INTRODUCTION
Microorganisms are ubiquitous, both inside and outside the human body, eventually settling on surrounding surfaces, including floors. Most common floor microflora are opportunistic and can cause infections at higher concentrations [1]. Staphylococcus aureus, a ubiquitous and pathogenic organism to humans, triggers various skin infections such as carbuncles, impetigo, cellulitis, furuncles, and so on. It can also lead to pneumonia, endocarditis, osteomyelitis, sepsis, and so on. [2]. Pseudomonas aeruginosa, an opportunistic human pathogen, causes diseases such as generalized inflammation and sepsis [3] Candida albicans can survive on hospital surfaces and fomites, causing opportunistic infections like oral thrush, vaginal infection, and so on [4,5]. Aspergillus niger, an opportunistic pathogen, can lead to allergic bronchopulmonary aspergillosis or invasive aspergillosis [6]. Mucor mold, an opportunistic fungus, causes mucormycosis [7]. Due to the COVID-19 pandemic in India, mucormycosis has emerged as a serious complication during its second wave [8]. Disinfectants play a significant role in preventing infections and maintaining environmental health [9]. The usage of chemical disinfectants has significantly increased in hospitals, laboratories, and households due to the earlier COVID-19 pandemic. However, it causes various side effects, such as irritation to the respiratory system, skin, and eyes. Cases of poisoning due to excessive usage and reports of disinfectant adulteration have also been documented [10]. Overuse and abuse of chemical disinfectants lead to antibiotic resistance genes and resistance to disinfectants [9]. Therefore, finding a natural disinfectant is crucial to reducing the problems caused by chemical disinfectants. A natural disinfectant should be easy to use, noncorrosive, harmless on most surfaces, safe on the skin and for breathing, and eco-friendly [11]. Studies were carried out effectiveness of different plant extracts such as neem leaves [12,13], turmeric [14], aloe vera, and gotu kola [15] as a natural disinfectant. However, ascertaining the cytotoxic nature of herbal plants is essential, which is why the MTT test is frequently employed to assess the viability of cells at plant concentrations [16].
There is a demand for an effective natural disinfectant which is of societal demand for safer alternatives to chemical disinfectants, that are known to have significant side effects. Syzygium cumini (Jamun) is a large evergreen tree of the Myrtaceae family with various medicinal properties [17,18]. Its leaf extract has antibacterial [17,19], antifungal [17], antidiabetic activity [20]. To the best of our knowledge, the effectiveness of S. cumini leaf extract as a disinfecting agent was not investigated. Therefore, evaluating the antibacterial and antifungal activity of Syzygium cumini leaf extract (SCLE), determining the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) of the extract, calculating MBC/MIC and MFC/MIC ratios, and assessing the effectiveness of SCLE as a disinfecting agent were the focuses of this study. Additionally, Gas Chromatography-Mass Spectroscopy (GC-MS) analysis and in vitro toxicity tests of SCLE by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay were conducted.
MATERIALS AND METHODS
Collection and preparation of extract
Syzygium cumini (L.) Skeel leaves were collected from Perumbakkam, Chennai, Tamil Nadu, and authenticated by Dr. P. Palani, Centre for Advanced Studies in Botany, University of Madras, Chennai, Tamil Nadu. Syzygium cumini extract was prepared according to Elfadil et al. [17] with slight modification. The collected leaves were washed properly, shade dried, powdered, and used for the extraction with distilled water (1:10). It was heated at 60°C for 2 hours. Then, it was filtered, dried, and used for the study.
Cultures
Bacterial cultures such as P. aeruginosa (ATCC 27853) and S. aureus (ATCC 29213) were obtained from Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu. Fungal cultures such as C. albicans, Mucor sp., and A. niger were obtained from the Department of Microbiology, University of Madras, Chennai, Tamil Nadu.
Antibacterial and antifungal activity
The antibacterial and antifungal activity of SCLE was evaluated by the agar well diffusion method [21]. Muller Hinton agar plates were inoculated with the test organisms such P. aeruginosa, S. aureus, Mucor sp., C. albicans., and A. niger. A stock solution of 500,000 μg/ml concentration of SCLE was prepared. From the stock solution, 100 µl was loaded into the wells. Ciprofloxacin and Nystatin were used as a positive control for the tested bacterial and fungal cultures, respectively. As a negative control, distilled water was used. The diameter of the inhibition zone was measured after incubation at 37°C for 1 day for bacterial cultures and for 3 days at room temperature (RT) for fungal cultures, respectively. The assay was conducted in triplicates.
MIC
MIC of SCLE was determined by the agar well diffusion method [22]. Different concentrations of SCLE (µg/ml) such as 38, 78, 156, 312.5, 625, 1,250, 2,500, 5,000, and 10,000 were prepared. Lawn cultures of the used organisms were made onto the sterile Muller Hinton agar plates. 100 µl of different concentrations were loaded into the respective wells. The plates were then incubated.
MBC and MFC
Streaks were taken from the inhibition zone of MIC plates and inoculated onto respective nutrient agar (NA) and Sabouraud Dextrose agar (SDA) plates and then incubated. The concentration of the extract which did not show microbial growth on the respective agar plates was determined [23].
Then, the MBC/MIC and MFC/MIC ratio of SCLE against the test organisms were calculated to determine if the extract has bactericidal/fungicidal (MBC/MIC≤4 or MFC/MIC≤4) or bacteriostatic/fungistatic (MBC/MIC>4 or MFC/MIC>4) activity [24,25].
Efficacy of SCLE as a disinfecting agent
5%, 10%, and 50% concentrations of the plant extract were prepared to evaluate its effectiveness as a disinfecting agent. The procedure was followed according to Welk et al. [26] with some modifications. 10 µl of bacterial (1 × 105CFU/ml) or fungal suspension (1 ×104CFU/ml) was added separately to 0.99 ml of each concentration at RT, respectively. The control contained 0.99 ml of distilled water instead of extract. After 15 and 30 minutes of contact time, it was serially diluted and plated onto NA and SDA plates which were then incubated at 37°C for 24 hours for bacterial cultures and 48 hours at RT for fungal cultures, respectively. After incubation, colonies were counted.
The percentage decrease in microorganism counts following treatment with varying concentrations of extract for 15 minutes and 30 minutes was calculated [27].
GC-MS analysis
GCMS-QP 2010 Ultra (Shimadzu, Japan) was used to detect the compounds present in the extract. Their mass spectrum was compared with the reference spectrum available in the Willey 8 and NIST 11 and 17 libraries.
In vitro toxicity test
The in vitro cytotoxicity test of S. cumini leaves was evaluated using Vero cell lines by MTT assay [28]. The cells were seeded in Dulbecco’s modified eagle medium (100 µl) including 10% fetal bovine serum, streptomycin, penicillin, gentamycin, amphotericin B, and L-glutamine in a 96-well plate at 1.0 × 104 cells/ml and incubated at 37°C for 1 day. 1–1,000 μg/ml concentrations of SCLE were used for cytotoxicity. After incubation with the extract, the MTT reagent was added into each well and incubated for 4 hours. Then, dimethyl sulphoxide was added after removing the supernatant. The absorbance was then measured at 580 nm. The cell viability (%) was calculated according to Fouda et al. [29].
STATISTICAL ANALYSIS
One-way ANOVA and post hoc Duncan test were used to determine the significance level (p < 0.05) of data (IBM SPSS statistics version 25.0).
RESULTS AND DISCUSSION
Antibacterial and antifungal activity of extract
SCLE demonstrated antimicrobial activity against S. aureus, P. aeruginosa, C. albicans, Mucor sp., and A.niger. The results of antibacterial and antifungal activity are shown in Figure 1. Table 1 displays the zone of inhibition (mm) against the test organisms. SCLE inhibited all the tested microbial cultures with an inhibition zone range from 20–35 mm but was not effective on A. niger. Elfadil et al. [17] revealed that S. cumini water extract has antimicrobial activity against S. aureus and C. albicans but none against Pseudomonas sp. and A. niger. Pareek et al. [30] reported that the aqueous extract of S. cumini exhibits antifungal activity against C. albicans and A. niger. The difference in the results might be due to the difference in the extraction process. Consistent with our findings, SCLE has antifungal activity against C. albicans. [31].
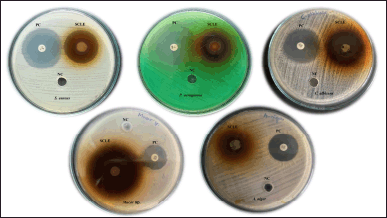 | Figure 1. Antibacterial and antifungal activity of SCLE. [Click here to view] |
 | Table 1. Zone of inhibition (mm) of SCLE against test organisms and data were expressed as mean ± SD. [Click here to view] |
MIC
The inhibitory concentration of SCLE against test organisms is illustrated in Figure 2. The MIC value of SCLE ranged from 0.078–1.25 μg/ml. MIC of SCLE against S. aureus, P. aeruginosa, Mucor sp., and C. albicans was found to be 625 μg/ml, 1,250 μg/ml, 78 μg/ml, and 1,250 μg/ml, respectively. The lowest inhibitory concentration was found against Mucor sp. A previous study reported that S. cumini aqueous extract had MIC of 1,560 μg/ml against C. albicans [31]. Chanudom et al. [32] found that the MIC of the aqueous extract of S. cumini against S. aureus was 6.25 mg/ml. Oliveira et al. [33] reported MIC of S. cumini hydroalcoholic extract against S. aureus, P. aeruginosa, and C. albicans as 20 µg /ml, 90 µg/ml, and 90 µg /ml, respectively]. Furthermore, hydroalcoholic extract of S. cumini leaves has 1,296.8μg/ml against S. aureus. The variation in the values might be due to the difference in extraction process and concentration tested.
MBC
The MBC of SCLE against S. aureus and P. aeruginosa was 1,250 μg/ml and 2,500 μg/ml, respectively. Chanudom et al. [32] reported an MBC of the aqueous extract of S. cumini against S. aureus as 12.5 mg/ml. In this study, the MBC/MIC ratio for SCLE was determined to evaluate its bactericidal or bacteriostatic activity. SCLE. Its MBC/MIC against S. aureus and P. aeruginosa was 2, indicating bactericidal activity which could be due to its bioactive constituents. Consistent with our finding, hydroalcoholic extract of S. cumini leaves had a bactericidal effect against S. aureus [34].
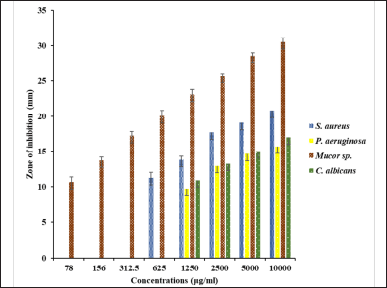 | Figure 2. MIC of SCLE against test organisms. [Click here to view] |
MFC
The MFC of SCLE against C. albicans and Mucor sp. was 5,000 μg/ml and 156 μg/ml, respectively. The MFC/MIC ratio for SCLE was further analyzed to evaluate its fungicidal or fungistatic activity. The MFC/MIC ratio of SCLE against C. albicans and Mucor sp. was 4 and 2, signifying fungicidal activity. This fungicidal activity against all tested fungi could be due to the bioactive constituents in SCLE. Figueirêdo Junior et al. [35] reported that the hydroalcoholic extract of S. cumini leaves had a fungistatic effect against C. albicans [35].
Efficacy of SCLE as a disinfecting agent
The effectiveness of SCLE as a disinfecting agent against bacterial and fungal cultures is illustrated in Figure 3. SCLE was effective against P. aeruginosa and S. aureus within the tested contact times. Against fungi, SCLE was also effective against Mucor sp. and C. albicans. Reduction percentages after treatment with SCLE are detailed in Table 2.
In this study, tested bacterial and fungal cultures were significantly reduced after treatment with SCLE. The disinfectant effectiveness of SCLE against the tested organisms was arranged as (P. aeruginosa > S. aureus> Mucor sp.> C. albicans). SCLE exhibited higher disinfectant effectiveness on bacterial cultures than on fungal cultures. Among the tested concentrations, 50% SCLE with a 30-minute contact time showed the most significant reduction in the tested organisms (99.99% reduction in P. aeruginosa, 90.71% in S. aureus, 92% in Mucor sp., and 73.45% in C. albicans). This study represents the first report evaluating the efficacy of S. cumini leaf extract as a disinfecting agent against test organisms. Previous studies reported disinfectant effectiveness of different plant extracts [12–15]. Hidayati et al. [12] found that 50%, 75%, and 100% concentration of neem leaves reduced the bacteria in poultry incubators by 56.16%, 57.94%, and 62.20, respectively, and fungi in poultry incubators by 57.63%, 58.11, and 57.65%, respectively [12]. The same author also revealed that neem leaf extract decreased the Mucor sp. by 50.75% [12]. Rios et al. [13] reported that 5% concentrations of neem leaves inhibited S. aureus by 99.96% at 5 minutes of contact time. Mayefis et al. [15] reported that 25%, 50%, and 100% concentration of aloe vera reduced 44.6%, 89.2%, and 95.3% of germs on dinner plates, respectively, and also reported that a combination of gotu kola and aloe vera at 50% and 100% concentration reduced 73.3% and 93% of the germs [15].
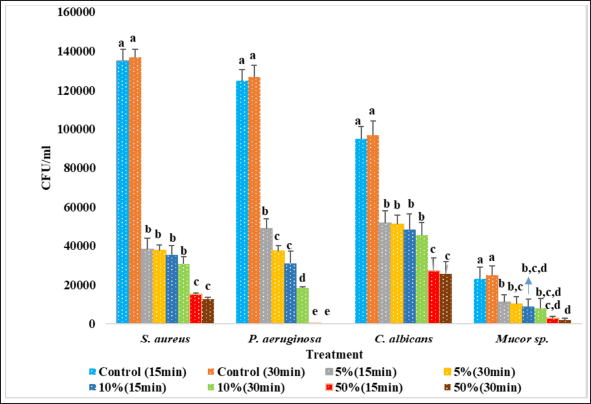 | Figure 3. Efficacy of SCLE as a disinfecting agent against test organisms. N = 3. Mean ± SD. Different letters at same organism denotes significantly different (p < 0.05), according to Duncan’s test. [Click here to view] |
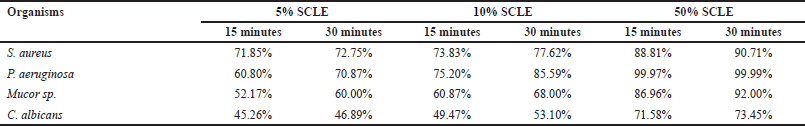 | Table 2. Reduction percentage of microorganisms after treated with SCLE. [Click here to view] |
GC-MS analysis
Below Figure 4 displays the GC-MS chromatogram for SCLE, identifying 16 compounds presented in Table 3. Thymine has bactericidal activity against gram-negative pathogens [36]. 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- has antimicrobial activity [37] Cyclohexane 1,3,5-triphenyl- has industrial applications as a surfactant and emulsifier [38] 1-Hexacosanol possess acetylcholinesterase inhibitory activity [39]. Gamma-sitosterol has antidiabetic hypolipidemic, anticancer, antibacterial, and antiviral activity [21]. 2,2’Benzylidenebis(3-methylbenzofuran) has been reported for its anti-diabetic property [40] 5,5’-Dithiobis-(2-nitrobenzoic acid) has antiviral property [41].
In vitro cytotoxicity test
In Figure 5, the percentage of cell viability is illustrated. SCLE exhibited nontoxicity on Vero cell lines with an IC50 value of 320 µg/ml. Masfra and Hafni [42] reported that all extracts were considered nontoxic if the IC50 value was >30 µg/ml [42]. Ribeiro et al. [43] demonstrated low toxicity was showed in rodent macrophages, showing an IC50 value of 31.64 µg/ml for the hexane extract of S. cumini leaves. Pereira et al. [44] found that concentrations ranging from 10 to 200 µg/ml of S. cumini exhibited no toxicity on macrophage cells.
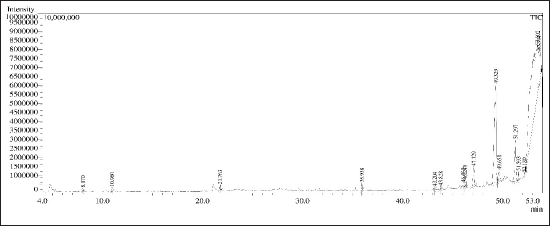 | Figure 4. GC-MS chromatogram for SCLE. [Click here to view] |
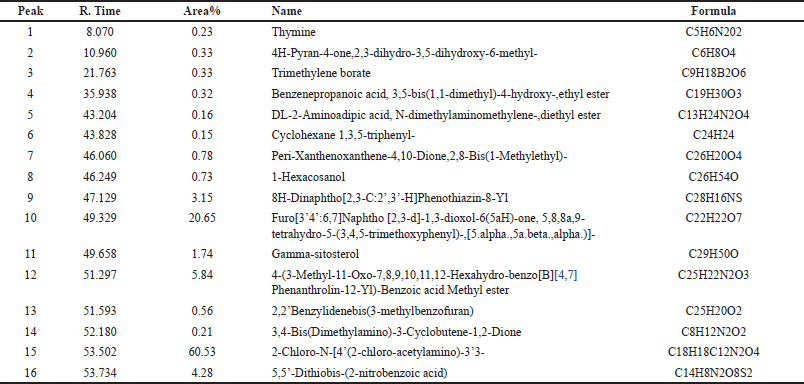 | Table 3. GC-MS spectral analysis of SCLE. [Click here to view] |
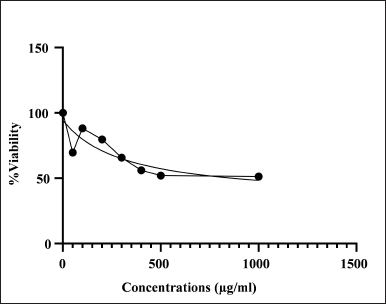 | Figure 5. Percentage of viability of SCLE on vero cell lines. [Click here to view] |
Isolation of bioactive compounds from the crude extract of SCLE should be done for future studies.
CONCLUSION
Syzygium cumini leaf extract showed a bactericidal effect against P. aeruginosa and S. aureus as well as a fungicidal effect against Mucor sp. and C. albicans. Additionally, it demonstrated a notable decrease in microorganisms following SCLE treatment. The bactericidal and fungicidal activities of SCLE may be attributed to its bioactive components. It was found to be nontoxic on Vero cell lines. Thus, SCLE could be used as a potent natural disinfecting agent.
ACKNOWLEDGMENT
The authors are very thankful to the Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai, and the Department of Microbiology, University of Madras, Chennai, Tamil Nadu, for providing the cultures for this study.
AUTHOR CONTRIBUTIONS
All the authors made significant contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Vaishali C, Jivitesh B, Shalini R, Sonal D. Sensitivity of floor Microflora towards various Disinfectants. Electron J Biol. 2011; 7:44–8. Available from: https://ejbio.imedpub.com/sensitivity-of-floor-microflora-towards-various-disinfectants.php?aid=6005
2. Bush LM and Vazquez-Pertejo MT. Staphylococcal infections [Internet]. MSD Manual; 2023 May [cited 2023 Dec 5]. Available from: https://www.msdmanuals.com/en-gb/professional/infectious-diseases/gram-positive-cocci/staphylococcal-infections
3. Cigana C, Lorè NI, Bernardini ML, Bragonzi A. Dampening host sensing and avoiding recognition in Pseudomonas aeruginosa Pneumonia. BioMed Res Int. 2011;2011:10. CrossRef
4. Ferreira TR, Costa SM, De Souza JM, Castilho ACS, Eller LKW, Moris DV, et al. Viability of Candida albicans in different fomites and hospital surfaces under disinfectants and biological fluids influence. Res Soc Dev. 2021;10(5):1–7. CrossRef
5. Arya NR and Rafiq NB. Candidiasis. [Internet]. Treasure Island, FL: StatPearls; 2023 May [cited 2023 Dec7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560624/
6. Poulsen JS, Madsen AM, White JK, Nielsen JL. Physiological responses of Aspergillus niger challenged with Itraconazole. Antimicrob Agents Chemother. 2021;65(6):e02549–20. CrossRef
7. Ashley Hagen MS. COVID-19-Associated Mucormycosis: triple threat of the pandemic. Am Soc Microbiol. [Internet]. 2021 July [cited 2023 Nov 27]. Available from: https://asm.org/Articles/2021/July/COVID-19-Associated-Mucormycosis-Triple-Threat-of
8. Singla N, Sharma N, Sharma N, Behera A, Bhatia M. Clinical profile of patients admitted with Mucormycosis during the COVID-19 pandemic in medicine emergency of a tertiary care hospital care in North India. Cureus. 2022;14(9):e29219. CrossRef
9. Tong C, Hu H, Chen G, Li Z, Li A, Zhang J. Disinfectant resistance in bacteria: Mechanisms, spread, and resolution strategies. Environ Res. 2021;195:110897. CrossRef
10. Lachenmeier DW. Antiseptic drugs and disinfectants with special scrutiny of COVID-19 pandemic related side effects. Side Effects of Drugs Ann. 2021;43:275–84. CrossRef
11. Mandavgane SA, Rambhal AK, Mude NK. Development of cow urine based disinfectant. Nat Prod Radiance. 2005;4:410–5. Available from: http://nopr.niscpr.res.in/handle/123456789/8129
12. Hidayati YA, Yulia R, Rosita S, Rahmah KN, Marlina ET, Harlia E. The effect of neem leaves (Azadirachta indica A. Juss) application as a natural disinfectant on decreasing number of bacteria and fungi in poultry incubator. Walailak Procedia. 2019;2019(1):1–4. Available from: https://wjst.wu.ac.th/index.php/wuresearch/article/view/6588
13. Rios HJ, Claros BP, Rios AM, Mejia JC, Arias AF. In vitro effectiveness of an aqueous extract of neem (Azadirachta indica A. Juss) leaves on bacteria causing healthcare associated infection in Valledupar. Vitae. 2021;27(3):1–6. CrossRef
14. Verma RK, Kumari P, Maurya RK, Kumar V, Verma RB, Singh RK. Medicinal properties of turmeric (Curcuma longa L.): a review. Int J Chem Stud. 2018;6(4):1354–7. Available from: https://www.chemijournal.com/archives/2018/vol6issue4/PartV/6-4-314-742.pdf
15. Mayefis D, Gainil S, Dahlia AB, Syukrillah GS, Oktaviyanti N. Effectiveness of combination of Gotu Kola (Centella Asiatica (L.) Urban) and aloe vera herb extracts as a natural disinfectant. Jurnal Eduhealth. 2023;14(1):182–93. Available from: http://ejournal.seaninstitute.or.id/index.php/healt/article/view/1466
16. Vajrabhaya Lo and Korsuwannawong S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J Anal Sci Technol. 2018;9:15. CrossRef
17. Elfadil AG, Karamallah AA, Abualhassan AM, Hamid AA, Sabahelkhier MK. Antimicrobial activities of Syzygium cumini leave extracts against selected microorganisms. Nova J Med Biol Sci. 2015;4:1–8. Available from: https://api.semanticscholar.org/CorpusID:87668526
18. Chhikara N, Kaur R, Jaglan S, Sharma P, Gat Y, Panghal A. Bioactive compounds and pharmacological and food applications of Syzygium cumini–a review. Food Funct. 2018;9:6096–115. doi: https://doi.org/10.1039/C8FO00654G
19. Devi HJ, Gnanasekaran P, Devi YA. Selection of effective plant extract as a disinfecting agent using hot and cold-water extraction. Eco Env Cons. 2022;28(4):1874–81. CrossRef
20. Alam Md R, Rahman AB, Moniruzzaman Md, Kadir MF, Haque Md A, Alvi RaziUl-Hsan M, et al. Evaluation of antidiabetic phytochemicals in Syzygium cumini (L.) Skeels (Family: Myrtaceae). J App Pharm Sci. 2012;2(10):094–8. CrossRef
21. Sofi MA, Nanda A, Raj E, Sofi MA. Phytochemical profiling of the methanolic root extract of Lavatera cashmeriana using GC-MS and evaluation of its potential antimicrobial activity. Res J Pharm Technol. 2022;15(12):5707–3. CrossRef
22. Venkateswarulu TC, Srirama K, Mikkili I, Nazneen Md B, Dutta JB, Alugunutta VN, et al. Estimation of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of antimicrobial peptides of Saccharomyces boulardii against selected pathogenic strains. Karbala Int J Mod Sci. 2019;5(4):Article 8. CrossRef
23. Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25(2):361–6. CrossRef
24. Caruso C, Porta A, Tosco A, Eletto D, Pacente L, Bartollino S, et al. A novel vitamin E TPGS-based formulation enhances chlorhexidine bioavailability in corneal layers. Pharmaceutics. 2020;12(7):642. CrossRef
25. Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther. 2020;20:55. CrossRef
26. Welk A, Meller C, Schubert R, Schwahn Ch, Kramer A, Below H. Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate hydrogen peroxide combination in a quantitative suspension test. BMC Microbiol. 2019;9:134. CrossRef
27. Saad AH, Gamil SN, Kadhim RB, Samour R. Formulation and evaluation of herbal hand wash from Matricaria chamomilla flowers extracts. Int J Res Ayurveda Pharm. 2011;2(6):1811–3. Available from: https://ijcrt.org/papers/IJCRT2005404.pdf
28. Njeru SN and Muema JM. In vitro cytotoxicity of Aspilia pluriseta Schweinf. extract fractions. BMC Res Notes. 2021;14:57. CrossRef
29. Fouda A, Al-Otaibi WA, Saber T, AlMaotwaa SM, Alshallash KS, Elhady M, et al. Antimicrobial, antiviral and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of Selenium Nanoparticles. J Funct Biomat. 2022;132(3):157. CrossRef
30. Pareek A, Meena RK, Yadav B. Antimicrobial activity of Syzigium cumini. Indian J Appl Res. 2015;5(8):751753. Available from: https://www.worldwidejournals.com/indian-journal-of-applied-research-(IJAR)/recent_issues_pdf/2015/August/August_2015_1438869504__222.pdf
31. Adelakun AO, Awosika A, Adabanya U, Omole AE, Olopoda AI, Bello ET. Antimicrobial and synergistic effects of Syzygium cumini, Moringa oleifera, and Tinospora cordifolia against different Candida infections. Cureus. 2024;16(1):e52857. CrossRef
32. Chanudom L, Bhoopong P, Khwanchuea R, Tangpong J. Antioxidant and antimicrobial activities of aqueous & ethanol crude extracts of 13 Thai traditional plants. Int J Curr Microbiol App Sci. 2014;3(1):549–58. Available from: https://ijcmas.com/vol-3-1/Lanchakon%20Chanudom,%20et%20al.pdf
33. Oliveira GF, Furtado NAJC, Filho AAS, Martins CHG, Bastos JK, Cunha WR, et al. Antimicrobial activity of Syzigium cumini (Myrtaceae) leaves extract. Braz J Microbiol. 2007;38(2):381–4. CrossRef
34. Bernardo WLC, Boriollo MFG, Tonon CC, da Silva JJ, Oliveira MC, de Moraes FC, et al. Biosynthesis of silver nanoparticles from Syzygium cumini leaves and their potential effects on odontogenic pathogens and biofilms. Front Microbiol. 2022;13:995521. CrossRef
35. Figueirêdo Junior EC, Cavalcanti YW, Lira AB, Pessôa HLF, Lopes WS, da Silva DR et al. Phytochemical composition, antifungal activity, in vitro and in vivo toxicity of Syzygium cumini (L.) Skeels leaves extract. Bol Latinoam Caribe Plant Med Aromat. 2021;20:536–57. CrossRef
36. Liu Y, Yang K, Jia Y, Shi J, Tong Z, Wang Z. Thymine sensitizes Gram negative pathogens to antibiotic killing. Front Microbiol. 2021;12:622798. CrossRef
37. Amala VE and Jeyaraj M. Determination of antibacterial, antifungal, bioactive constituents of Triphala by Ft-IR and GC-MS analysis. Int J Pharm Sci. 2014;6(8):123–6. Available from: https://www.academia.edu/87764889/Determination_of_Antibacterial_Antifungal_Bioactive_Constituents_of_Triphala_by_FT_Ir_and_GC_MS_Analysis
38. Human Metabolome Database [Internet]. Showing metabocard for 1,3,5-Triphenylcyclohexane (HMDB0037518). 2022 Jul 3;[cited 2023 Nov 27]. Available from: https://hmdb.ca/metabolites/HMDB0037518
39. Gade S, Rajamanikyam M, Vadlapudi V, Nukala KM, Aluvala R, Giddigari C, et al. Acetylcholinesterase inhibitory activity of stigmasterol & hexacosanol is responsible for larvicidal and repellent properties of Chromolaena odorata. Biochimica et Biophysica Acta General Subjects. 2017;1861(3):541–50. CrossRef
40. Ladokun OA, Abiola A, Okikiola D, Ayodeji F. GC-MS and molecular docking studies of Hunteria umbellate methanolic extract as a potent anti-diabetic. Inform Med Unlocked. 2018;13:1–8. CrossRef
41. Lara HH, Ixtepan Turrent L, Garza Trevino EN, Flores Tevino SM, Borkow G, Rodriguez Padilla C. Antiviral propierties of 5,5’-Dithiobis-2-nitrobenzoic acid and bacitracin against T-tropoc human immunodeficiency virus type 1. Virol J. 2011;8:137. CrossRef
42. Masfra and Hafni A. Cytotoxicity of “Ekor naga” Leaf (Rhaphidophora pinnata (Lf) Shcott) Chloroform extract against T47D Cancer cells. Int J PharmTech Res. 2014-2015;7(2):238–42. Available from: https://api.semanticscholar.org/CorpusID:88916695
43. Ribeiro TG, Chávez Fumagalli MA, Valadares DG, Valadares DG, Franca JR, Lage PS, et al. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp Parasitol. 2014;143:60–8. CrossRef
44. Pereira JV, Freires IA, Castilho AR, da Cunha MG, Alves HDS, Rosalen PL. Antifungal potential of Sideroxylon obtusifolium and Syzigium and their mode of action against Candida albicans. Pharm Biol. 2016;54(10):2312–9. CrossRef