INTRODUCTION
Cancer remains one of the main causes of morbidity and mortality in the world [1], and although it has been considered one of the most preventable diseases, its incidence continues to show an increasing trend. In line with global trends, according to the GLOBOCAN database report for the year 2022, this pathology was the second cause of death exceeded only by cardiovascular diseases, accounting for nearly 10 million deaths by 2020 for men and women combined [2]. Global patterns show that lung cancer was the leading cancer accounting for 18% of all cancer deaths, followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers.
Although the drugs currently used in cancer therapy prevent the transformation of pre-malignant cells into adenomas, and ultimately into carcinoma, their use can lead to resistance, as well as significant systemic toxicity with effects such as neurotoxic, gastrointestinal disorders, alopecia, renal dysfunction, hypertension, and skin toxicity. In addition, most cancer patients eventually suffer from cachexia and anorexia during the course of the disease leading to metabolic dysfunction, suppression of the immune response, emaciation, weakness, and fatigue with a significant impact on quality of life [3]. In this scenario, promising therapeutic alternatives for treating various cancers have been investigated. The fruit of these efforts, emerging drugs, such as melatonin (MLT), which have demonstrated great potential to combat various types of cancer (Fig. 1A). MLT (N-acetyl-5-methoxy-triptamine), is an endogenous neurohormone synthesized by the pineal gland. MLT plays a very critical role in determining homeostasis, neurohumoral stability, and regulation of circadian rhythms in synergy with other hormones and neuropeptides. The biological properties of MLT range beyond circadian rhythm regulation. MLT has received increasing attention in recent years because of its versatile regulatory actions as a dietary supplement or medication growing in popularity in the pharmaceutical market as an antioxidant, anxiolytic, antihypertensive, sedative, analgesic anti-inflammatory, neuroprotective effects, and of particular interest in this paper, the ability to prevent, treat, and delay tumor development [4–6]. Regarding the relationship between the structure of MLT and its antitumoral effects, a recent SAR study has demonstrated that the N-acetyl side chain plays a crucial role in the antiproliferative and oncolytic effects of MLT because removal of the N-acetyl group produces an important loss of cytotoxic response. The role of the 5-methoxy group is to potentiate the antiproliferative effects of MLT, while the tryptamine core is mostly responsible for its anticancer benefits [7].
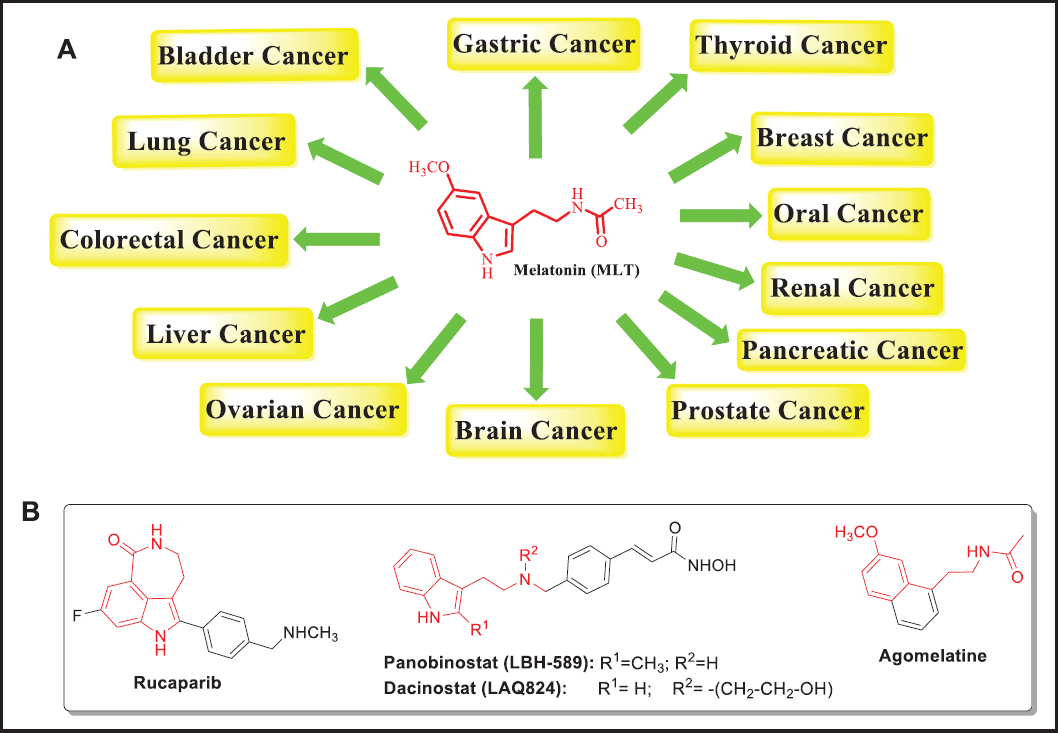 | Figure 1. (A). In vitro, in vivo, and clinical action of MLT against various types of cancer. (B). Structures of selected clinically approved drug molecules incorporating an MLT portion. [Click here to view] |
Regarding the biological mechanism, the promising therapeutic potential of MLT for many types of malignancies in vitro, in animal models, and in cancer patients, have been closely associated via the direct and indirect modulation of recognized cell-intrinsic proapoptotic mechanisms implicated in cancer progression (Fig. 2) [8–10]. From an initial commentary in 2004 in which MLT could modulate cancer initiation and progression [11], the cytotoxic effects exerted by MLT have inspired the development of potential antineoplastic agents incorporating key features of MLT. In fact, various of them have been approved by the Food and Drug Administration (FDA) (Fig. 1B). Thus, for example, in 2020 Rucaparib (Rubraca®), a conformationally restrained MLT-analog was approved for the treatment of metastatic prostate cancer and recurrent ovarian carcinomas. Likewise, recently Dacinostat and Panobinostat were approved as chemotherapeutics agents in the treatment of prostate/breast cancer and multiple myeloma, respectively. Moreover, the naphthalenic bioisostere of MLT, Agomelatine, an FDA-approved antidepressant, has been also suggested recently as a new repurposed strategy for the treatment of colorectal cancer [12].
In addition to its anti-tumoral benefits, mounting evidence has indicated that MLT could be used as an adjuvant or protective anticancer drug in current chemotherapeutic regimes. In the first case, it was reported that MLT is able to synergize the chemotherapeutic effects of the anti-tumor drugs 5-FU or oxaliplatin in metastatic cancer [13–16]. Second, MLT has shown relevant protective benefits in clinical trials against the detrimental injuries of chemotherapy drug-induced toxicity in patients with cancer [17–21]. Moreover, convincing evidence has shown that in cancer patients daily intake of oral MLT before bedtime improves the quality of sleep, and reduces the incidence of depressive symptoms [22–24]. Despite MLT being currently considered as a new and promising molecule that could be used in clinical oncological intervention, there are serious limitations due to its bioavailability, oral absorption, and rapid metabolism. When MLT was administered orally in normal healthy volunteers, it showed a poor absolute bioavailability of less than 3% while the intravenous MLT administration revealed serum values ranging between 9% and 33 % [25,26]. Recent research studying alternative routes for enhancing the pharmacokinetic response of MLT has been envisaged for cancer therapy, among them nanostructured lipid encapsulation [27], soft gel encapsulation [28], bioisosteric modifications on MLT structure, or through the design of large library of MLT-derivatives. In addition to these approaches, MLT also fulfills most of the requirements of a typical lead compound for rational drug design. In fact, from 1998 to nowadays, MLT has emerged as a promising structural prototype for developing hybrids and derivative molecules, particularly with anticancer benefits in both in vitro and in vivo models [29]. These efforts have led to a vast number of compounds containing an MLT portion, most of them have demonstrated to have not only potent antiproliferative and marked cytotoxic effects against the most common types of cancer, but are also more active than MLT. Findings, limitations, arguments, and comparison of derivatives and hybrids incorporating MLT will be pointed out in the discussion section.
 | Figure 2. The most common pro-apoptotic mechanisms by which MLT mitigates cancer at the initiation, progression, and metastasis phases. [Click here to view] |
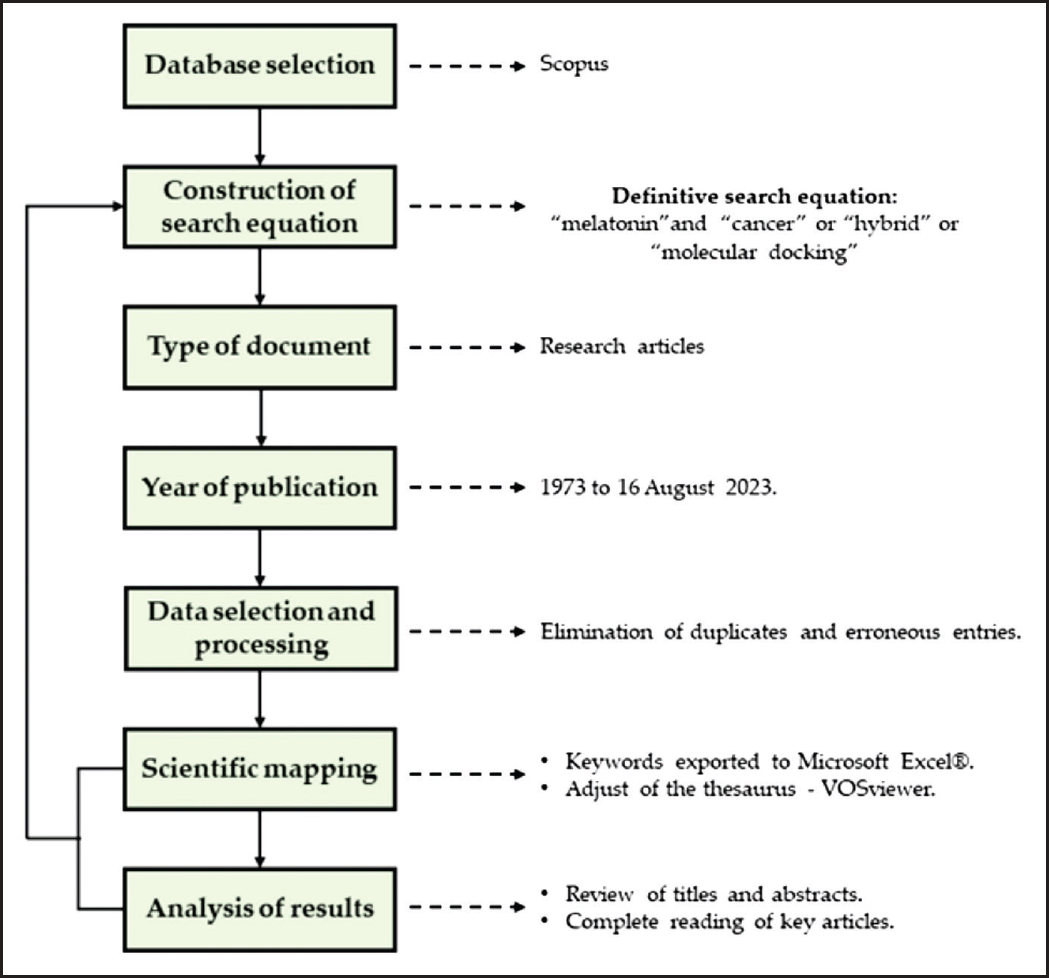 | Figure 3. Methodological scheme for bibliometric analysis studies. [Click here to view] |
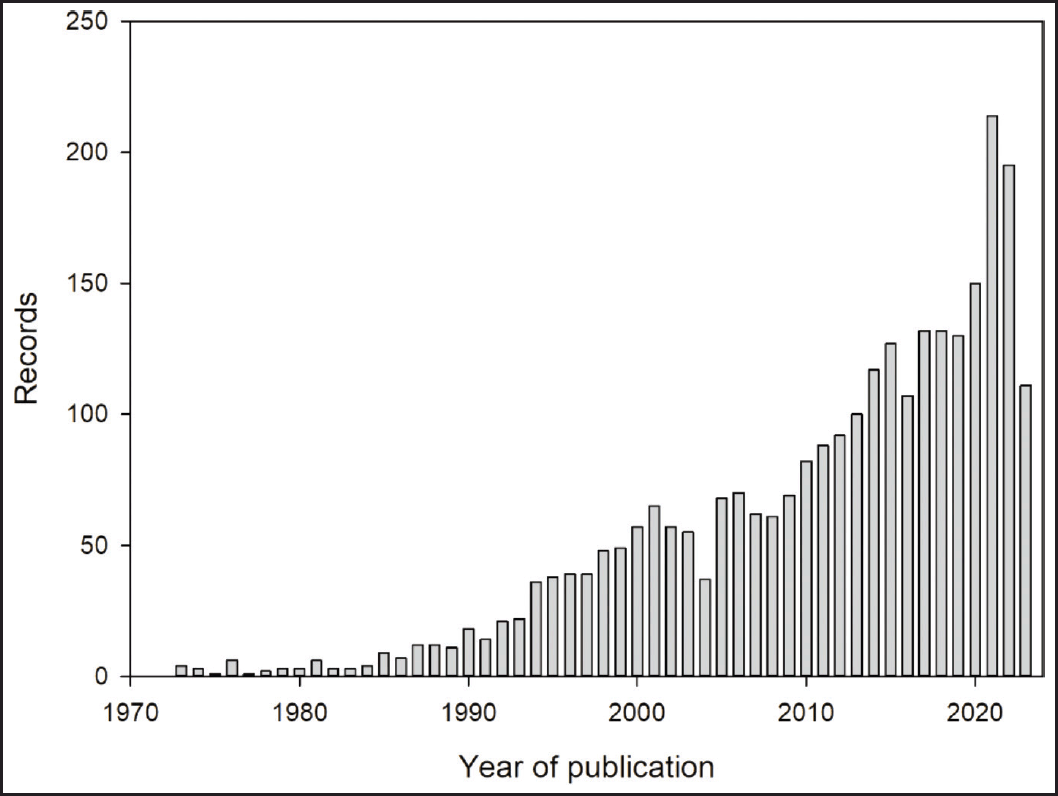 | Figure 4. Evolution of published studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. [Click here to view] |
 | Figure 5. Top 10 areas of knowledge related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. [Click here to view] |
MLT is synthesized in almost all living organisms including yeasts, bacteria, plants, and animals; however, the levels of MLT are significantly lower in nature. Due to this limitation and the current extensive pharmaceutical use and application of MLT in cancer, an economical large-scale production of MLT is necessary. Consequently, novel synthetic pathways have been proposed for the production of MLT via cheap and commercially available starting materials. Currently, simple synthetic methods have been devised for the preparation of MLT basically taking a compound containing an indole ring as synthon [30,31].
Taken altogether, MLT and its derivatives and hybrids showed high significance toward the development of new cancer drugs because of its diverse and potent oncolytic properties. A vast volume of information in the scientific literature suggests that MLT, its hybrids, and its derivatives are currently hotspots in many areas of research, mainly cancer. Because of that, a systematic approach that allows valuable insights into the quantitative aspects of scientific literature, including trends, emerging topics, and the evolution of research over time, is an alternative for presenting a general overview of MLT. Bibliometric analysis is a technique that helps to provide a macroscopic overview of large amounts of scientific literature, as is the case of MLT. This information can be used to assess the research patterns during a defined timespan. In the case of MLT, there are several reviews available, including studies as a promising anticancer drug lead [9,32]. Nevertheless, and to the best of our knowledge, a bibliometric analysis of MLT regarding cancer is still lacking. Therefore, the aim of this study is to carry out a bibliometric analysis of MLT and their derivatives and hybrids in cancer.
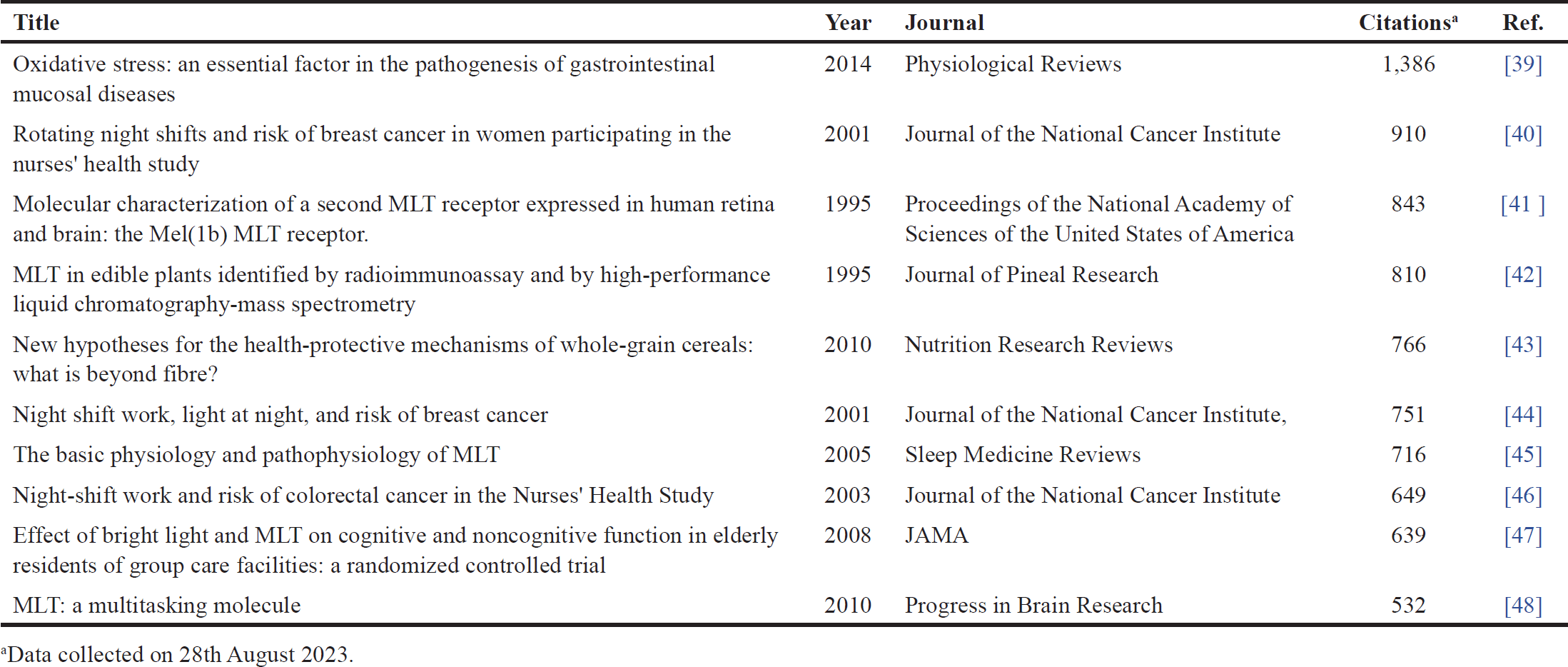 | Table 1. The top 10 most cited studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. [Click here to view] |
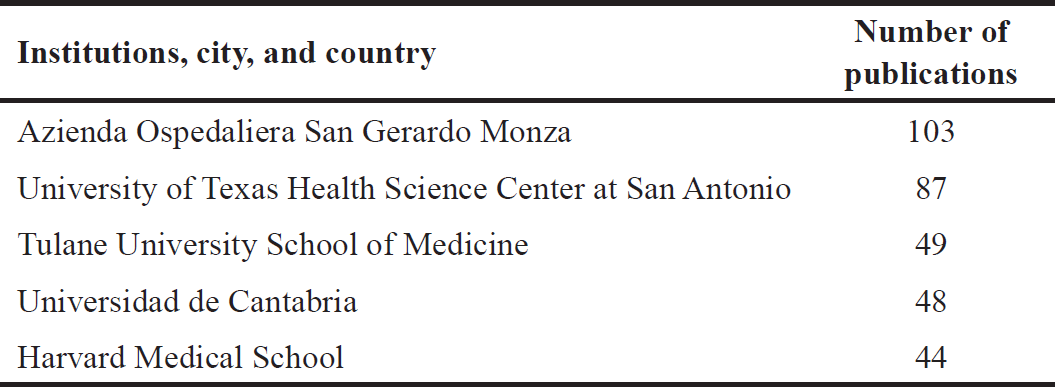 | Table 2. The top five institutions publishing studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. [Click here to view] |
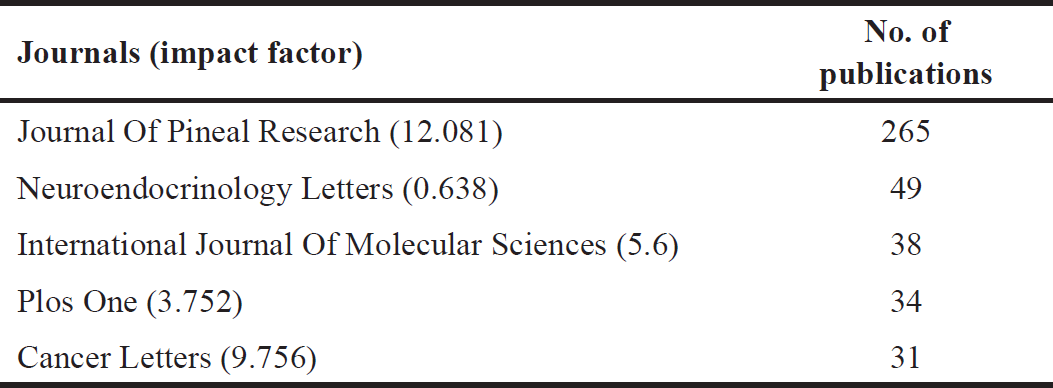 | Table 3. The top five journals publishing studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. [Click here to view] |
METHODS
Database selection and search strategy
This study has imported/collected data from the Scopus database (on 16 August 2023), which is one of the largest databases of peer-reviewed literature worldwide in the field of science and technology. The retrieved bibliographic data contains the most relevant studies related to both MLT and cancer as the main topics. The search equation was designed using the terms “melatonin,” “cancer,” “hybrid,” and “molecular docking” and connecting them by using the Boolean operators “AND” and “OR”: The final query was as follows:
Query: ((TITLE-ABS-KEY (melatonin) AND TITLE-ABS-KEY (cancer) OR TITLE-ABS-KEY (hybrid) OR TITLE-ABS-KEY (“molecular docking”)) AND (LIMIT-TO (DOCTYPE,”ar”))).
Timespan: 1973 to 16 August 2023.
The search equation was queried within the fields of article title, abstract, and keywords, and further refined to only articles. Elimination of duplicate records was conducted by the medeley reference manager to address limitations or potential biases in the bibliometric analysis. See [33–37] for further details of the bibliometric analysis methodology. Figure 3 shows a scheme of the methodology applied during this study.
Data export and bibliometric indicators
The bibliographic data retrieved for the research articles were abstract, keywords, and both citation and bibliographical information. Data were downloaded from Scopus in CSV format and exported to Excel®. Subsequently, VOSviewer 1.6.19 was used for data analysis and visualization [38]. In addition, bibliometric indicators such as the volume and growth of issued articles, subject areas, co-occurrence keywords network visualization, co-occurrence keywords overlay visualization, leading institutions and journals (and their impact factors), the top 10 most cited studies, and collaboration networks among the countries, were evaluated.
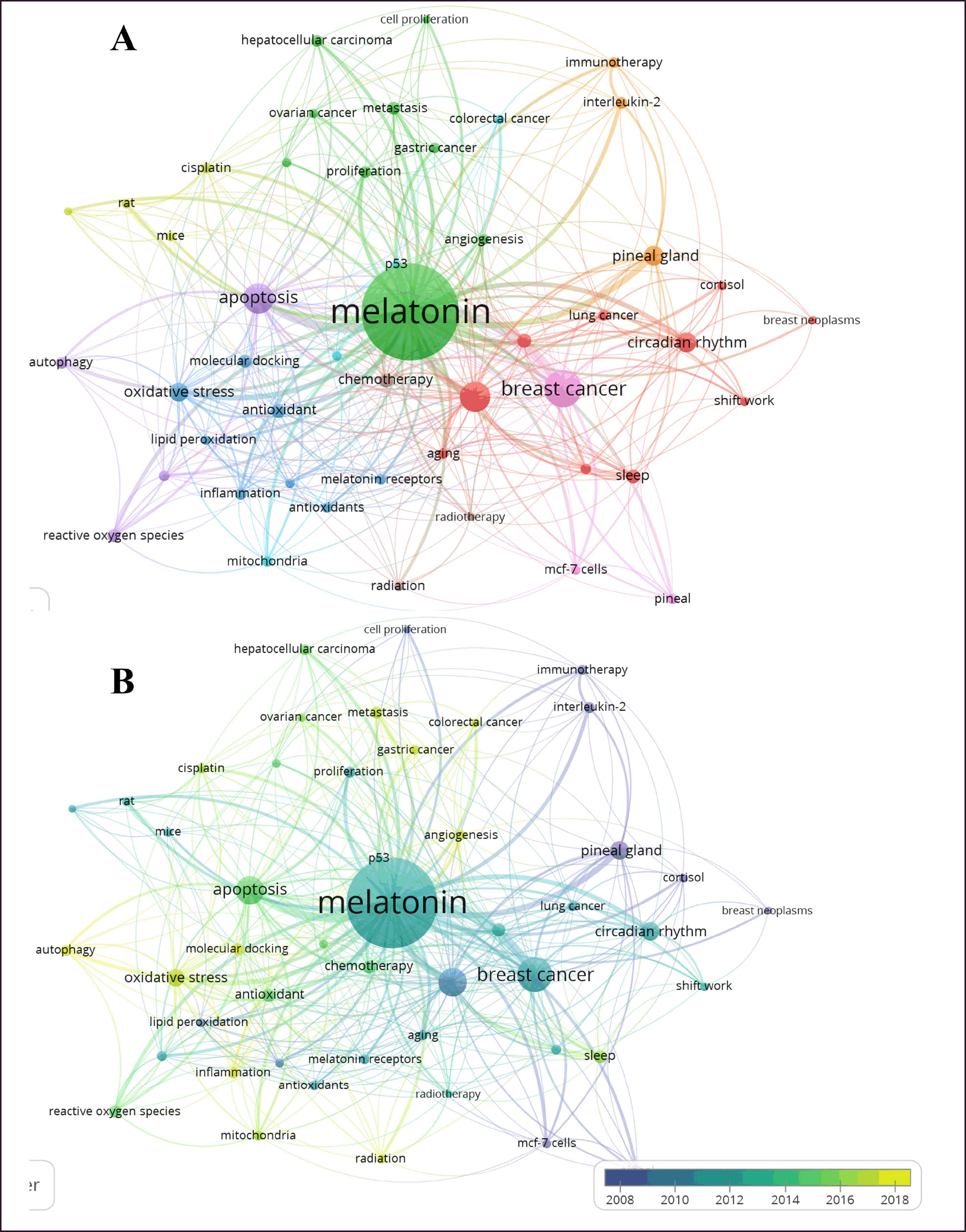 | Figure 6. Bibliometric network of studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. A) Research-topic map. B) Research-topic map with time overlap. The nodes represent the author´s keywords while the size of the circle indicates their occurrences (i.e., the number of publications that have the corresponding term in their title or abstract). In addition, the width of the line linking them is proportional to the strength of the relationship between the author´s keywords, and the distance between them indicates the relatedness of the nodes. Note: minimum number of occurrences of a keyword is 18. [Click here to view] |
 | Figure 7. Bibliometric map of global collaboration network among countries researching on MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. A) Global collaboration network among countries. B) Global collaboration network among countries with time overlap. The nodes represent the countries while the size of the circle denotes the number of publications. In addition, the width of the line linking them is proportional to the strength of the relationship between countries, and the distance between them indicates the relatedness of the nodes. The minimum number of documents in a country is 10. [Click here to view] |
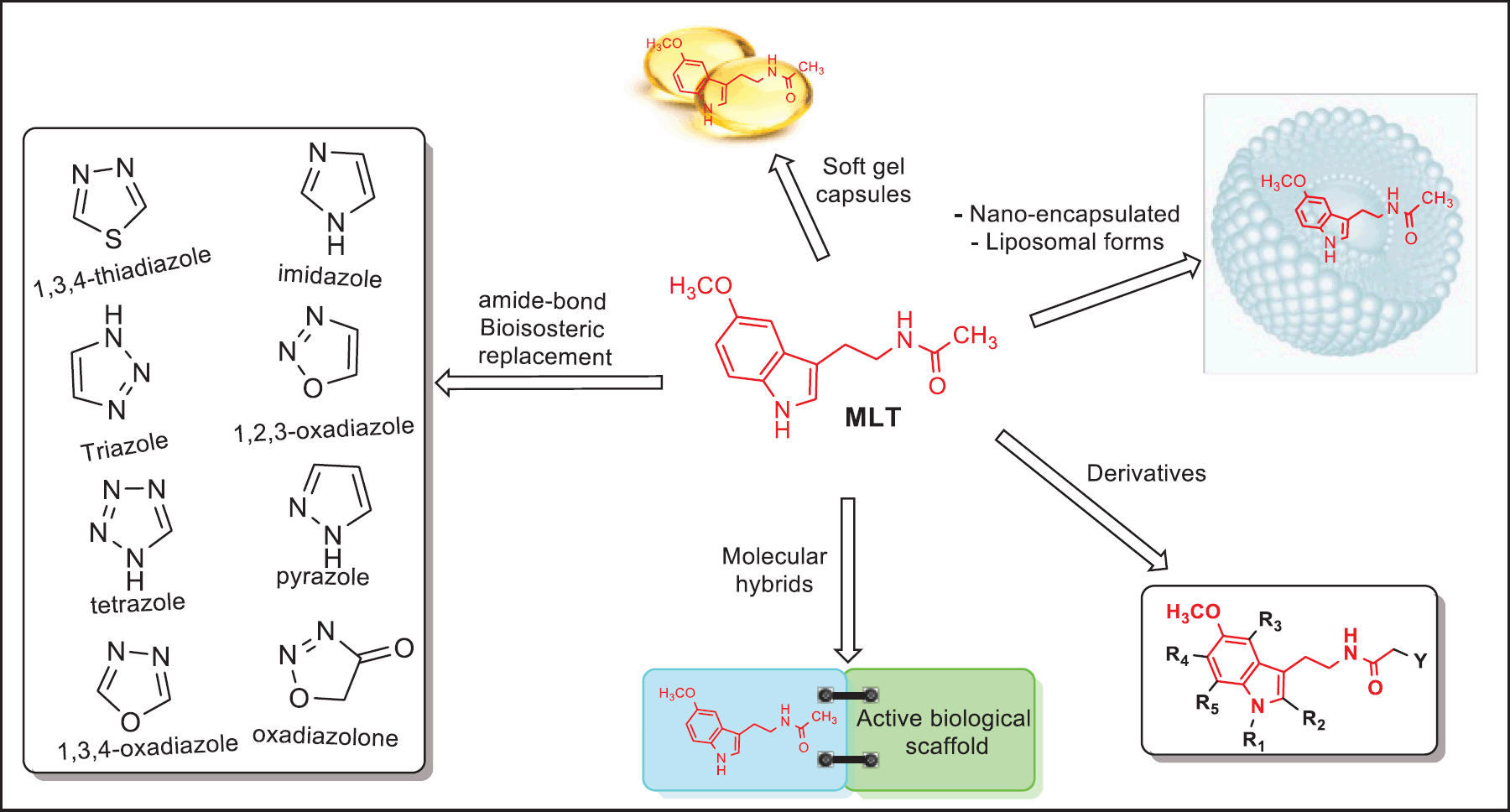 | Figure 8. Different approaches for improving MLT pharmacokinetics properties: molecular hybridization, structure modification (derivatives), bioisosterism, nanotechnology, and softgel capsules. [Click here to view] |
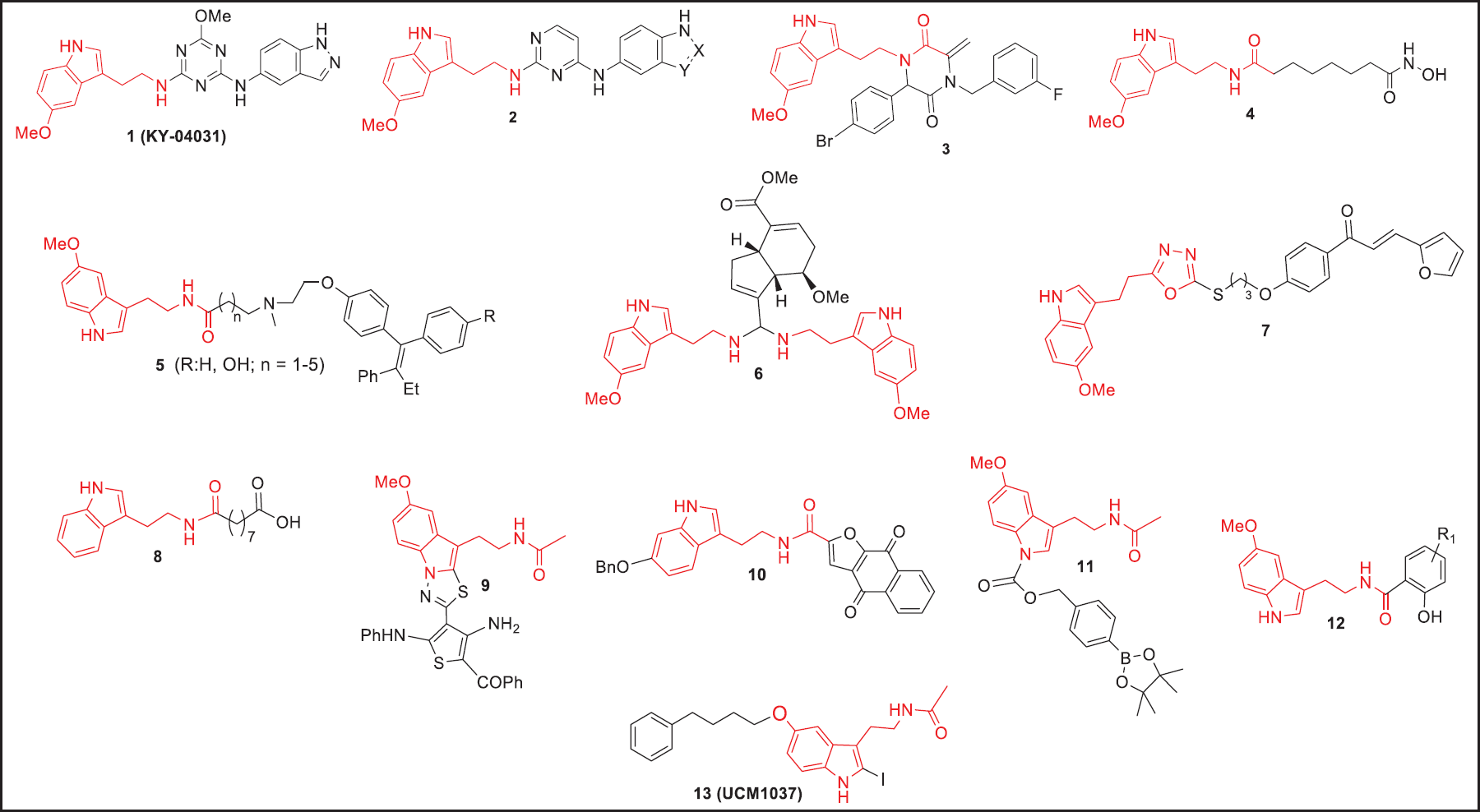 | Figure 9. Molecular structures of highly potent derivatives and hybrid molecules incorporating MLT as emerging drugs for cancer intervention. [Click here to view] |
RESULTS
Evolution of publishing papers related to MLT
A total of 2,792 articles were retrieved from the scopus database. Figure 4 shows the growth of published studies related to MLT, cancer, hybrid, and molecular docking from 1973 to 16 August 2023. During this period, an increase in the number of articles issued was observed since 2004. Besides, the main areas of knowledge of those studies were: (i) Biochemistry, Genetics, and Molecular Biology, (ii) Medicine, and (iii) Pharmacology, Toxicology, and Pharmaceutics, with 33.9%, 32.4% and 10.1% of the records, respectively (Fig. 5). Furthermore, the United States and China ranked first and second, respectively, followed by Italy, Spain, and Germany as the leading countries in the numbers of published articles in this field.
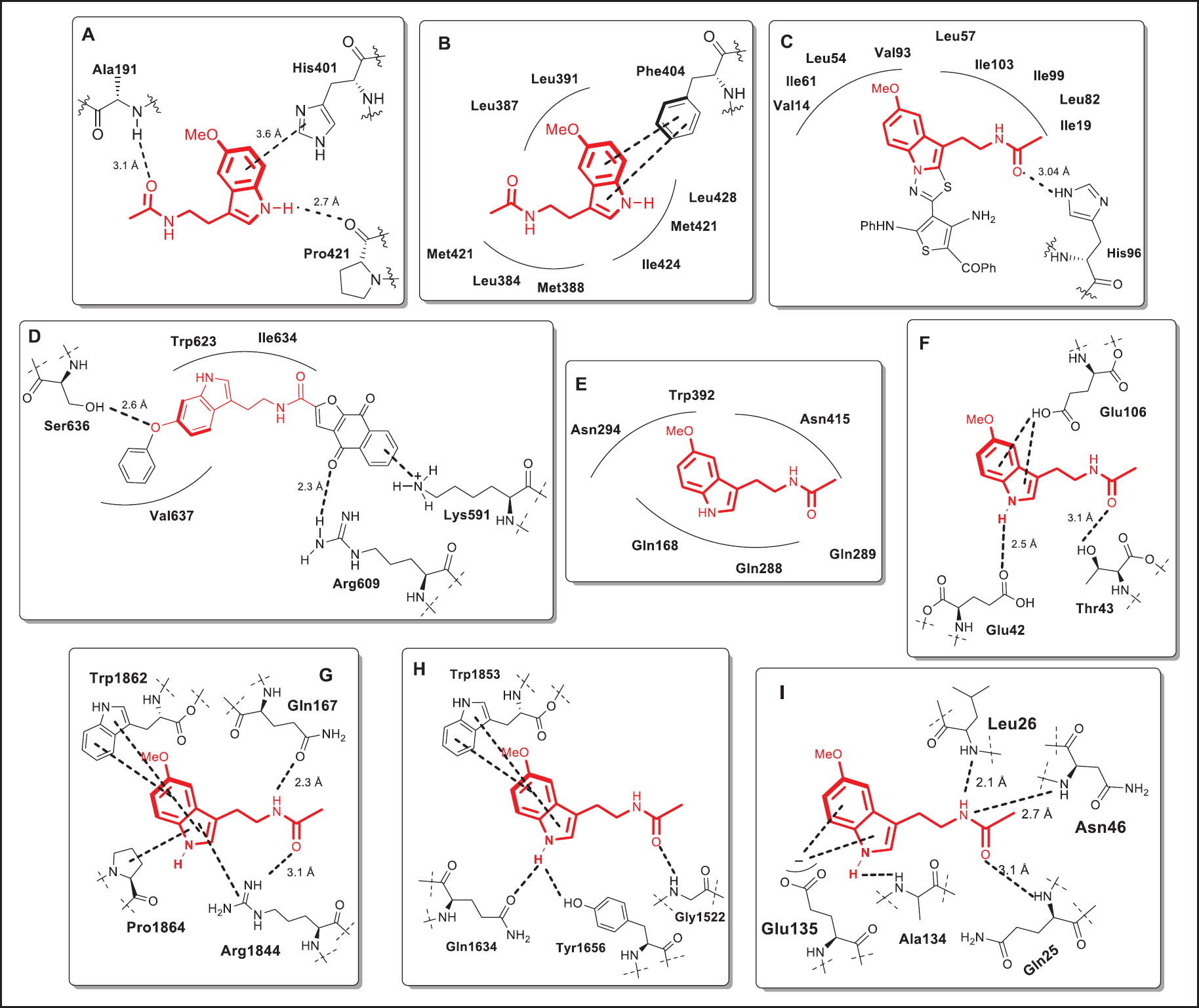 | Figure 10. 2D-ligand interaction plots after docking studies for MLT, its hybrids, and its derivatives within the active site of five critical target-proteins associated with the initiation and progression of tumors. A) Binding of MLT with MMP-9. B) Binding of MLT with ESR1. C) Binding of MLT with MDM2. D) Binding of MLT with STAT3. E) Binding of MLT with GLUT1. F) Binding of MLT with Ile6. G) Binding of MLT with TP53. H) Binding of MLT with mTOR. I) Binding of MLT with TNF-α. [Click here to view] |
Journals, institutions, and publications with the highest impact
Table 1 shows the top 10 cited studies related to m MLT elatonin, cancer, hybrid, and molecular docking, while Table 2 displays the top five leading institutions in this field. Here, and as expected, the leading institutions are in the top countries previously mentioned (Italy, the United States, and Spain). Furthermore, Table 3 shows the leading journals that issued the articles on this topic, and their impact factor.
Bibliometric networks of MLT, cancer, hybrid, and molecular docking
The co-occurrence of the author’s keywords network contains nine clusters and 48 nodes (Fig. 6A). Among them, ovarian cancer, gastric cancer, colorectal cancer (green and light blue clusters), breast cancer (pink cluster), lung cancer (red cluster), chemotherapy (brown cluster), inflammation, and molecular docking studies (blue clusters) are highlighted. In addition, Figure 6B shows the topics on which the researchers centered their studies between 2008 and 2018. It is noticeable that in 2018 the studies concentrated on molecular docking and inflammation (see yellow nodes in Fig. 6B). Thus, this could indicate that they are emerging topics in this field. Furthermore, Figure 7 shows the countries’ collaboration networks. Clearly, the United States, China, Italy, Spain, and Germany displayed the biggest nodes in this network. Figure 7B shows a research-topic map with time overlap. Here, China, Iran, Iraq, Saudi Arabia, and Egypt are the leading countries between 2018 and 2020. Based on the described results, and thanks to bibliometric indicators, the discussion of this study was concentrated on molecular docking studies of MLT and their anticancer activities against several types of cancer.
DISCUSSION
Pharmacokinetic limitations of MLT inspired its hybrids and its derivatives
Undoubtedly, credible evidence has proven that MLT possesses a range of beneficial effects against cancer. Nevertheless, MLT exhibits a serious pharmacokinetic limitation mainly because of its poor oral bioavailability and short half-life, as a result of its rapid metabolic inactivation, thereby restraining its therapeutic use [49]. There is conflicting evidence on the pre- and clinical efficacy of exogenous MLT. The pharmacokinetic properties of MLT have been extensively studied in critically ill patients, evidencing that following oral and intravenous administration, the bioavailable amount of MLT was generally low ranging from 3% to 33% critically affecting the drug response critically [50,51]. In line with this, many strategies have been rationalized to facilitate selective applications in medicine and improve the pharmacokinetic properties of MLT (Fig. 8). Interestingly, these novel approaches have successfully prolonged the release, efficacy, and safety of MLT. In this regard, first, the acetamido group of MLT has been bioisosterically replaced by classical amide bond bioisosteres units such as 1,2,3-triazole, 1,n,ω-oxadiazole, oxadiazolone, 1,3,4-thiadiazole, imidazole, tetrazole, and pyrazole [52–54]. In addition to their classical bioisosteres, the pharmacokinetics of alternative administration routes such as nano encapsulated, liposomal forms, and soft gel capsules of MLT are now emerging exhibiting superior effectiveness in many pathological processes [28,55,56,27,57]. Finally, another point of special interest in this bibliometric analysis is the structure of MLT has been used in medicinal chemistry as a pharmacophoric motif for the design of new potential derivatives and hybrid compounds for anticancer intervention which have shown to possess potent in vitro and in vivo anti-tumoral effects in a variety of malignancies [58,59]. In this scenario, numerous investigations, reports, and articles have demonstrated that the incorporation of an MLT portion in diverse active cores increases the effectiveness (Fig. 9). These efforts have highlighted MLT-triazino, -pyrimido, and -piperazine-2,5-dione conjugates, 1 (KY-0403), 2, and 3, respectively, with potent cytotoxic responses against prostate, lung, colon, breast, and pancreatic cancer cells [60–62]. A recent investigation in MCF-7, PC-3M-Luc, and HL-60 cancer cell lines treated with the MLT–vorinostat hybrid 4 showed strong oncostatic action which is driven by the histone deacetylase (HDAC) inhibition [63]. MLT was also linked to the antitumoral FDA-approved Tamoxifen given hybrids 5, which exhibited potent anticancer activity (2.2 nM–3.0 pM) in a mouse model [64]. Furthermore, its ability to alter tumor cell metabolism was evidenced when MLT was combined with the anticancer natural occurrent monoterpenoid Genipin to afford the hybrid ligands 6, which exhibited potent cytotoxic activities through induction of apoptosis and arrested the cell cycle in five cancer cell lines (SW-480, A-549, HL-60, SMMC-7721, and MCF-7) with IC50 ranging from 0.90 μM to 0.43 μM [65]. More recently, it was reported that a series of hybrids containing the oxadiazole-bioisostere MLT and chalcones showed a potent antiproliferative effect against SW480 human colon cancer cell, highlighting the compound 7, which displayed an IC50 value very close to 260 nM [53]. In this same line, it was reported that the MLT-derivative 8 was very potent against the human colorectal adenocarcinoma HT29 cell line revealing an IC50 = 6 nM [66]. In 2021, novel MLT derivatives were also reported, particularly compound 9, which possesses valuable cytotoxic effects against two human cancer cell lines, breast cancer (MCF7) and colon cancer (HCT-116) with an IC50 value ranging from 11 to 37 μM [59]. Taking advantage of the oncostatic benefits of MLT, recently the Zhang Group and collaborators reported a new series of hybrids incorporating in the core structure of MLT a portion of napabucasin, an FDA-approved drug for treating patients with metastatic colorectal cancer. Of those hybrids, compound 10 showed greater growth inhibitory effects against different tumor cells (HepG2, MDA-MB-231, and A549) than napabucasin, with IC50 values of 1.06, 1.38, and 1.3 µM, respectively [67]. Furthermore, it was reported that hybrids of MLT containing ROS-responsive arylboronate 11 portions exhibited strong toxic effects in cervical cancer cells (HeLa) [68]. Potential anticancer benefits of MLT-salicylic acid hybrids 12 were also investigated against a human gastric carcinoma MGC-803 cell line, showing marked antiproliferative and cytotoxic activity with IC50 in the range of 43 to 62 µM [69]. The antiproliferative and pro-apoptotic activity of MLT analogs was also demonstrated against melanoma and breast cancer in both in vitro and in vivo models, that, particularly compound named UCM 1037 (13) induced apoptosis on human breast cancer cell line (MCF-7), and caused suppression of tumor growth in a mice model [70].
Biochemical mechanism of MLT, its hybrids, and derivatives: highlights and computational studies
As mentioned above, MLT, its hybrids, and its derivatives provide a new direction in the development of new antineoplastic agents. Recent experimental evidence has revealed that this valuable proapoptotic action of MLT as well as of these MLT-based compounds on tumor cells could be strongly related to targeting specific receptors mainly involved in cancer cell proliferation and survival (Fig. 2). Therefore, including MLT, numerous investigations have unequivocally demonstrated that these molecules are capable of modulate at least 19 proapoptotic mechanisms, thus, 1) by acting as a free radical scavengers and antioxidant at the mitochondrial level [71,72]; 2) by helping to regulate the immune system [73] activating the T cells by increasing the secretion of interleukin-2, interleukin-10 [73,74]; 3) in affecting DNA damage response by interacting with the direct mediators of cell cycle arrest, apoptosis and autophagy [75–77]; 4) by downregulating the amounts of vascular endothelial growth factor (VEGF) [78]; 5) disrupting the p53/MDM2 complex formation by inhibiting the phosphorylation of MDM2 [59,79,]; 6) inhibiting the progression of gastric cancer by direct binding to the estrogen receptor 1 (ESR1) [80]; 7) in suppressing lung adenocarcinoma cells growth by targeting HDAC1 signaling [81]; 8) by inhibiting directly the activation of the signal transducer and activator of transcription 3 (STAT3) in metastatic colorectal cancer, pancreatic cancer, non-small cell lung cancer, and gastric cancer [67]; 9) on human prostate cancer, promoting cell death through a competitive inhibition for the binding sites into the glucose transporter 1 (GLUT1) [82,83]; 10) by enhancing the BAX expression, reducing Bcl-xL production and activating caspase-3 and caspase-9 [84]; 11) by suppressing the activation of the nuclear factor κB (NF-κB) signaling [85]; 12) in decreasing breast cancer metastasis by modulation of Rho-associated kinase protein-1 expression [86]; 13) by suppressing the MMP-13 expression in metastatic prostate cancer cells [87]; 14) by interfering with MMP-9 activity through a direct docking into the active site of MMP-9 [88]; 15) in reducing the expression of cyclins and CDKs (D1, CDK4, cyclin B1, and CDK1) in human osteosarcoma cells [89]; 16) by inhibiting breast cancer cell lines through a suppressive role on aromatase activity [90,91]; 17) by exerting a potent and sustained anti-angiogenic action by inhibiting HIF-1α and VEGF expression [10, 92–95]; 18) by regulating the epigenetic response in altering the status of DNA methylation in different cancer cells and models, which is nowadays a potent and effective strategy for treatment of cancer patients [96]; and 19) in preventing survival of neoplastic cells mimicking the role of sexual hormones on hormonal receptors, particularly in the cell proliferation of prostate, breast, and ovarian cancer cells [97–100].
More specifically, activation of these above-mentioned pro-apoptotic mechanisms by MLT occurs through ligand binding to specific receptors. Various studies that included enzymatic and molecular docking protocols have been carried out to understand at the molecular level the anti-tumoral role of MLT. The first of these results revealed that MLT is able to interfere with the matrix metallopeptidase 9 (MMP-9) activity by interacting with key residues within the catalytic site of MMP-9 (Fig. 10A) affecting the catalytic contribution of the histidine-coordinated zinc-binding site, as well as two surrounded crucial amino acid residues (Pro421 and Ala191) [88]. MMP-9 is a proteolytic enzyme that is implicated in diverse roles in the growth and proliferation of malignant cells in the host organ. Second, based on molecular docking, it was also evidenced that MLT could inhibit the progression of gastric cancer by blocking the ESR1 with a binding affinity of 7.1 kcal/mol making key contacts with those crucial catalytic residues in the ESR1-active site (Fig. 10B). Currently, ESR1 is a crucial target to fight gastric cancer [80]. Third, in breast cancer, another computational study has evidenced that anticancer activity of MLT and MLT-based derivatives would be closely related to inhibition of the MDM2, by interacting with those crucial residues in the p53-binding pocket of MDM2 in a manner sufficient to disrupt the MDM2 and p53 interaction and enhance the functions of p53 (Fig. 10C) [59,79]. Blocking the MDM2–p53 interaction has become a new direction to combat different human cancers.
Fourth, recent studies have also evidenced that MLT-derived possess a repressive effect on the coding function of the STAT3 protein, by binding into the STAT3-SH2 domain through two hydrogen bonds with key Ser636 and Arg609 residues, one cation-π contact (with Lys591 residue), and several hydrophobic interactions (Fig. 10D). Targeting STAT3 activity has emerged as a rationale drug target to combat tumor progression in most human cancers involving organs such as the pancreas, brain, breast, ovaries, and prostate, among others [101]. Fifth, to confirm the potential mechanism by which MLT represses the growth of human prostate cancer cells, in vitro, enzymatic followed by molecular docking studies also evidenced that MLT is able to reduce the uptake of glucose by blocking the glucose-binding site into the GLUT1 receptor making key interactions with those amino acids residues (Fig. 10E) crucial for facilitating glucose transportation (Gln168, Gln288, Gln289, Asn294, Trp392, and Asn415) [83]. In prostate cancer, GLUT1 is overexpressed, then compounds acting as competitive inhibitors for the glucose-binding site of GLUT1 could become promising drug candidates to be used in prostate cancer intervention [102]. Finally, target analysis and molecular mechanism studies suggested that MLT would mitigate cancer by directly binding to at least four other proapoptotic target proteins, as reported recently by Suriagandhi and collaborators [103]. Thus, MLT would interact with good binding affinity to IL-6 (Fig. 10F), TP53 (Fig. 10G), mTOR (Fig. 10H), and TNF-α (Fig. 10I) receptors, which are overexpressed in many types of cancer cells and play a critical role in promoting tumor initiation and progression.
FUTURE PERSPECTIVES
Global collaboration
Given the interdisciplinary nature of cancer research and its impact on society, future endeavors may involve increased collaboration between countries, researchers, clinicians, and industry partners on a global scale. Open sharing of data could accelerate progress in the field. In addition, data science could play a crucial role in advancing the studies of MLT. Data integration and mining (omics data integration), bioinformatics analysis (pharmacogenomics), and machine learning for prediction could be useful.
Development of novel derivatives and hybrids
Future research may involve the design and synthesis of novel MLT derivatives and hybrids with enhanced bioavailability, stability, and targeted delivery properties. This could eventually improve the pharmacokinetics and therapeutic potential of MLT -based anti-cancer agents.
Preclinical and clinical trials
As promising preclinical results accumulate, more emphasis may be placed on conducting rigorous clinical trials to assess the safety and efficacy of MLT derivatives and hybrids in diverse cancer populations.
CONCLUSION
From an initial commentary in 2004 in which MLT could modulate cancer initiation and progression, it has seen how MLT its hybrids, and its derivatives have gained special interest in the scientific community. Our bibliometric analysis revealed that:
i) A total of 2792 articles related to MLT and MLT-derived compounds have been reported exhibiting valuables anticancer properties issued in journals indexed in the Scopus database from 1973 to 16 August 2023.
ii) A clear rise in the number of articles publishing MLT in cancer was observed from an initial commentary in 2004.
iii) The main areas of knowledge of those records were: Biochemistry, genetics and molecular biology, medicine and pharmacology, toxicology, and pharmaceutics, while the United States, China, Italy, Spain, and Germany are the leading countries in the numbers of published articles.
iv) It was found that MLT and MLT-derived compounds possess potent responses against at least 13 cancer cell lines (gastric, thyroid, breast, oral, renal, pancreatic, prostate, brain, ovarian, liver, colorectal, lung, and bladder cancers) in both in vitro and in vivo models.
v) Current advances in molecular, biochemical, and computational analysis have documented that the antitumoral benefits provided for MLT, its hybrids, and derivatives not only is caused by their well-known antioxidant properties but it also has been well-documented that these compounds are able to modulate o to interfere with at least 19 proapoptotic signaling pathways.
vi) Three compounds incorporating an MLT moiety have recently received approval by the FDA as chemotherapeutics for clinical use in metastatic prostate cancer, breast cancer, and multiple myeloma treatments.
vii) Regarding MLT and MLT-derived compounds, the present network analysis also revealed that apoptosis, chemotherapy, breast cancer, gastric cancer, colorectal cancer, prostate cancer, and molecular docking studies are emerging topics in this field.
From these bibliometric findings, undoubtedly MLT, its derivatives and hybrids possess great potential to be considered in new avenues toward cancer research and treatment. More importantly, bibliometric results covering the scenario of MLT, derivatives, and hybrids in cancer, play a significant role in identifying the value of these scaffolds in future research directions aimed at developing new anti-cancer drug candidates.
ACKNOWLEDGMENTS
The authors thank the University of Antioquia (grant CODI 2020-34590; Código BUPP ES84200123)) for financial support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
INFORMED CONSENT
For this type of study, formal consent is not required.
REFERENCES
1. World Health Organization. Cancer fact sheet [Internet]. Geneva, Switzerland: World Health Organization; 2022 [cited 2023 May 20]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
2. Global Cancer Observatory. Cancer fact sheet/all cancers [Internet]. Lyon, France: Global Cancer Observatory; 2020 [cited 2023 May 20]. Available from: https://gco.iarc.fr/
3. Gupta A, Eisenhauer EA, Booth CM. The time toxicity of cancer treatment. J Clin Oncol. 2022;40(15):1611–5. CrossRef
4. Baranowska A, Matyka K, Matyszewska A, Slowiaczek A, Sutkowski J, Wilczewska E, et al. Melatonin—the new multipotential drug of the future? J. Edu. Health and Sport. 2023;13(4):330–8. CrossRef
5. Pohanka M. New uses of melatonin as a drug; a review. Curr Med Chem. 2022;29(20):3622–37. CrossRef
6. Mehrzadi S, Pourhanifeh MH, Mirzaei A, Moradian F, Hosseinzadeh A. An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 2021;21:188–205. CrossRef
7. Letra-Vilela R, Sánchez-Sánchez AM, Rocha AM, Martin V, Branco-Santos J, Puente-Moncada N, et al. Distinct roles of N-acetyl and 5-methoxy groups in the antiproliferative and neuroprotective effects of melatonin. Mol Cell Endocrinol. 2016;434:238–49. CrossRef
8. Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI. Melatonin in cancer treatment: current knowledge and future opportunities. Molecules. 2021;26:2506–15.
9. Wang L, Wang C, Choi WS. Use of melatonin in cancer treatment: where are we? Int J Mol Sci. 2022;23:3779–93. CrossRef
10. Goradel NH, Asghari MH, Moloudizargari M, Negahdari B, Haghi-Aminjan H, Abdollahi M. Melatonin as an angiogenesis inhibitor to combat cancer: mechanistic evidence. Toxicol Appl Pharmacol. 2017;335:56–63. CrossRef
11. Reiter RJ. Mechanisms of cancer inhibition by melatonin. J Pineal Res. 2004;37(3):213–4. CrossRef
12. Moreno-SanJuan S, Puentes-Pardo JD, Casado J, Escudero-Feliu J, Khaldy H, Arnedo J. et al. Agomelatine, a melatonin-derived drug, as a new strategy for the treatment of colorectal cancer. Antioxidants (Basel). 2023;12(4):926–41. CrossRef
13. Mafi A, Rezaee M, Hedayati N, Hogan SD, Reiter RJ, Aarabi MH, et al. Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun Signal. 2023;21:33–59. CrossRef
14. Adeya C, Siregar MFG, Putra IB, Hasibuan PA, Andrijono A, Bachtiar A, et al. Promising effect of cisplatin and melatonin combination on the inhibition of cisplatin resistance in ovarian cancer. F1000Res. 2023;12:313–26. CrossRef
15. Polat S, Topal H, Aydemir CN, Erbas E, Kara A. Melatonin and cisplatin synergistically enhance apoptosis via autophagy-dependent alteration of P53 transcription in human colorectal cancer cells. Eurasian Mol Biochem Sci. 2022;1(2):11–8. CrossRef
16. Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal Res. 2017;62(2):321–40. CrossRef
17. Aboelwafa HR, Ramadan RA, El-Kott AF, Abdelhamid FM. The protective effect of melatonin supplementation against taxol-induced testicular cytotoxicity in adult rats. Braz J Med Biol Res. 2022;55:11614. CrossRef
18. Karvan S, Sadeghi A, Farrokhi P, Nekouee A, Sharifi M, Moghaddas A. Melatonin in the prevention of cisplatin-induced acute nephrotoxicity: a randomized, controlled clinical trial. Res Pharm Sci. 2022;17(2):176–88. CrossRef
19. Xing F, Wang M, Ding Z, Zhang J, Ding S, Shi L. et al. Protective effect and mechanism of melatonin on cisplatin-induced ovarian damage in mice. J Clin Med. 2022;11(24):7383–99. CrossRef
20. Ma Z, Xu L, Liu D, Zhang X, Di S, Li W, et al. Utilizing melatonin to alleviate side effects of chemotherapy: a potentially good partner for treating cancer with ageing. Oxid Med Cell Longev. 2020;2020:6841581–99. CrossRef
21. Haghi-Aminjan H, Farhood B, Rahimifard M, Didari T, Baeeri M, Hassani S, et al. The protective role of melatonin in chemotherapy-induced nephrotoxicity: a systematic review of non-clinical studies. Expert Opin Drug Metab Toxicol. 2018;14(9):937–50. CrossRef
22. Seo K, Kim JH, Han D. Effects of melatonin supplementation on sleep quality in breast cancer patients: a systematic review and meta-analysis. Healthcare (Basel). 2023;11(5):675–701. CrossRef
23. Etedali A, Hosseni AK, Derakhshandeh A, Mehrzad V, Sharifi M, Moghaddas A. Melatonin in the management of mood and sleep problems induced by androgen deprivation therapy in prostate cancer patients: a randomized double-blinded, placebo-controlled clinical trial. Innov J Pharm Res. 2022;21(1):128817–39. CrossRef
24. Innominato PF, Lim AS, Palesh O, Clemons M, Trudeau M, Eisen A, et al. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support Care Cancer. 2016;24(3):1097–105. CrossRef
25. DeMuro RL, Nafziger AN, Blask DE, Menhinick AM, Bertino JS Jr. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40(7):781–4. CrossRef
26. Andersen LP, Werner MU, Rosenkilde MM, Harpsøe NG, Fuglsang H, Rosenberg J, et al. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol. 2016;17:8–16 CrossRef
27. Mirza -Aghazadeh-Attari M, Mihanfar A, Yousefi B, Majidinia M. Nanotechnology-based advances in the efficient delivery of melatonin. Cancer Cell Int. 2022;22:43–62. CrossRef
28. Proietti S, Carlomagno G, Dinicola S, Bizzarri M. Soft gel capsules improve melatonin’s bioavailability in humans. Expert Opin Drug Metab Toxicol. 2014;10(9):1193–8. CrossRef
29. Lesieur D, Leclerc V, Chavatte P, Marot C, Renard P, Guardiola-Lemaitre B. La mélatonine, prototype pertinent pour l’innovation thérapeutique [Melatonin, a pertinent prototype for therapeutic innovation]. Therapie. 1998;53(5):429–37
30. Jockers R, Cecon E. Melatonin: methods and protocols. 1st ed. Louisville, KY: Humana; 2022.
31. Masanori S, Yoshikazu F, Masakazu H, Naoki O, Toshikatsu H. Syntheses of melatonin and its derivatives. Heterocycles. 2000;53(8):1725–36. CrossRef
32. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, et al. Melatonin, a full-service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18(4):843–57. CrossRef
33. Sanchez-Ledesma LM, Ramírez-Malule H, Rodríguez-Victoria JA. Volatile fatty acids production by acidogenic fermentation of wastewater: a bibliometric analysis. Sustainability. 2023;15:2370–87. CrossRef
34. Ramírez-malule H, López-Agudelo VA, Gómez-ríos D. Candida auris: a bibliometric analysis of the first ten years of research (2009–2018). J Appl Pharm Sci. 2020;10:12–21. CrossRef
35. Natalia A, Adriana J-E, Howard R-M. Bibliometric analysis of bacterial resistance on periodontal disease. J Appl Pharm Sci. 2021;11:118–24. CrossRef
36 Gómez-ríos D, Ramírez-malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm Sci. 2019;9:112–6. CrossRef
37. Ramírez-Malule H, Quiñones-Murillo DH, Manotas-Duque D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg Contam. 2020;6:179–93. Doi: https://doi.org/10.1016/j.emcon.2020.05.001
38. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–38. CrossRef
39. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–54. CrossRef
40. Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. JNCI J Natl Cancer Inst. 2001;93:1563–8. CrossRef
41. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella J. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci. 1995;92:8734–8. CrossRef
42. Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, et al. Melatonin in edible plants identified by radioimmunoassay and by high-performance liquid chromatography-mass spectrometry. J Pineal Res 1995;18:28–31. CrossRef
43. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134. CrossRef
44. Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. JNCI J Natl Cancer Inst. 2001;93:1557–62. CrossRef
45. Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. CrossRef
46. Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–8. CrossRef
47. Riemersma-van der Lek RF. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities. JAMA. 2008;299:2642–54. CrossRef
48. Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127–51. CrossRef
49. Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9(1):11–24. CrossRef
50. Harpsøe NG, Andersen, LPH, Gögenur I. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71:901–9. CrossRef
51. Mistraletti G, Sabbatini G, Taverna M, Figini MA, Umbrello M, Magni P, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–7. CrossRef
52. Herrera-Arozamena C, Estrada-Valencia M, Pérez C, Lagartera L, Morales-García JA, Pérez-Castillo A, et al. Tuning melatonin receptor subtype selectivity in oxadiazolone-based analogues: discovery of QR2 ligands and NRF2 activators with neurogenic properties. Eur J Med Chem. 2020;190:112090–9 CrossRef
53. Yepes AF, Arias JD, Cardona-GW. New class of hybrids based on chalcone and melatonin: a promising therapeutic option for the treatment of colorectal cancer. Med Chem Res. 2021;30:1–16. CrossRef
54. De la Fuente Revenga M, Fernández-Sáez N, Herrera-Arozamena C, Morales-García JA, Alonso-Gil S, Pérez-Castillo A, et al. Novel N-Acetyl bioisosteres of melatonin: melatonergic receptor pharmacology, physicochemical studies, and phenotypic assessment of their neurogenic potential. J Med Chem. 2015;58(12):4998–5014. CrossRef
55. Niyaz A, Mohammed S, Ayman M, Mohammed R, Mohd F, Ziyad S, et al. Preparation of melatonin nanoparticles and their enhancement of lungs bioavailability in the treatment of lungs cancer. J Young Pharm. 2023;15(2):314–25. CrossRef
56. Said DE, Amer EI, Sheta E, Makled S, Diab HE, Arafa FM. Nano-encapsulated melatonin: a promising mucosal adjuvant in intranasal immunization against chronic experimental T. gondii infection. Trop Med Infect Dis. 2022;7:401–13. CrossRef
57. Chuffa LGA, Seiva FRF, Novais AA, Simão VA, Martín Giménez VM, Manucha W, et al. Melatonin-loaded nanocarriers: new horizons for therapeutic applications. Molecules. 2021;26(12):3562–74. CrossRef
58. Phiboonchaiyanan PP, Puthongking P, Chawjarean V, Harikarnpakdee S, Sukprasansap M, Chanvorachote P, et al. Melatonin and its derivative disrupt cancer stem-like phenotypes of lung cancer cells via AKT downregulation. Clin Exp Pharmacol Physiol. 2021;48(12):1712–23. CrossRef
59. Alsayed MS, El-kady DS, Tantawy MA, Abdelhalim MM, Elazabawy SR, Abdallah AE. et al. Novel melatonin derivatives: synthesis, anticancer evaluations and molecular-docking study. Egypt J Chem. 2021;64(3):1517––33. CrossRef
60. Meng JP, Li SQ, Tang Y, Xu ZG, Chen ZZ, Gao LX. Facile synthesis and biological evaluation of tryptamine-piperazine-2,5-dione conjugates as anticancer agents. RSC Adv. 2021;11(45):27767–71. CrossRef
61. Ryu BJ, Kim S, Min B, Kim KY, Lee JS, Park WJ, et al. Discovery and the structural basis of a novel p21-activated kinase 4 inhibitor. Cancer Lett. 2014;349(1):45–50. CrossRef
62. Hoegberg M, Dahlstedt E, Smitt O, Johansson T. Novel pyrimidine derivatives. US Patent 0045845Al. 2014.
63. Helmi YY, Papenkordt N, Rennar G, Gbahou F, El-Hady AK, Labani N, et al. Melatonin-vorinostat hybrid ligands show higher histone deacetylase and cancer cell growth inhibition than vorinostat. Arch Pharm (Weinheim). 2023;356(9):e2300149–59. CrossRef
64. Witt-Enderby PA, Davis VL, Lapinsky D, Anti-cancer tamoxifen-melatonin hybrid ligand. US patent US8785501B2. 2014.
65. Jiaqi F, Tao H, Mengyuan X, Lulu D, Xiaojiang H, Yuehu W, et al. Design and synthesis of novel monoterpenoid indole alkaloid-like analogues and their antitumour activities in vitro. Org Biomol Chem. 2018;16:3026–37. CrossRef
66. Simonetti G, Boga C, Durante J, Micheletti G, Telese D, Caruana P, et al. Synthesis of novel tryptamine derivatives and their biological activity as antitumor agents. Molecules. 2021;26(3):683–98. CrossRef
67. Zhang C, Yang L, Yang X, Gao Q, Qu Y, Wu L. Design, synthesis, and biological evaluation of novel napabucasin-melatonin hybrids as potent STAT3 inhibitors. Bioorg Chem. 2023;136:106541–58. CrossRef
68. Bedini A, Fraternale A, Crinelli R, Mari M, Bartolucci S, Chiarantini L, et al. Design, synthesis, and biological activity of hydrogen peroxide responsive arylboronate melatonin hybrids. Chem Res Toxicol. 2019;32(1):100–12. CrossRef
69. Xiong R, He D, Deng X, Liu J, Lei X, Xie Z, et al. Design, synthesis and biological evaluation of tryptamine salicylic acid derivatives as potential antitumor agents. Medchemcomm. 2019;10(4):573–83. CrossRef
70. Gatti G, Lucini V, Dugnani S, Calastretti A, Spadoni G, Bedini A, et al. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget. 2017;8(40):68338–53. CrossRef
71. Florido J, Rodriguez-Santana C, Martinez-Ruiz L, López-Rodríguez A, Acuña-Castroviejo D, Rusanova I. Understanding the mechanism of action of melatonin, which induces ROS production in cancer cells. Antioxidants (Basel). 2022;11:1621–31. CrossRef
72. Núñez Martínez P, Zapico S. Melatonin: a double-edged sword for cancer treatment. Free Radicals and Health. Hauppauge, NY: Nova Science Publishers, Inc; 2016. pp. 75–98
73. Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, Asghari MH. Immunoregulatory role of melatonin in cancer. J Cell Physiol. 2020;235(2):745–57. CrossRef
74. Srinivasan V, Pandi-Perumal SR, Brzezinski A, Bhatnagar KP, Cardinali DP. Melatonin, immune function and cancer. Recent Patents Endocr Metab Immune Drug Discov. 2011;5(2):109–23. CrossRef
75. Majidinia M, Sadeghpour A, Mehrzadi S, Reiter RJ, Khatami N, Yousefi B. Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J Pineal Res. 2017;63(1):e12416–26. CrossRef
76. Hong Y, Won J, Lee Y, Lee S, Park K, Chang KT, et al. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res. 2014;56(3):264–74. CrossRef
77. Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45(4):532–40.
78. González-González A, González A, Rueda N, Alonso-González C, Menéndez JM, Martínez-Campa C, et al. Usefulness of melatonin as complementary to chemotherapeutic agents at different stages of the angiogenic process. Sci Rep. 2020;10(1):4790–809.
79. Proietti S, Cucina A, Dobrowolny G, D’Anselmi F, Dinicola S, Masiello MG, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014;57(1):120–9. CrossRef
80. Wang Y, Song J, Li Y, Lin C, Chen Y, Zhang X, et al. Melatonin inhibited the progression of gastric cancer induced by Bisphenol S via regulating the estrogen receptor 1. Ecotoxicol Environ Saf. 2023;259:115054–68. CrossRef
81. Fan C, Pan Y, Yang Y, Di S, Jiang S, Ma Z, et al. HDAC1 inhibition by melatonin leads to suppression of lung adenocarcinoma cells via induction of oxidative stress and activation of apoptotic pathways. J Pineal Res. 2015;59(3):321–33. CrossRef
82. Shen D, Ju L, Zhou F, Yu M, Ma H, Zhang Y, et al. The inhibitory effect of melatonin on human prostate cancer. Cell Commun Signal. 2021;19:34. CrossRef
83. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, et al. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015;58(2):234–50. CrossRef
84. Song J, Ma SJ, Luo JH, Zhang H, Wang RX, Liu H, et al. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol Rep. 2018;39:1975–83. CrossRef
85. Qin W, Lu W, Li H, Yuan X, Li B, Zhang Q, et al. Melatonin inhibits IL1β-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-κB activation. J Endocrinol. 2012;214(2):145–53. CrossRef
86. Borin TF, Arbab AS, Gelaleti GB, Ferreira LC, Moschetta MG, Jardim-Perassi BV, et al. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res. 2016;60(1):3–15. CrossRef
87. Wang SW, Tai HC, Tang CH, Lin LW, Lin TH, Chang AC, et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J Cell Physiol. 2021;236:3979–90. CrossRef
88. Rudra DS, Pal U, Maiti NC, Reiter RJ, Swarnakar S.Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J Pineal Res. 2013;54:398–405. CrossRef
89. Liu L, Pan Y, Chen D, Xia L, Liu Y, Xingyu P, et al. Melatonin inhibits the proliferation of human MG-63 osteosarcoma cells via downregulation of cyclins and CDKs. J China Med Univ. 2017;46:131–5.
90. Jin Y, Choi YJ, Heo K, Park SJ. Melatonin as an oncostatic molecule based on its anti-aromatase role in breast cancer. Int J Mol Sci. 2021;22(1):438–47. CrossRef
91. Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, et al. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. 2014;28(7):1215–21. CrossRef
92. Cheng J, Yang HL, Gu CJ, Liu YK, Shao J, Zhu R, et al. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int J Mol Med. 2019;43(2):945–55. CrossRef
93. Jardim-Perassi BV, Arbab AS, Ferreira LC, Bonin TF, Varma NR, Iskander AS, et al. Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLos One. 2014;9(1):85311–22. CrossRef
94. Lv D, Cui PL, Yao SW, Xu YQ, Yang ZX. Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin J Cancer Res. 2012;24(4):310–6. CrossRef
95. Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni GJ. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol Lett. 2001;22(1):45–7.
96. Davoodvandi A, Nikfar B, Reiter RJ, Asemi Z. Melatonin and cancer suppression: insights into its effects on DNA methylation. Cell Mol Biol Lett. 2022;27(1):73–97. CrossRef
97. Cipolla-Neto J, Amaral FG, Soares JM Jr, Gallo CC, Furtado A, Cavaco JE, et al. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology. 2022;112(2):115–29. CrossRef
98. Menndez-Menndez J, Mart. melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol. 2018;2018:20–31. CrossRef
99. Cos S, Gonzalez A, Martinez-Campa C, Mediavilla MD, Alonso-Gonzalez C, Sanchez-Barcelo EJ. Melatonin as a selective estrogen enzyme modulator. Curr Cancer Drug Targets. 2008;8(8):691–702. CrossRef
100. Gonzalez A, Cos S, Martinez-Campa C, Alonso-Gonzalez C, Sanchez-Mateos S, Mediavilla MD. Selective estrogen enzyme modulator actions of melatonin in human breast cancer cells. J Pineal Res. 2008;45(1):86–92. CrossRef
101. Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19:145–67. CrossRef
102. Pliszka M, Szablewski L. Glucose transporters as a target for anticancer therapy. cancers (Basel). 2021;13(16):4184–98. CrossRef
103. Suriagandhi V, Nachiappan V. Therapeutic target analysis and molecular mechanism of melatonin—treated leptin resistance induced obesity: a systematic study of network pharmacology. Front Endocrinol. 2022;13:92757689. CrossRef