INTRODUCTION
Cosmetics, designed to enhance beauty, cleanse the skin, and reduce the effects of aging [1–3], have witnessed a remarkable surge in popularity, with the industry growing by approximately 4.5% annually over the past two decades [4]. This growth can be attributed to a growing consumer demand for novel and improved products. The allure of appearing aesthetically pleasing, coupled with limited public awareness regarding the safety of cosmetic products, has led to a massive increase in their usage [5]. A diverse range of commonly utilized cosmetic products such as lipsticks, creams, lotions, nail polishes, perfumes, hair colors, eye and face makeup, deodorants, shampoos, and toothpaste contribute to this developing trend [1]. With the ever-increasing use of cosmetic products, adverse events associated with these formulations are also on the rise [6].
Despite the skin’s inherent protective mechanisms, certain cosmetic ingredients possess the ability to permeate the skin barrier and induce systemic effects [7,8]. The presence of potentially harmful substances such as heavy metals, nitrosamines, phenols, hydroquinone, and steroids in cosmetic formulations highlights potential health risks for individuals [9–11]. Specifically, heavy metals, a common component in cosmetics, can cause skin irritation, damage epithelial cells, and pose risks to mucous membranes [12]. Moreover, the incorporation of preservatives, fragrances, surfactants, and dyes in cosmetic formulations aims to enhance quality and prolong shelf life [13]. However, it has been found that these substances may impart varying degrees of toxicity, ranging from mild hypersensitivity reactions to severe adverse effects [14,15]. Adverse events, ranging from minor issues such as skin irritation to severe complications such as infections, scarring, or even life-threatening conditions, contribute to the burden on healthcare resources.
In recent years, there has been a rising emphasis on conducting tests and closely monitoring the potential adverse effects of cosmetics [16]. Regulatory authorities for cosmetics are actively engaged in setting standards to mitigate unwanted reactions by ensuring the effectiveness, safety, and quality of these products [17]. Despite these efforts, certain challenges persist in developing countries, including the unauthorized sale of products, inadequate awareness about appropriate cosmetic use, and underreporting of adverse events [18]. Addressing these challenges is crucial for effective regulation and consumer protection.
Global reporting indicates relatively low numbers of adverse events associated with cosmetics; however, potential underestimation remains a concern, primarily due to factors such as self-diagnosis, self-medication, and limited medical consultation, especially for mild to moderately harmful events [19,20]. Consequently, there is a need to examine and evaluate the prevalence of adverse events associated with cosmetics. To address this gap, our study aimed to conduct a comprehensive analysis of existing studies on the prevalence and risk factors associated with cosmetic-induced adverse events. Through this approach, we anticipate providing a holistic understanding of cosmetic-induced adverse events, thereby establishing a basis for evidence-based guidelines, regulatory decisions, and public health initiatives. These efforts are aimed at ensuring the safety and well-being of consumers in the growing landscape of cosmetic usage.
METHODOLOGY
Study design
We performed a meta-analysis of primary studies that reported the prevalence of adverse events caused by the application of various cosmetics, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. The study protocol is not registered in any database.
Literature search
A computerized search of the relevant literature published until August 2023 was conducted using different databases such as PubMed, Embase, Scopus, and Google Scholar. We utilized the PICO (Population, Intervention, Comparison, Outcome) strategy to guide our search, addressing the question: “What is the prevalence of adverse events among cosmetics users, and what are the associated factors?” The PICO characteristics were defined as follows: Population (P): cosmetic users; Intervention (I): not applicable; Comparison (C): not applicable; and Outcome (O): prevalence of adverse events. Keywords related to “adverse events,” “cosmetics,” and “prevalence” were used and linked with Boolean operators such as OR and AND [22]. Google Scholar search results were screened up to the initial 20 pages (200 results). The complete search strategy for each database is provided in Supplementary Table 1. An additional search was also conducted to identify pertinent studies by examining the reference lists of full-text articles.
Eligibility criteria
The review included studies published in the English language that reported the prevalence of adverse events due to various cosmetic products. Articles were excluded if they reported reactions to specific cosmetic products, targeted only a particular adverse reaction, failed to report the prevalence of adverse events, or if the prevalence of events could not be calculated as a percentage of the sample that used cosmetics. Additionally, clinical reports, meta-analyses, systematic reviews, literature reviews, conference abstracts, summary articles, letters to the editor, case reports or case series, and animal studies were excluded. Furthermore, research protocols for which only abstracts were available for analysis, and whose results were not published or not available in the database, were excluded. Moreover, studies reporting adverse events due to cosmetics in the pediatric population were not considered in this review.
 | Supplementary Table 1. Search strategy used for each database. [Click here to view] |
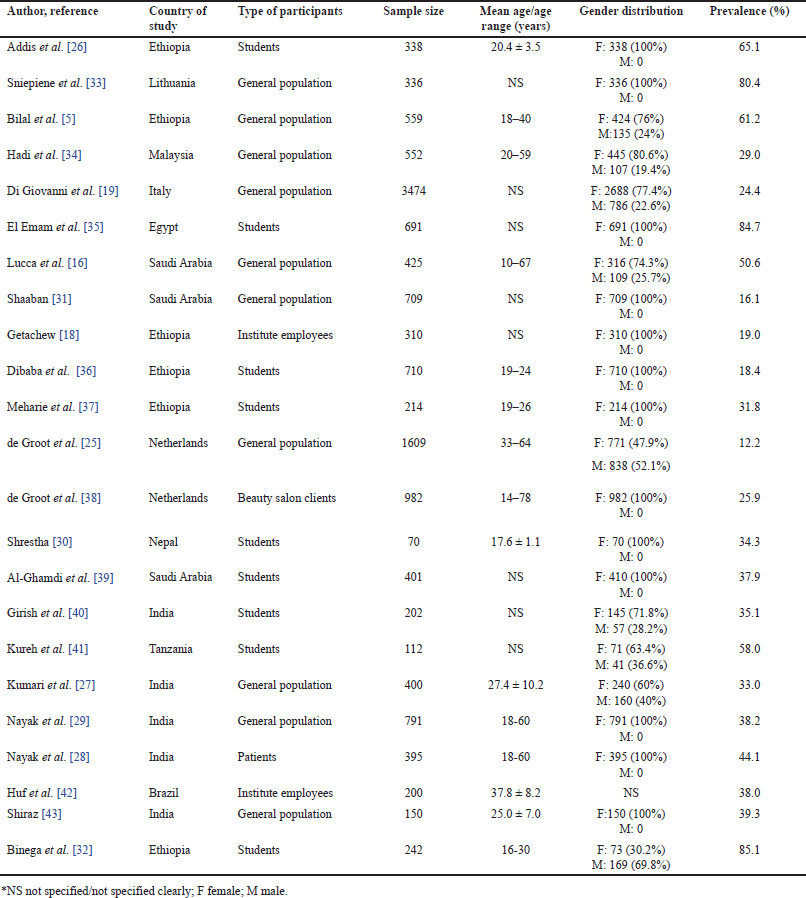 | Table 1. Characteristics of included studies. [Click here to view] |
Selection of studies and data extraction
Following the predetermined eligibility criteria, two investigators (SK and SKS) independently screened the titles and abstracts of retrieved papers from the databases. In the next step, full texts of potentially relevant papers were evaluated to identify studies that met the eligibility criteria. Both reviewers separately assessed each study, and a final decision was reached by referring to a senior reviewer (BP) regarding the inclusion of each study before the data collection process. Data were extracted from all selected studies using a well-structured data collection form. The extracted data included general characteristics of the studies, such as author’s name, year of publication, country of study, participants’ age, gender distribution, type of participants, sample size, and prevalence of adverse events. To explore the determinants of adverse events caused by cosmetics, we specifically extracted data from studies that performed bivariate or multivariate analyses and reported their results using adjusted odds ratios (AORs) if found statistically significant.
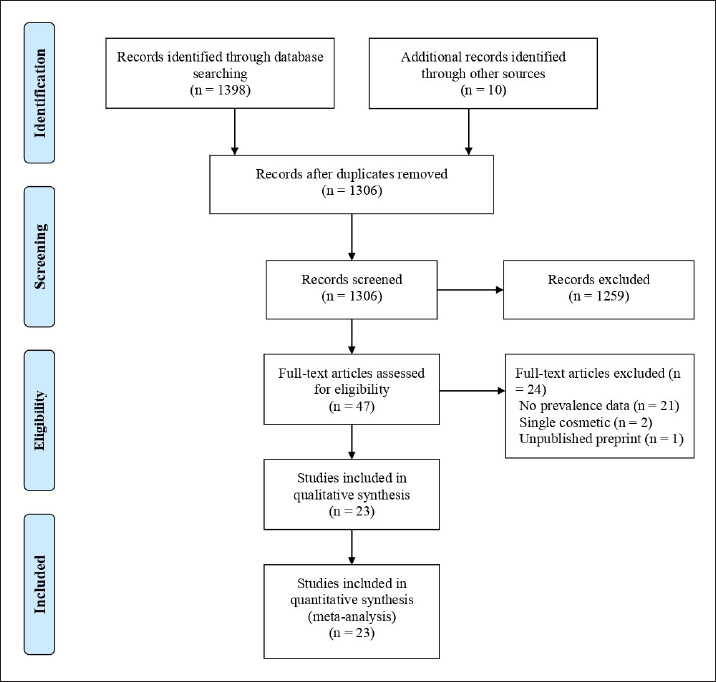 | Figure 1. PRISMA flow diagram showing the process of record selection. [Click here to view] |
Quality assessment
Two reviewers (SK and SKS) independently evaluated the methodological quality of the studies using a critical assessment checklist developed by the Joanna Briggs Institute (JBI) [23]. This checklist, consisting of nine questions (Q1–Q9), focused on aspects such as the sampling frame, study subjects, sample size, methods, and statistical analysis. In cases where disagreements arose between the reviewers, a third investigator (BP) was consulted for resolution. The total scores on the JBI checklist ranged from 0 to 9. For inclusion in this review, studies with a total score of more than 5, indicating “Yes” ratings to the checklist questions, were considered.
Synthesis of findings
R software (v. 4.2.2) was used for the analysis. Quantitative synthesis was done utilizing a random-effects model (maximum likelihood estimator—MLE) due to the observed high heterogeneity (I2 = 99%) among the studies. The outcomes were visually presented using forest plots, and the pooled prevalence was calculated at a 95% confidence level. To estimate the impact of individual studies on the pooled prevalence, a sensitivity analysis was carried out by systematically excluding one study at a time. Additionally, to further explore regional influences on heterogeneity, a subgroup analysis based on study regions was conducted. Another subgroup analysis based on the type of participants involved in the study was also carried out. The assessment of publication bias was conducted using a visual assessment of the funnel plot and Egger’s test [24].
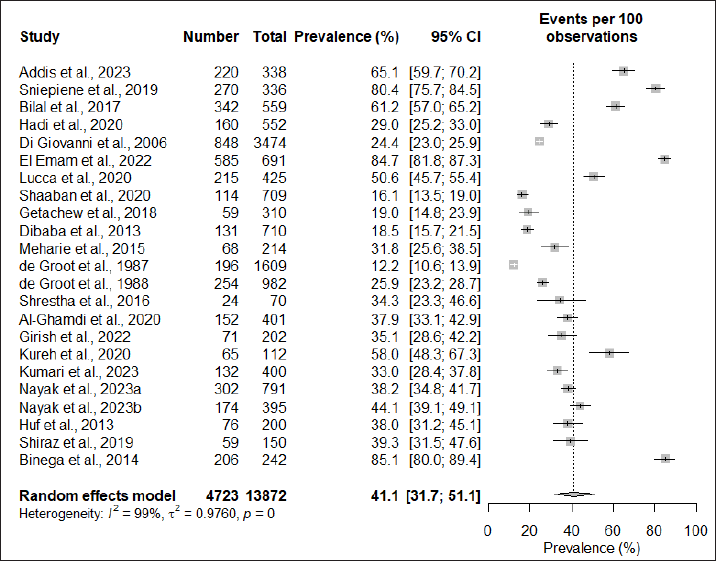 | Figure 2. The overall global prevalence of cosmetic-induced adverse events. [Click here to view] |
RESULTS
Study screening
The database search originally identified 1,398 potentially relevant citations. Additionally, a comprehensive search of the reference lists of potentially eligible articles found 10 more relevant articles. After eliminating duplicates, 1,306 citations remained for subsequent evaluation. During the screening of titles and abstracts, 1,259 articles were excluded as they did not meet the predefined criteria. Subsequently, a full-text examination of 47 studies led to the exclusion of 24 papers for various reasons. Ultimately, 23 studies that aligned with the inclusion criteria were considered for inclusion in this study. An adapted PRISMA diagram (Fig. 1) was used to illustrate the study selection process.
Characteristics of included studies
In this analysis, 23 papers published between 1987 [25] and 2023 [26–29] were included to examine the prevalence of adverse events resulting from cosmetic use. These studies, predominantly conducted in community settings, presented sample sizes ranging from 70 [30] to 3,474 [19], collectively encompassing 13,872 cosmetic users. Most participants were of younger ages (<30 years). The gender distribution, based on 22 studies (one lacking gender specification), revealed a predominantly female participant base (82.5%). The prevalence of adverse events associated with cosmetics within the included studies exhibited a wide range, from 16.1% [31] to 85.1% [32]. The characteristics of these studies are outlined in Table 1.
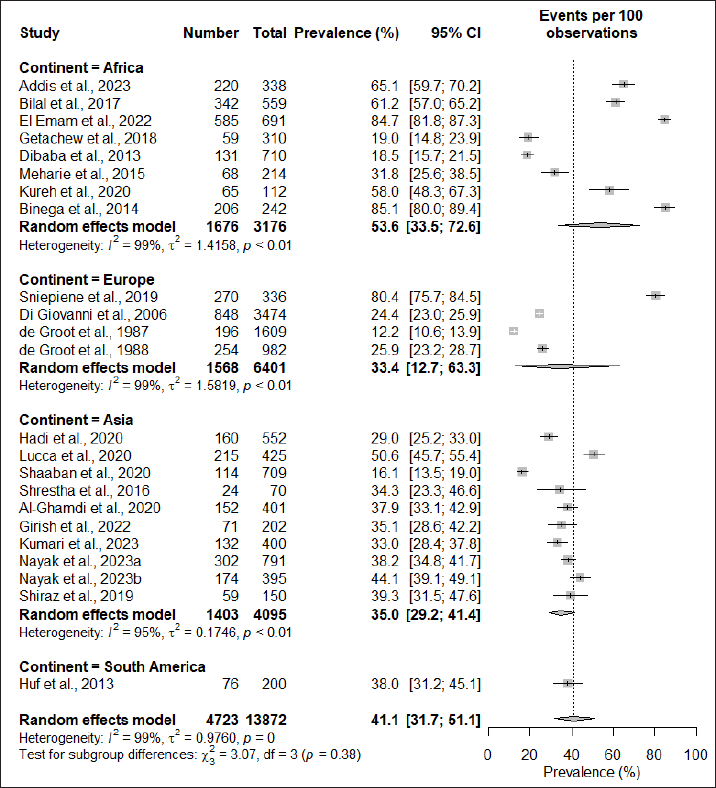 | Figure 3. Subgroup anaysis based on study region. [Click here to view] |
Prevalence of cosmetic-induced adverse events
The pooled prevalence of cosmetic-induced adverse events among cosmetic users was found to be 41.1% (95% CI: 31.7; 51.1). However, significant heterogeneity was observed among the studies (I2 = 99%; τ2 = 0.9760; p = 0). The forest plot showing the pooled prevalence is depicted in Figure 2.
Subgroup analysis and sensitivity analysis
Subgroup analysis based on the region of study found that the African continent had the highest prevalence at 53.6% (95% CI: 33.5; 72.6), followed by South America at 38% (95% CI: 31.2; 45.1), Asia at 35.0% (95% CI: 29.2; 41.4), and Europe having the lowest prevalence of 33.4% (95% CI: 12.7; 63.3). However, the subgroup difference test did not demonstrate significant regional variations in the prevalence of cosmetic-induced adverse events (p = 0.38) (Fig. 3). When subgrouping the studies based on the types of participants recruited for the assessment, it was observed that students had the highest rate of adverse events induced by cosmetics (51.1%; 95% CI: 34.1; 67.9), while studies conducted in the general population had a prevalence rate of 36.8% (95% CI: 24.4; 51.1), and the difference in the subgroup was also found to be statistically significant (p < 0.01) (Fig. 4).
The results of the sensitivity analysis confirmed that removing individual studies did not considerably affect the pooled prevalence of adverse reactions caused by using cosmetics.
Publication bias and quality assessment
The visual inspection of the funnel plot found that the included studies did not exhibit complete symmetry, indicating the presence of publication bias (Fig. 5), and this was further confirmed by the result of Egger’s test (p = 0.041).
Each of the included studies received a grade of “Yes,” exceeding the threshold set at 5, indicating their suitability for inclusion in the study due to their strong methodological quality. The results of the evaluation of methodological quality can be found in Supplementary Table 2.
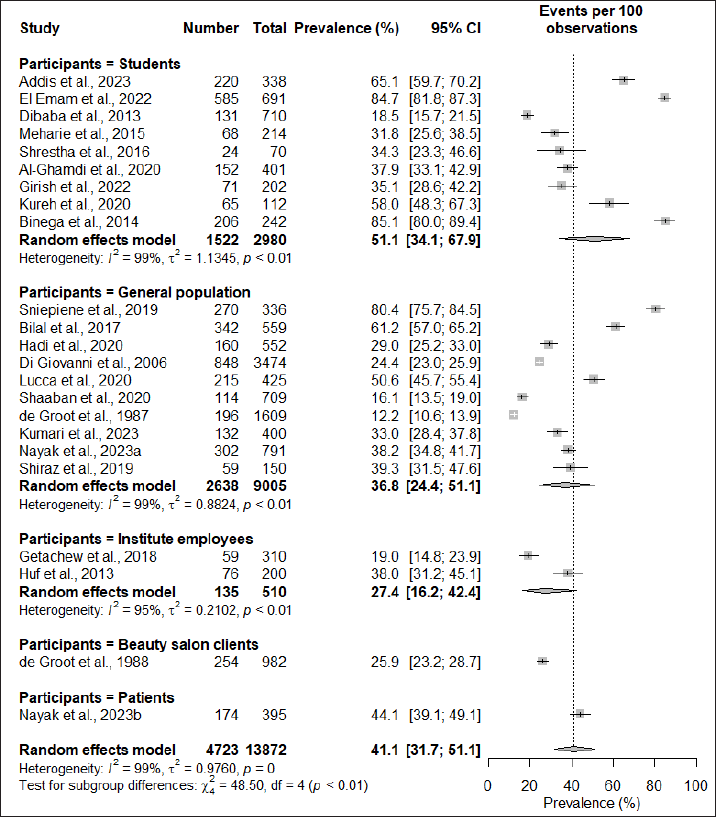 | Figure 4. Subgroup analysis based on types of participants. [Click here to view] |
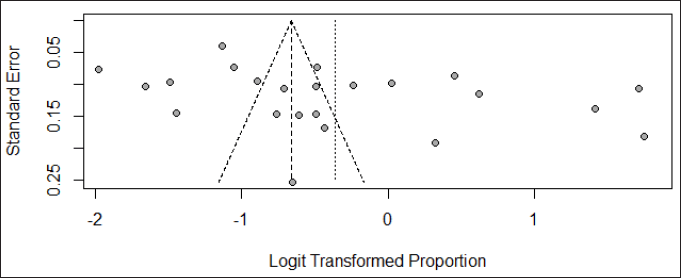 | Figure 5. Funnel plot for assessing publication bias. [Click here to view] |
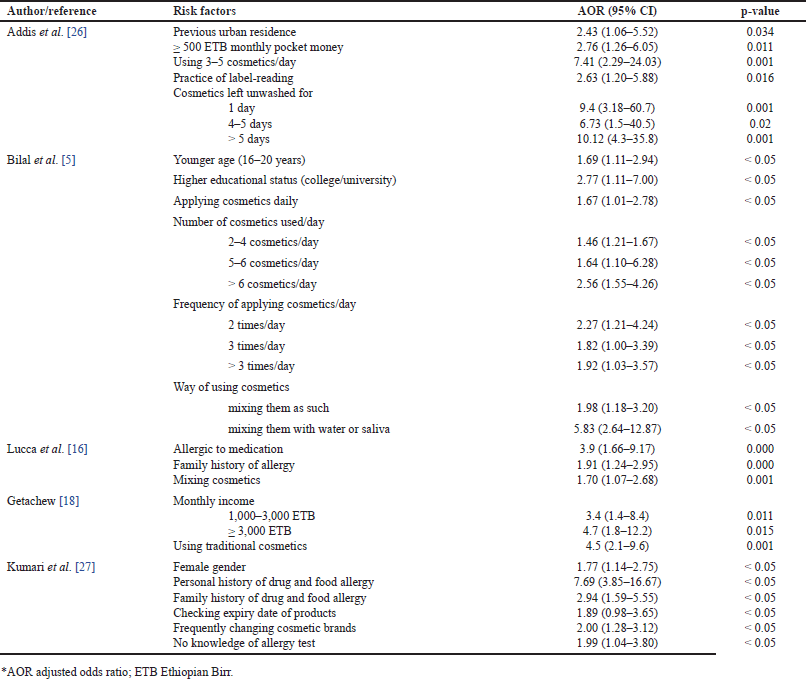 | Table 2. Factors associated with cosmetic-induced adverse events. [Click here to view] |
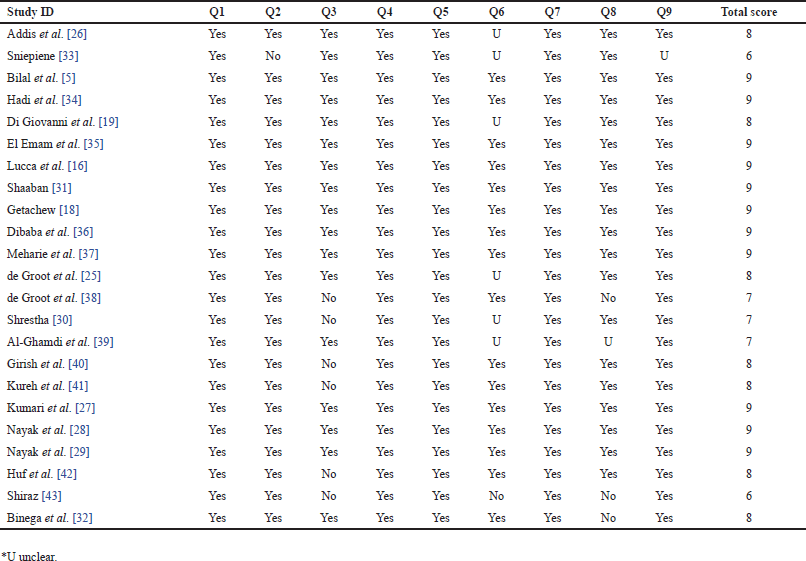 | Supplementary Table 2. Quality assessment of included studies using JBI critical appraisal checklist. [Click here to view] |
Determinants of cosmetic-induced adverse events
Several factors have been identified as contributors to the increased occurrence of cosmetic-induced adverse events. Habits such as the daily use of multiple cosmetics [5,26], frequent application [5], and a personal or family history of allergies [16,27] to specific medications and foods are among these contributing factors. Furthermore, prolonged intervals without washing applied cosmetics [26] were found to be substantially associated with a higher occurrence of adverse events. Urban residence [26], higher educational status [5], and good financial condition [18,26] were additional factors correlated with a high susceptibility to adverse events. Moreover, the data indicated that individuals of the female gender [27] and younger age [5] were more prone to both the use of cosmetics and subsequently exhibiting adverse reactions to them. Additionally, the act of mixing different cosmetic products [5,16] and combining them with saliva or water [5] were identified as significant determinants contributing to the occurrence of cosmetic-induced adverse events. The various determinants of adverse events reported in the included studies are presented in Table 2.
DISCUSSION
The meta-analysis of cosmetic-induced adverse events has shown a concerning prevalence of 41.1%. The regional analysis showed varying prevalence rates of cosmetic-induced adverse events, with the highest rate observed in the African region at 53.6%, followed by comparatively lower rates in South America (38.0%), Asia (35.0%), and Europe (33.4%). These inconsistencies can be attributed to differences in cultural practices, regulatory frameworks, healthcare infrastructure, and socioeconomic factors. In the African region, the higher prevalence is related to lower literacy levels, limited emphasis on safety evaluations for nonmedicated cosmetics [5,44], and challenges in proper storage and handling due to the warm climate [5,37]. Similarly, an analysis based on participant types found a higher prevalence of cosmetic-induced adverse events among the student community at 51.1%, compared to 36.8% in the general population. This discrepancy may arise from students’ interest in beauty trends [18], leading to riskier cosmetic practices without strict adherence to safety guidelines [35]. Students are more likely to experiment with various products, potentially increasing their exposure to substances that can trigger adverse reactions. Limited awareness and education on safe cosmetic use among students further contribute to the higher prevalence [45]. Beyond the promises of beautiful skin and exciting colors, cosmetics can sometimes create problems for our skin [46]. Certain ingredients, such as fragrance alcohol or harsh surfactants, have the potential to directly harm our skin, causing redness and dryness [15,47]. For some individuals, elements like nickel or formaldehyde in cosmetics can trigger the immune system, leading to itchy rashes or swelling [48]. Exposure to sunlight can result in issues like burns or dark spots due to specific ingredients [49]. Additionally, cosmetics containing substances like parabens or phthalates may disrupt hormonal balance, potentially resulting in long-term health consequences [50,51]. The complexity does not always lie in isolated ingredients but in the unexpected interaction of multiple chemicals within a product, giving rise to unintended and undesirable reactions.
The prevalence of cosmetic-induced adverse events is influenced by various determinants, as found in our qualitative analysis. Habitual use of multiple cosmetics per day [5,26] and frequent daily application [5] emerged as significant risk factors due to prolonged and repetitive exposure to diverse product formulations, increasing the likelihood of skin irritation or sensitization. The interaction between cosmetic products or their ingredients may contribute to these adverse events [5,36,52]. Individuals with a personal or family history of allergies [16,27] showed enhanced susceptibility, indicating that pre-existing allergic conditions may cause reactions to cosmetic ingredients. Additionally, a higher risk of adverse events was linked to both higher educational status [5] and urban residence [26], potentially arising from increased exposure among urban, educated populations to numerous cosmetic products [27]. Conversely, individuals with lower educational status reported fewer adverse events [5], possibly influenced by cultural, religious, or social factors that result in reduced cosmetic usage [5,27]. Moreover, good financial conditions were correlated with enhanced susceptibility [18,26], implying that increased financial resources might be related to the use of a broader range of cosmetic products due to the ability to afford more expensive items [18]. This, in turn, raises the risk of experiencing adverse events. Adverse events were also associated with the female gender [27] and younger age [5], likely due to higher cosmetic consumption driven by beauty concerns in these populations [16,18,27]. Reading product labels [26] and checking expiry dates [27] were important determinants influencing adverse event reporting, as those who encountered adverse events were more inclined to check product composition and expiration dates [27]. Conversely, adverse events were associated with mixing different cosmetic products [5,16], combining them with saliva or water [5], frequent brand changes [27], and a lack of knowledge about allergy tests [27]. Combining cosmetic products with substances not specified by the manufacturer can alter the physical and chemical properties of the product, leading to unintended chemical reactions and potential unexpected adverse events [5]. Furthermore, water and saliva, being conducive environments for bacterial growth, can also impact the concentrations of preservatives [35,36].
Simplifying daily routines with fewer products can decrease the risk of adverse events [28]. Additionally, testing cosmetics before applying them to detect potential adverse reactions, including allergies, is imperative [36]. Targeted education campaigns, especially focusing on female populations, should encourage safe cosmetic practices. Emphasizing the selection of products based on skin type and sensitivity, along with promoting the practice of reading labels, including double-checking ingredient lists and expiry dates, is essential [5,27]. Raising awareness about the risks associated with mixing different products is also necessary. Addressing the impact of adverse events on healthcare systems, individual health, and financial considerations is important for reducing healthcare costs and ensuring the safety of cosmetic product users. Collaborative efforts among healthcare professionals, regulatory bodies, and the industry are crucial to focus on patient safety and uphold public trust. This study supports the establishment of strong regulatory frameworks, including licensing requirements and a strict product approval process, to respond effectively to adverse events.
LIMITATIONS
This study has limitations that should be acknowledged. The reliance on self-recall data in primary studies introduces the potential for recall bias. Potential publication bias and study heterogeneity need to be considered when interpreting results. Additionally, the assessment of determinants relied on data from a limited number of studies reporting AORs, which may affect interpretation.
CONCLUSION
This study found a high prevalence of cosmetic-induced adverse events, affecting both healthcare systems and user well-being. To mitigate these risks, users should adopt a cautious approach to cosmetic applications, including reducing the quantity and frequency and testing products for potential reactions before use. The study advocates for global cosmetovigilance, regulatory enhancements, and targeted consumer education to decrease the incidence of adverse events. Implementing these measures will contribute to a safer cosmetics practice and ensure the long-term well-being of cosmetic users.
ACKNOWLEDGMENTS
The authors would like to express appreciation to Embase for providing access
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data generated and analyzed are included within this article.
CONSENT FOR PUBLICATION
All authors have read and approved the final manuscript.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Khan AD, Alam MN. Cosmetics and their associated adverse effects: a review. J Appl Pharm Sci Res. 2019;2(1):1–6. CrossRef
2. Ahsan H. The biomolecules of beauty: biochemical pharmacology and immunotoxicology of cosmeceuticals. J Immunoass Immunochem. 2019;40(1):91–108. CrossRef
3. Bilal M, Iqbal HM. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—a review. Sci Total Environ. 2019;670:555–68. CrossRef
4. Zulaikha S. Hazardous ingredients in cosmetics and personal care products and health concern: a review. J Public Health Res. 2015;5(1):7–15. CrossRef
5. Bilal AI, Tilahun Z, Osman ED, Mulugeta A, Shekabdulahi M, Berhe DF. Cosmetics use-related adverse events and determinants among Jigjiga town residents, eastern Ethiopia. Dermatol Ther. 2017;7(1):143–53. CrossRef
6. Nayak M, Sreedhar D, Prabhu SS, Ligade VS. Global trends in cosmetics use-related adverse effects: a bibliometric analysis of literature published during 1957–2021. Cosmetics. 2021;8(3):75. CrossRef
7. Mohiuddin AK. Heavy metals in cosmetics: the notorious daredevils and burning health issues. Am J Biomed Sci Res. 2019;4(5):333–7. CrossRef
8. Marie C, Cabut S, Vendittelli F, Sauvant-Rochat MP. Changes in cosmetics use during pregnancy and risk perception by women. Int J Environ Res Public Health. 2016;13(4):383. CrossRef
9. Nduka JK, Kelle HI, Odiba IO. Review of health hazards and toxicological effects of constituents of cosmetics. IntechOpen. 2019. CrossRef
10. Bamidele OD, Kayode BA, Eniayewu OI, Adegbola AJ, Olatoye RS, Njinga NS, et al. Quality assessment of hydroquinone, mercury, and arsenic in skin-lightening cosmetics marketed in Ilorin, Nigeria. Sci Rep. 2023;13(1):20992. CrossRef
11. Pal B, Kumari S, Kumari A, Singh SK, Babbar H. Allergic contact dermatitis to lip care cosmetic products—a systematic review. Cutan Ocul Toxicol. 2024;43(1):13–21. CrossRef
12. Borowska S, Brzóska MM. Metals in cosmetics: implications for human health. J Appl Toxicol. 2015;35(6):551–72. CrossRef
13. Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit-Otmani Z, Boucherit K, et al. Cosmetics preservation: a review on present strategies. Molecules. 2018;23(7):1571. CrossRef
14. Pereira JX, Pereira TC. Cosmetics and its health risks. Glob J Med Res. 2018;18(B2):63–70. CrossRef
15. Lourith N, Kanlayavattanakul M. Natural surfactants used in cosmetics: glycolipids. Int J Cosmet Sci. 2009;31(4):255–61. CrossRef
16. Lucca JM, Joseph R, Al Kubaish ZH, Al-Maskeen SM, Alokaili ZA. An observational study on adverse reactions of cosmetics: the need of practice the Cosmetovigilance system. Saudi Pharm J. 2020;28(6):746–53. CrossRef
17. Ferreira M, Matos A, Couras A, Marto J, Ribeiro H. Overview of cosmetic regulatory frameworks around the world. Cosmetics. 2022;9(4):72. CrossRef
18. Getachew M, Tewelde T. Cosmetic use and its adverse events among female employees of Jimma University, southwest Ethiopia. Ethiop J Health Sci. 2018;28(6):717–24. CrossRef
19. Di Giovanni C, Arcoraci V, Gambardella L, Sautebin L. Cosmetovigilance survey: are cosmetics considered safe by consumers? Pharmacol Res. 2006;53(1):16–21. CrossRef
20. Sautebin L. Understanding the adverse effects of cosmetics: a pilot project in cosmetovigilance. Drug Saf. 2008;31(5):433–6. CrossRef
21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. CrossRef
22. Padmakar S, Murti K, Pandey K, Kumari S, Kumar R, Siddiqui NA, et al. Suicidal ideation associated with vitiligo-a systematic review of prevalence and assessment. Clin Epidemiol Glob Health. 2022;17:101140. CrossRef
23. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. JBI Manual Evid Synth. JBI, 2020. CrossRef
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. CrossRef
25. De Groot AC, Nater J, van der Lender R, Rijcken B. Adverse effects of cosmetics and toiletries: a retrospective study in the general population. Int J Cosmet Sci. 1987;9(6):255–9. CrossRef
26. Addis GT, Yimer YS, Dagnew SB, Moges TA, Assefa AN, Alemu MA, et al. Cosmetic use and related negative effects among graduate university female students in Ethiopia: a multicenter cross-sectional study. J Public Health. 2023;32:237–45. CrossRef
27. Kumari S, Pal B, Tewari D, Sahu SK. Cosmetics utilization and associated adverse events: a community based cross-sectional study. Clin Epidemiol Glob Health. 2023;23:101382. CrossRef
28. Nayak M, Ligade VS, Prabhu SS. Awareness level regarding adverse reactions caused by cosmetic products among female patients: a cross-sectional study. J Cosmet Dermatol. 2023;22:2512–9. CrossRef
29. Nayak M, Prabhu SS, Sreedhar D, Muragundi PM, Janodia MD, Ligade VS. Awareness level regarding adverse reactions caused by cosmetic products among Indian women: a cross-sectional study. J Cutan Aesthet Surg. 2023;22(9):2512–9. CrossRef
30. Shrestha R, Shakya J. Knowledge regarding adverse effects of selected cosmetic products among higher secondary level girl students, Chitwan. J Chitwan Med Coll. 2016;6(2):27–32. CrossRef
31. Shaaban H, Alhajri W. Usage patterns of cosmetic and personal care products among female population in Saudi Arabia: important factors for exposure and risk assessment. J Environ Public Health. 2020:8:434508. CrossRef
32. Binega G, Tegegne G, Gelaw B, Defersha M. Assessment of cosmetic use and its skin reaction among post graduate students in University of Gondar, Gondar, North East Ethiopia. Int J Cur Res Chem Pharm Sci. 2014;1(7):08–12. CrossRef
33. Sniepiene G, Jonuševi?ien? J. Cosmetics usage habits and related side effects among females: Lithuanian case. CBU Int Conf Proc. 2019,7:824–32. CrossRef
34. Hadi H, Ai Na, Zamli M, Awadh AI, Zafar MZ, Jamshed S. Cosmetic use-related adverse events: findings from lay public in Malaysia. Cosmetics. 2020;7(2):41. CrossRef
35. El Emam Hafez El Emam F, Samy Bauomy E, El-Gilany A-H, Nabeh Taref N. Prevalence, determinants and adverse events of cosmetics use among university female students: a study in Egypt. Egypt J Health Care. 2022;13(1):1376–86. CrossRef
36. Dibaba H, Yadesa D, Legesse B, Shewamene Z, Gerima BW. Cosmetics utilization pattern and related adverse reactions among female university students. Int J Pharm Sci Res. 2013;4(3):997–1004. CrossRef
37. Meharie BG, Ambaye AS, Thaimanot Y. A cross sectional study on assessment of cosmetics utilization and self-reported adverse reactions among Wollo University, Dessie campus female students, Dessie, North East Ethiopia. Eur J Pharm Med Res. 2014;2:49–63. CrossRef
38. De Groot AC, Beverdam EG, Ayong CT, Coenraads PJ, Nater JP. The role of contact allergy in the spectrum of adverse effects caused by cosmetics and toiletries. Contact Dermatitis. 1988;19(3):195–201. CrossRef
39. Ghamdi HSA, Abukhelaif AE, Croft M, Yusif M, Ghamdi HAA, Mangi AA. Assessment of prevalence, awareness and practices regarding cosmetics harmful effects among Saudi female university students of Albaha; Saudi Arabia. J Pharm Res Int. 2020;32(13):184–94. CrossRef
40. Girish K, Vasundara K, Jyothi R, Arunnair V. Knowledge and practice towards cosmetics and their adverse reactions among undergraduate medical, pharmacy, and physiotherapy students. Asia J Pharm Clin Res. 2022;15(9):118–22. CrossRef
41. Kureh GT, Ndesangia A, Opio RD, Umoh IO, Aruwa JO, Okoruwa GA. Use of cosmetic products and related adverse reactions among health science students. J Young Pharm. 2020;12(3):271–4. CrossRef
42. Huf G, Rito PdN, Presgrave RdF, Bôas MHSV. Adverse reactions to cosmetic products and the Notification System in Health Surveillance: a survey. Rev Bras Epidemiol. 2013;16(4):1017–20. CrossRef
43. Shiraz A, Rahaman A. Study on awareness about adverse health effects of cosmetics among females of different age groups. J Med Sci Clin Res. 2019;7(11):503–10. CrossRef
44. Amasa W, Santiago D, Mekonen S, Ambelu A. Are cosmetics used in developing countries safe? use and dermal irritation of body care products in Jimma town, southwestern Ethiopia J Toxicol. 2012;2012:204830. CrossRef
45. Olcer Z, Cal A, Unal N, Oztas B, Oge G. Examining the use of cosmetic products and the awareness of healthy life among university students. Turk J Dermatol. 2023;17(3):79–87. CrossRef
46. Rodan K, Fields K, Majewski G, Falla T. Skincare bootcamp: the evolving role of skincare. Plast Reconstr Surg Glob Open. 2016;4(12 Suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):e1152. CrossRef
47. Panico A, Serio F, Bagordo F, Grassi T, Idolo A, M DEG, et al. Skin safety and health prevention: an overview of chemicals in cosmetic products. J Prev Med Hyg. 2019;60(1):E50–7. CrossRef
48. Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: human health and environmental toxicology. Int J Environ Res Public Health. 2020;17(3):679. CrossRef
49. D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–48. CrossRef
50. Wang Y, Qian H. Phthalates and their impacts on human health. Healthcare (Basel). 2021;9(5):603. CrossRef
51. Nowak K, Ratajczak–Wrona W, Górska M, Jab?o?ska E. Parabens and their effects on the endocrine system. Mol Cell Endocrinol. 2018;474:238–51. CrossRef
52. Nigam PK. Adverse reactions to cosmetics and methods of testing. Indian J Dermatol Venereol Leprol. 2009;75(1):10–8. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: Link here [https://japsonline.com/admin/php/uploadss/4399_pdf.pdf]