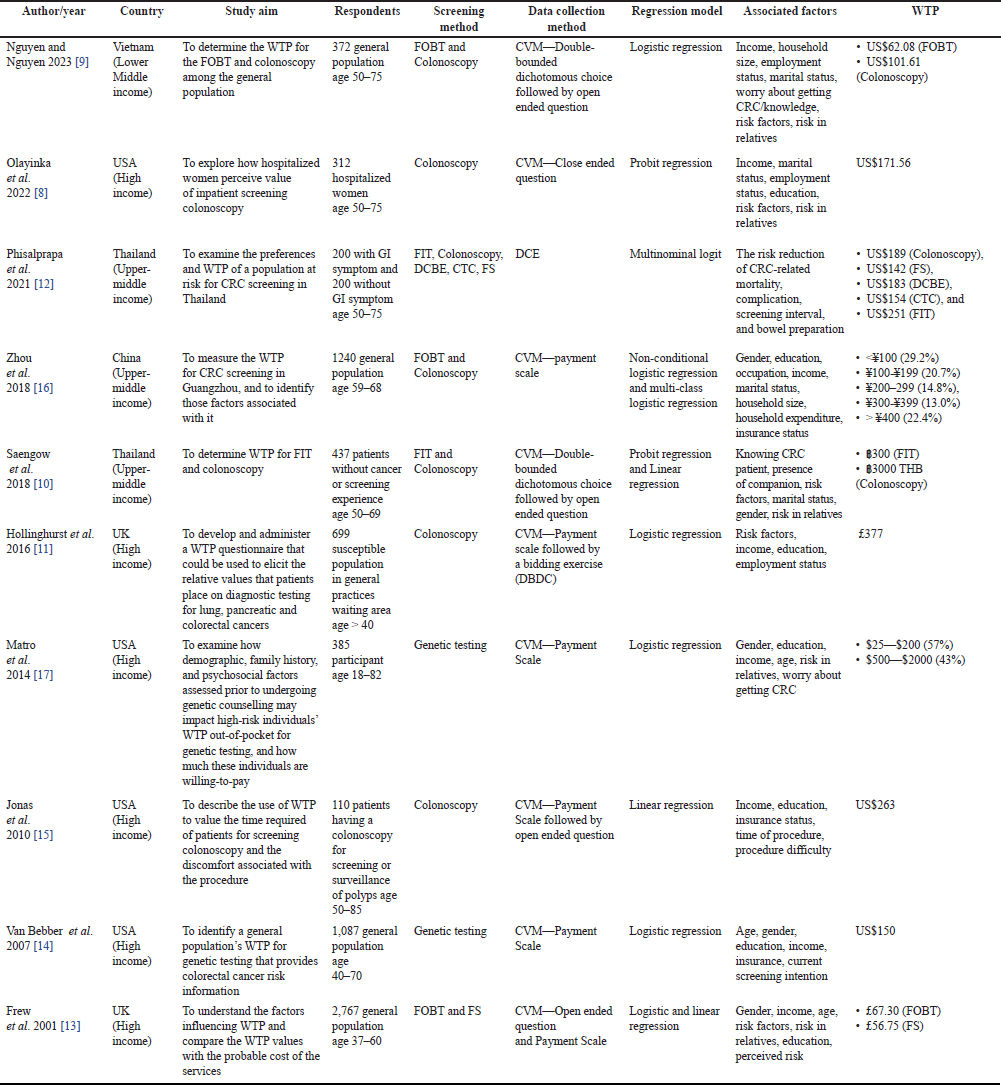 | Table 1. Characteristics of studies. [Click here to view] |
The quality of the final studies was assessed using the JBI (Joanna Briggs Institute) checklist for observational studies [19]. Key aspects of assessment include 8 points that are scored 1 or 0 (yes = 1), (no = 0), and (unclear or not applicable = 0) [20]. Quality evaluation was evaluated as “high,” “moderate,” or “low” for studies with more than 7 scores, 4–6 scores, or <4 scores, respectively [21]. The Characteristics of studies, the WTP value and its associated factors were extracted from each study. Subsequently, the co-payment rate was calculated based on the proportion of WTP to the examination cost. All currency values were converted to 2023 US dollars, using a country-specific consumer price indices calculator and US government Treasury converter. Furthermore, the correlation of associated factors to the WTP value was summarized using the symbols “+” and “-” to signify direct and inverse relationships, respectively.
RESULTS
Figure 1 depicts a PRISMA diagram of the search procedure used in this review. As shown in the PRISMA diagram, 699 results were listed from the initial search (PubMed = 97, ScienceDirect = 495, Scopus = 107). Screening of titles and abstracts was conducted to eliminate duplicates, non-English articles, inaccessible articles, and irrelevant studies. Thereafter, 19 relevant studies were assessed for eligibility, resulting 10 articles included in this review.
According to the study quality assessment results, out of the ten studies that were examined, five of them fulfilled six points [13–17], three studies received a score of 7 [8,9,12], and two studies scored 8 [10,11]. Frequently missing points were the clarity of confounding factor identification and its dealing strategy. It is challenging to identify the confounding variables in WTP studies because the main objective of these studies is to assess people’s monetary appraisal of a particular good or service. Thus, participant behaviors, attitudes, and lifestyle choices are critical to the assessment [19].
Characteristics of the studies
The assessment shown in Table 1 indicated that the studies were published in the years 2023–2001 and were conducted in high-income (USA, UK) [8,11,13–15,17] and lower/upper-middle-income countries (Thailand, Vietnam, and China) [9,10,12,16]. The contingent valuation method (CVM) was the most common method used in data collection, with various types of elicitation methods that consist of double-bounded dichotomous choice (DBDC), payment scale (PS), open-ended (OE) questions, and closed-ended (CE) questions [8–11,13–17]. The other method was a discrete choice experiment (DCE) that also examined the preference for CRC screening [12]. The respondent types consist of a general population [9,13,14,16,17] and some specific population including susceptible patients [8,10–12,15] with an age range of around 40–80 years old. The examination methods include colonoscopy [8–12,15,16], FOBT [9,13,16], genetic testing [14,17], FIT [10,12], FS [12,13], and DCBE [12].
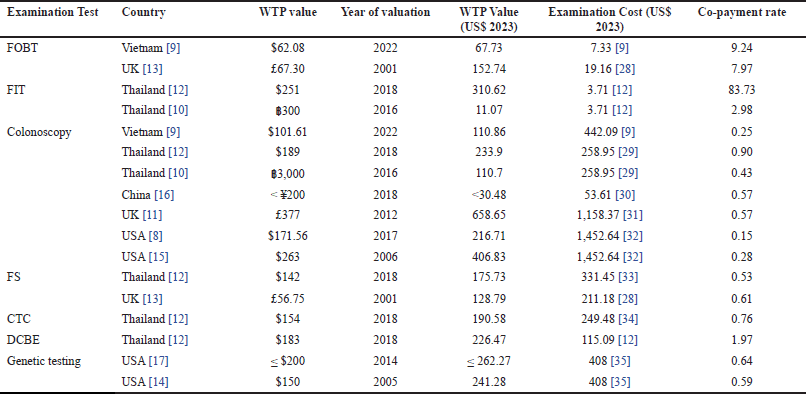 | Table 2. Summary of willingness to pay for colorectal cancer screening. [Click here to view] |
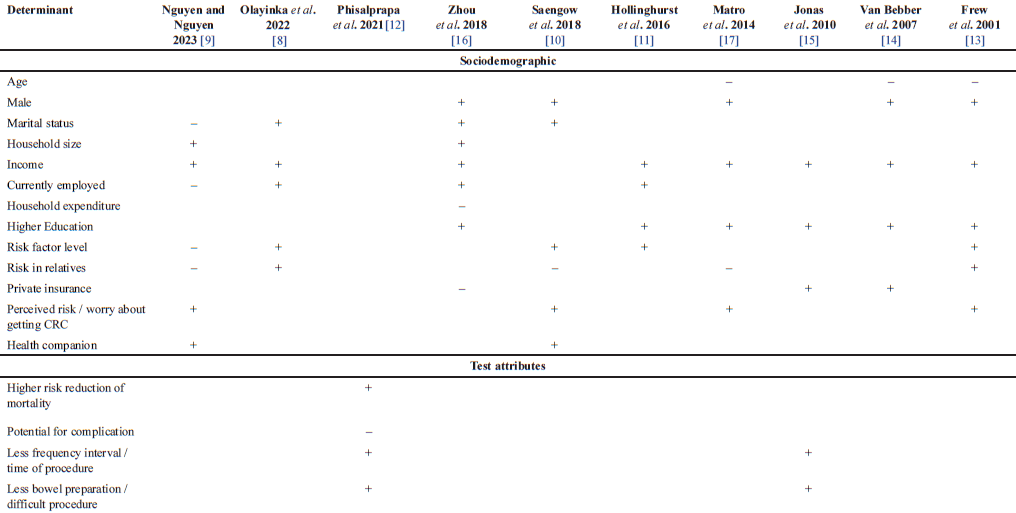 | Table 3. Factors influencing willingness to pay for colorectal cancer screening. [Click here to view] |
WTP for CRC screening test
The systematic review reveals substantial variability in WTP for CRC screening across different countries and screening methods. This variability is influenced by factors such as economic conditions and the effectiveness and efficiency of the screening methods. The data for WTP for CRC screening is shown in Table 2. It is classified based on the type of screening method. Colonoscopy is the most measured test among the studies. China scored the lowest WTP value at < $30.48 [16], while the UK recorded the highest value at $658.65 [11]. Other countries reported the value at around $100–$400. The co-payment rate ranged from 0.15 [8] to 0.9 [12]. Still, in the visual method, FS displayed the WTP value at $130–$170 with a co-payment rate of about 0.6 [12,13]. Meanwhile, the radiologic tests CTC and DCBE had a co-payment rate of more than 0.7 and were valued at around $200 [12]. Moreover, the genetic testing in the USA showed a WTP result of about $250, which is estimated to be 60% of the examination cost [14,17]. Furthermore, the study revealed the stool-based test was notably valued higher than the examination cost (co-payment rate > 2), with a maximum WTP value of $152.74 and $310.62 for FOBT and FIT, respectively [12,13].
Associated factors affecting WTP for CRC screening
In exploring the factors influencing individuals’ decisions surrounding WTP for CRC screening, our analysis incorporates findings of evidently associated determinants from diverse studies conducted by different researchers across various countries. Table 3 depicts the structured presentation, which is divided into sociodemographic factors and test attributes. The sociodemographic factors include demographic information, economic status, health awareness, risk factors, and education. While the test attributes analyze the risk of the screening procedure, the discomfort, and the time spent toward WTP for CRC screening.
Among the studies, sociodemographic factors are commonly examined as the determinant toward WTP for CRC screening. It is shown that male [10,13,14,16,17], household size [9,16], education [11,13–17], income [8,9,11,13–17], health companion [9,10], and perceived risk or worry about getting CRC [9,10,13,17] are positively associated to the WTP for CRC screening. On the other hand, household expenditure [16] and age [13,14,17] are consistently giving an inverse relationship toward the WTP value. Meanwhile, variative results are identified for marital status [8–10,16], employment status [8,9,11,16], risk factor level [8–11,13], risk in relatives [8–10,13,17], and insurance status [14–16].
Regarding the test attributes, the potential for complications caused by the screening procedure reduces the WTP value [12]. This finding is in line with the point of risk reduction in mortality that increases the WTP value [12]. Moreover, the lower frequency interval as well as the time of screening examination notably increase the WTP value. Besides, people are willing to pay more for an easier procedure, including less bowel preparation [12,15].
DISCUSSION
According to the results of the study characteristics, willingness-to-pay studies that also examined its associated determinants were quite limited globally. Overall, this review recorded studies from five countries that were predominantly conducted in the USA, a high-income country. In upper and lower—middle-income countries, all the studies were identified in Asia region (Thailand, Vietnam, and China). The observed limited global representation in WTP studies for CRC screening is noteworthy given the substantial burden of CRC across different regions. Among the selected studies, the USA, UK, and China were reported as top ten countries with the highest incidence cases in 2020 [2]. According to Goodarzi et al. [22] the distribution of CRC cases varies globally, with a significant number in countries characterized by a high or very high human development index. More than two-thirds of all CRC cases and approximately 60% of deaths related to CRC are reported in these economically developed nations [22]. Supporting this finding, the USA had the most significant predicted number of CRC new cases in 2020, and the number of new cases is projected to continue increasing over the next 20 years due to demographic reasons. Similar to the United States, the number of CRC event cases in China is expected to rise by 64%, or around 0.35 million, from 2020 to 2040 [2].
WTP FOR CRC SCREENING
Our study revealed some variations in willingness-to-pay (WTP) values for each examination method. The diverse range of WTP values might be influenced by key determinants of sociodemographic factors and examination attributes. Besides, variations in WTP values for the identical examination procedure between countries tend to be influenced by their respective countries’ gross domestic product (GDP) per capita. Considering colonoscopy as a screening procedure, the WTP value of high-income countries [8,11,15] were relatively higher than those of upper- and lower-middle-income countries [9,10,16]. Correspondingly, the WTP value of FOBT in the UK [13] was around twice as high as the result in Vietnam [9]. However, the WTP for FS in Thailand [12] was unexpectedly higher than in the UK [13]. The possible explanation could be affected by the different elicitation methods between the studies, where the DCE method showed a higher WTP result than the CVM, in this case with PS options.
Looking further into the elicitation method of the WTP value, in the context of comparable screening methods in the same country, different instruments to elicit the WTP value show a notable disparity. In Thailand, the WTP result for the colonoscopy procedure was approximately twice as high when it was done through a DCE [12] as the CVM with double-bounded dichotomous choice [10]. The same phenomena also happened in the FIT screening method. Similar to Thailand, the WTP studies for colonoscopy conducted in the US showed that the PS method followed by an OE question [15] had a higher WTP result than using only CE questions [8]. Supporting the notion, the WTP studies for genetic testing in the US using a PS as the elicitation method yielded values that were roughly equivalent.
In this study, we provide an overview of the co-payment rate of the WTP result when it is compared to the estimated screening cost. The costs were retrieved based on previous studies in related countries or regions. The examination cost is either stated as a reference cost or retrieved from the direct medical cost of the examination procedure. All the costs were converted to 2023 USD using a country-specific consumer price indices calculator [23,24] and a US government Treasury converter [25,26]. It was shown that the lowest co-payment rate was from visual endoscopic examination, specifically colonoscopy, in the US [8,15] and Vietnam [9] (around 0.2). Except for the report from Phisalprapa et al. [12], the co-payment rate for this examination category ranges around 0.4–0.6. The copayment rate slightly increases with the genetic testing method, followed by radiologic testing CTC and DCBE. Furthermore, the stool-based tests (FOBT and FIT) consistently provide high co-payment rate values (>2). In general, this trend plausibly correlates to the characteristics of the examination procedure, particularly the invasion level to conduct the screening. FOBT as the least invasive method was evidently valued higher than the estimated cost, followed by radiologic testing as a non-invasive and painless procedure. Meanwhile, blood tests in genetic testing decreased the co-payment rate. Moreover, colonoscopy, as the most invasive procedure, increased the reluctance of participants to pay for the screening.
Determinants of WTP for CRC screening
As mentioned before, WTP studies are included in behavioral studies. Therefore, sociodemographic factors and test attributes inevitably influence the decisions of participants regarding the range of WTP. Through a thorough analysis of diverse studies and their findings, we elucidated key determinants impacting WTP for CRC screenings.
Sociodemographic factors
Based on the results, our analysis consistently highlighted several factors that positively influence individuals’ inclination to pay for CRC screening. Higher-income levels indicate the most important determinants in most studies. It makes sense that purchasing power is directly correlated with the amount they would pay for CRC screening. Alongside, larger household size also positively related to WTP value due to the financial support from family members to afford the screening test [9]. Another key determinant was higher education, which was impactful in providing better access to health information or a proactive approach to preventive healthcare, thus increasing the WTP value.
Moreover, the male participants were more willing to pay for CRC screening. This finding is interconnected with the previous discussion related to the income factor. According to Matro et al. [17] women were less likely to have a full-time job, resulting in less expendable personal income for optional healthcare needs despite overall adequate household finances. Friedemann-Saìnchez et al. [27] found that women were more likely to experience emotional fear, which includes emotions of exposure and vulnerability during operations, as well as anxiety about being in an uncomfortable circumstance and having their bodies visible to others. In addition, women thought of CRC as a disease that only affected men. This report is in accordance with our finding that perceived susceptibility to CRC increased awareness of the need for CRC screening, hence increasing the WTP value. Furthermore, the WTP value is higher when a health companion is present for the CRC screening. Thus, social support resulted in the participants’ positive perceptions of attending the CRC screening [10].
Our study revealed that factors like marital status, employment status, risk factor level, risk in relatives, and insurance status exhibited varying impacts on WTP value, suggesting complexities in their influence across different contexts and populations. Most of instances indicated that being currently employed had a positive association with higher WTP, suggesting that those with active employment might be more inclined to invest in these screenings due to potential financial stability or access to healthcare benefits. Similarly, most cases concluded that a higher risk factor level increased the WTP value. This heightened level of risk might lead individuals to express a higher WTP value for measures or solutions that can reduce or address the elevated risks. In contrast, the negative correlation reported by Nguyen [9] is presumably influenced by the belief in wasteful spending as an unavoidable consequence. Additionally, the presence of risk in relatives, including any history of cancer, implied both correlations with the decision on WTP. The positive correlation is strongly affected by prevention awareness, in line with the prior explanation related to risk factor level determinants. On the contrary, the inverse association could be explained by the assumption that individuals with a strong family history of CRC may expect a positive test result and therefore be less willing to pay, or that their familiarity with managing disease risk due to family history might make them feel that testing would not impact their strategy to prevention [10,17]. Furthermore, our analysis indicated that insurance status influences WTP values. Specifically, individuals with private insurance tend to exhibit an increase in WTP value. This suggests that having private insurance may contribute to a higher WTP, possibly indicating a greater financial capacity or a perception of enhanced access to healthcare services, thus influencing the willingness to invest in screenings and preventive measures.
The other factors inversely proportional to WTP for CRC screening were age and household expenditure. As age increased, there was a corresponding decrease in the WTP, suggesting a potential reluctance or lower priority among older individuals for investing in CRC screening. Additionally, higher household expenditures were associated with a reduced WTP, indicating that financial constraints or competing financial demands within the household might influence individuals to allocate less for CRC screening expenses.
Test attributes factors
Our findings indicate a positive correlation between increased WTP and several test attributes factors. We highlighted that positive WTP is determined by the least harmful procedure, including the potential for complications and mortality risk. Additionally, people would be willing to pay more for a more convenient and time-saving procedure, such as less bowel preparation and a lower frequency of examination.
Understanding the factors influencing WTP for CRC screening is crucial for healthcare practitioners and policymakers. Practitioners can adjust patient communication and improve screening experiences by addressing concerns such as perceived risk and procedure difficulty, thus improving participation in CRC screenings. For policymakers, these insights guide efficient resource allocation, ensure funds are directed toward highly valued screening methods, and inform targeted public health campaigns to raise awareness and reduce barriers, particularly for lower-income and less-educated populations. Additionally, policymakers can create incentive programs to encourage regular screening, enhancing participation rates. This patient-centred approach improves the effectiveness, accessibility, and acceptability of CRC screening. Future research should explore regional and demographic variations and conduct qualitative studies to understand deeper factors influencing WTP, eventually enhancing engagement strategies, health outcomes, and resource utilization.
This systematic review has limitations that affect the depth and applicability of its findings. A primary limitation is the restricted scope of countries studied, which limits the generalizability of the conclusions. By focusing on a limited set of countries, the review potentially disregards valuable insights from diverse healthcare systems and socio-economic contexts. Additionally, the review encountered variability in the methodologies and elicitation methods used to determine WTP values across the included studies. Although this variability introduces inconsistencies and complexities that obstruct direct comparisons and the synthesis of consistent trends, efforts were made to adjust the WTP values to the same currency and specific time periods to mitigate these issues. These limitations complicate policy relevance, and therefore, the study’s results should be cautiously interpreted when generalizing to populations outside the studied regions. Moreover, the study selection process only included English-language studies and of which full paper are accessible for researcher, potentially excluding valuable data. By addressing these limitations, future research endeavors can achieve a more comprehensive and globally representative analysis, offering insights that transcend regional boundaries and language barriers in understanding WTP dynamics for CRC screening.
CONCLUSION
The results of this study indicated a potential engagement of WTP for CRC screening among all the studies, although the amount of WTP varies across the studies related to the type of screening. Our analysis revealed the correlation of sociodemographic factors and test attributes in shaping individuals’ decisions surrounding CRC screenings. This review enhances existing literature by providing a comprehensive analysis of WTP for CRC screening, informing healthcare policy and practice by highlighting areas for customized interventions to increase screening uptake. Additionally, this review provides insights into co-payment rates for CRC screening, which indicate how people value the screening and highlight financial barriers, crucial for ensuring more equitable access to services. Future research should expand the geographical scope and standardize methodologies to enhance the generalizability and comprehensiveness of WTP for CRC screening findings. Additionally, employing mixed methods (qualitative and quantitative) can provide deeper insights into the reasons behind variations in WTP values.
LIST OF ABBREVIATIONS
CE: close-ended; CTC: computed tomographic colonography; CRC: colorectal cancer; CVM: contingent valuation method; DCE: discrete choice experiment; DCBE: double contrast barium enema; FS: flexible sigmoidoscopy; FOBT: fecal occult blood test; FIT: fecal immunochemical test; OE: open-ended; PS: payment scale; USA: United State of America; UK: United Kingdom; WTP: willingness to pay.
ACKNOWLEDGMENTS
This study was funded by the Indonesia Endowment Fund for Education (LPDP), Ministry of Finance, Republic of Indonesia.
AUTHORS CONTRIBUTIONS
SAK conceptualize the study and made critical revisions to the manuscript. ANN took the lead in drafting the article, conducting reviews, and editing the manuscript. The final version of the manuscript has been reviewed and approved by all authors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–49.
2. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Vol. 14, Translational Oncology. Amsterdam, The Netherlands: Neoplasia Press, Elsevier; 2021.
3. Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023 Apr 20;9(4):465–72.
4. Jayasinghe M, Prathiraja O, Caldera D, Jena R, Coffie-Pierre JA, Silva MS, et al. Colon cancer screening methods: 2023 Update. Cureus. 2023 Apr 13;15(4):e37509.
5. Gartlehner G, Schernhammer E, Lax SF, Preusser M, Bachler H, Titzer H, et al. Screening for colorectal cancer: A recommendation statement of the Austrian National Committee for Cancer Screening. Wien Klin Wochenschr. 2023 Sep 1;135(17–18):447–55.
6. Ebner D, Kisiel J, Barnieh L, Sharma R, Smith NJ, Estes C, et al. The cost-effectiveness of non-invasive stool-based colorectal cancer screening offerings from age 45 for a commercial and medicare population. J Med Econ [Internet]. 2023 Dec 31;26(1):1219–26. Available from: https://www.tandfonline.com/doi/full/10.1080/13696998.2023.2260681
7. Kim Y, Krishna H, Sagiraju R, Kim SY. Willingness-to-pay for vaccines in low-and middle-income Countries?: a systematic review sun [Internet]. 2014. Available from: https://www.researchgate.net/publication/328943317
8. Olayinka O, Gnanaraj J, Khaliq W. Hospitalized Women’s willingness to pay for inpatient screening colonoscopy. Women’s Health Rep. 2022 Sep 1;3(1):768–73.
9. Nguyen HT, Nguyen AQ. Willingness to pay for colorectal cancer screening in Vietnam: policy implications from a contingent valuation survey. Value Health Reg Issues. 2023 Nov 1;38:29–37.
10. Saengow U, Birch S, Geater A, Chongsuwiwatvong V. Willingness to pay for colorectal cancer screening and effect of copayment in Southern Thailand. Asian Pac J Cancer Prev. 2018 Jun 1;19(6):1727–34.
11. Hollinghurst S, Banks J, Bigwood L, Walter FM, Hamilton W, Peters TJ. Using willingness-to-pay to establish patient preferences for cancer testing in primary care. BMC Med Inform Decis Mak. 2016;16(1):105.
12. Phisalprapa P, Ngorsuraches S, Wanishayakorn T, Kositamongkol C, Supakankunti S, Chaiyakunapruk N. Estimating the preferences and willingness-to-pay for colorectal cancer screening: an opportunity to incorporate the perspective of population at risk into policy development in Thailand. J Med Econ. 2021;24(1):226–33.
13. Frew E, Wolstenholme JL, Whynes DK. Willingness-to-pay for colorectal cancer screening. Eur J Cancer. 2001;37(14):1746–51.
14. Van Bebber SL, Liang SY, Phillips KA, Marshall D, Walsh J, Kulin N. Valuing personalized medicine: willingness to pay for genetic testing for colorectal cancer risk. Per Med. 2007;4(3):341–50.
15. Jonas DE, Russell LB, Chou J, Pignone M. Willingness-to-pay to avoid the time spent and discomfort associated with screening colonoscopy. Health Econ. 2010;19(10):1193–211.
16. Zhou Q, Li Y, Liu HZ, Liang YR, Lin GZ. Willingness to pay for colorectal cancer screening in Guangzhou observational study. World J Gastroenterol. 2018;24(41):4708–15.
17. Matro JM, Ruth KJ, Wong YN, McCully KC, Rybak CM, Meropol NJ, et al. Cost sharing and hereditary cancer risk: predictors of willingness-to-pay for genetic testing. J Genet Couns. 2014;23(6):1002–11.
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021 Dec 1;10(1):89.
19. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z editors. Joanna Briggs Institute Reviewer’s Manual. Nashville, TN: The Joanna Briggs Institute; [Internet]. 2017; Available from: https://jbi.global/critical-appraisal-tools
20. Karim M, Husein A, Qamruddin I, Liszen T, Alam MK. Original article To evaluate the effects of Low-level laser therapy (LLLT) on wound healing of extraction socket: a systematic review. Bangladesh J Med Sci. 2023 Jun 16;22(3):585–97.
21. Wilairatana P, Masangkay FR, Kotepui KU, Milanez GDJ, Kotepui M. Prevalence and characteristics of malaria among covid-19 individuals: a systematic review, meta-analysis, and analysis of case reports. PLoS Negl Trop Dis. 2021 Oct 1;15(10):e0009766.
22. Goodarzi E, Beiranvand R, Naemi H, Momenabadi V, Khazaei Z. Worldwide incidence and mortality of colorectal cancer and human development index (HDI): an ecological study. WCRJ. 2019;6:e1433.
23. Chew R, Greer RC, Tasak N, Day NPJ, Lubell Y. Modelling the cost-effectiveness of pulse oximetry in primary care management of acute respiratory infection in rural northern Thailand. Trop Med Int Health. 2022 Oct 1;27(10):881–90.
24. FXTOP. Inflation calculator: FXTOP. Available from https://fxtop.com/en/inflation-calculator.php. 2023.
25. U.S. government. Currency exchange rates converter. Available from https://fiscaldata.treasury.gov/currency-exchange-rates-converter/. 2023.
26. Sukor N, Sunthornyothin S, Tran TV, Tarigan TJ, Mercado-Asis LB, Sum S, et al. Health care challenges in the management of primary aldosteronism in Southeast Asia. J Clin Endocrinol Metab. 2024 Jan 23;109(7):1718–25.
27. Friedemann-Sánchez G, Griffin JM, Partin MR. Gender differences in colorectal cancer screening barriers and information needs. Health Expect. 2007 Jun;10(2):148–60.
28. Sharp L, Tilson L, Whyte S, O’Ceilleachair A, Walsh C, Usher C, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012 Feb 28;106(5):805–16.
29. Joob B, Wiwanitkit V. Colonoscopy colorectal cancer screening: cost-effectiveness in Thailand. S Asian J Cancer. 2016;5:19.
30. Cenin D, Li P, Wang J, de Jonge L, Yan B, Tao S, et al. Optimising colorectal cancer screening in Shanghai, China: a modelling study. BMJ Open. 2022 May 16;12(5):e048156.
31. Public Health England. Cost-effective commissioning of colorectal cancer care [Internet]. PHE Publication; 2016. Available from: www.facebook.com/PublicHealthEngland
32. Subramanian S, Tangka FKL, Hoover S, Royalty J, DeGroff A, Joseph D. Costs of colorectal cancer screening provision in CDC’s colorectal cancer control program: comparisons of colonoscopy and FOBT/FIT based screening. Eval Program Plann. 2017 Jun 1;62:73–80.
33. Wong MCS, Ching JYL, Chan VCW, Lam TYT, Luk AKC, Wong SH, et al. Colorectal cancer screening based on age and gender: a cost-effectiveness analysis. Medicine (United States). 2016 Mar 4;95(10).
34. Hashimoto Y, Igarashi A, Miyake M, Iinuma G, Fukuda T, Tsutani K. Cost-effectiveness analysis of CT colonography for colorectal cancer screening program to working age in Japan. Value Health Reg Issues. 2014;3(1):182–9.
35. Unim B, Pitini E, De Vito C, D’Andrea E, Marzuillo C, Villari P. Cost-effectiveness of RAS genetic testing strategies in patients with metastatic colorectal cancer: a systematic review. Value Health. 2020;23:114–26.