INTRODUCTION
Fungi that are linked with the ocean have been shown a vital source of new natural products (NPs) with significant activities. Marine fungi are widely spread across the ocean and are particularly linked to marine life, such as coral, algae, sponges, sand, seawater, and submerged plants [1,2]. Marine-sediment fungi have been discovered in a variety of depth zones, including the epipelagic zone (surface to 200 meters), which has the most light, the mesopelagic zone (200–1,000 meters), which has the least sunlight and the greatest temperature fluctuations, the bathypelagic zone (1,000–4,000 meters), which temperature of 4°C and pressure over 5,850 pounds per square inch, and the abyssopelagic zone (4,000–6,000 meters), the deep, dark layer at the bottom with a persistently low temperature [3].
Their adaptation and survival are reflectors in the regulation of secondary metabolic pathways that are products of novel NPs [4]. In recent years, about 30,000 novel NPs have been reported and roughly 2% of them have been isolated from extreme environment organisms, and around 75% of that exhibited have biological activities [5]. The number of new NPs reported from marine organisms has been increasing over the past decade, with more than 200 new species reported every year [6]. In this report, we present a conscientious overview chemical structures and activities of 348 new compounds collected from marine sediment fungi from various depths including the deep sea.
CHARACTERISTICS OF COMPOUNDS
Foundation on their structures, the 348 new compounds provide be divisible to six groups: terpenes (165, 47%), chromones (44, 13%), alkaloids (44, 13%), polyketides, (43, 12%), lactones (37, 11%), and others (15, 4%) (Fig. 1). The compounds were obtained from a diverse range of marine sediments fungi inclusive to 23 genera as Arthrinium, Aspergillus, Botryotinia, Chaetomium, Cladosporium, Cladosporium, Cystobasidium, Diaporthe, Emericella, Engyodontium, Epicoccum, Eutypella, Graphostroma, Hypoxylon, Leptosphaeria, Myrothecium, Paraconiothyrium, Penicillum, Phomopsis, Pleosporales, Sarcopodium, Spiromastix, and Talaromyces. Botryotinia (20%, 71), Aspergillus (19%, 67), and Penicillum (14%, 48) are fungus each constituting more than 10% of produced of new compounds. Ten genera of fungus in the range of 2%–10%, including Phomopsis, Graphostroma, Eutypella, Spiromastix, Cladosporium, Cystobasidium, Arthrinium, Epicoccum, Paraconiothyrium, and Talaromyces. While the last 10 genera, cover less than 2% (≤4 compounds) (Fig. 1).
Based on the sea depth zone, bathypelagic and abyssopelagic that two zones constitution 90% of new compounds (173 and 142 compounds, respectively). Pipelagic and hadalpelagic are other zones that were represented by each 1% (each 4 compounds) and 8% (27 compounds) of the unidentified zone. In the biological activity assays, the new compounds from marine sediment fungi have one or more bioactivity. About 39% (135 compounds) of new compounds showed have biological activities, including antibacterial (13% 47), anti-inflammatory (13%, 46), cytotoxic (9%, 31), antiphytoplanton (1%, 4), antidiabetes (1%, 3), antifungal (3%, 2), and antioxidant (1%, 2). In total, 38% (51 compounds) of the active compounds displayed were obtained from Aspergillus, followed by Penicillium (13%, 17), Eutypella (10%, 14), Spiromastix and Graphostroma (each 7%, 9), Cladosporium (6%, 8) and Phomopsis (4%, 6). Other, covers of 21 compounds isolated from Cystobasidium, Epicoccum, Hypoxylon, Myrothecium, Sarcopodium, Alternaria, Arthrinium, Botryotinia, Chaetomium, Emericella, Paraconiothyrium, Pleosporales, and Talaromyces (Fig. 1).
DIVERSITY OF STRUCTURES
Marine sediment fungi are a wealthy source of structurally distinctive bioactive NPs. Marine fungi from the deep sediments are a comparatively untapped warehouse of NPs with structural variety waiting to be found due to deficiency of technique and the hardship for sampling. Corresponding to structure, the new compounds could be approximately classified as terpenes, chromones, polyketides, alkaloids, lactones, and other compounds.
Terpenes
Marine-derived fungi are important sources of terpenoids, which have interesting structure diversity and activity, as well as antimicrobial, cytotoxic, anti-inflammatory, and antioxidant activities. In the past years, there has been an enormous surge of discovery of novel terpene compounds in marine sources.
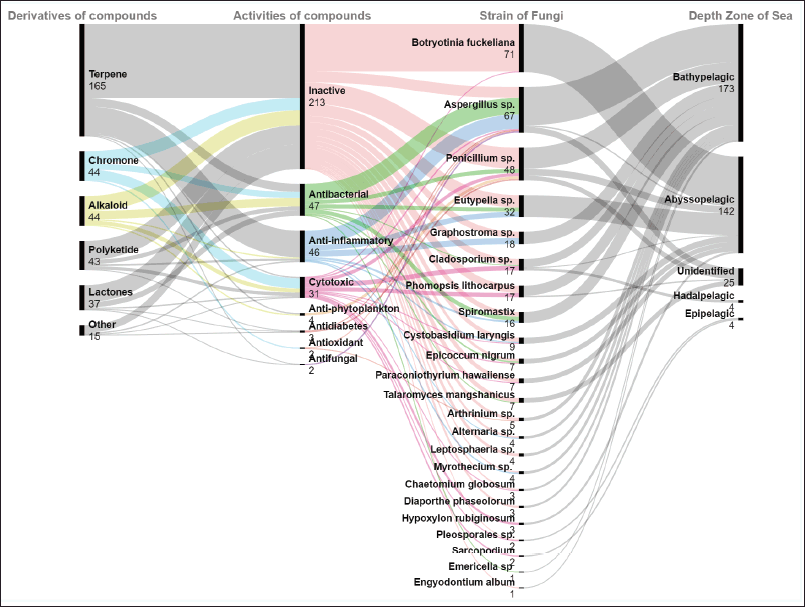 | Figure 1. Distributions derivatives, activities, strain, and depth zone of new compounds produced by marine sediments fungi. [Click here to view] |
Sesquiterpenes
Sesquiterpenes are the prime and largest resource group of terpenoids. A total of 72 Sesquiterpenes were reported in this review, including 23 compounds isolated from Aspergillus, (6R)-16,17,21,21-O-tetrahydroophiobolin G (1), (6R)-16,17-Dihydroophiobolin H (2), (5S,6S)-16,17-dihydroophiobolin H (3) [7], asperbisabolane A-N (4–17), aspercuparene A-C (18–20) [8], ent-aspergoterpenin C (21), 7-O-methylhydroxysydonic acid (22) [9], and 12-Hydroxysydowic acid (23) [10] (Fig. 2). Compounds 3-23 showed have activities which compounds 3 and 21–23 have antibacterial activities (MIC = 4.0–32.0 µg/ml) (Fig. 6) and 4–20 displayed anti-inflammatory activities (Fig. 8).
The newness of the sesquiterpenes isolated from marine sediment-derived fungus was still relatively high, two reports by Niu et al. [11] and one report by Guo et al. [12] The first report showed the 30 compounds isolated from Eutypella sp. MCCC 3A00281, 26 of them are new compounds namely eutyperemophilane A–Z (24–49). The second report identified 9 new sesquiterpenes from 11 isolated produced of Graphostroma sp. MCCC 3A00421, xylariterpenoid E-G (50–52), khusinol B-E (53–56), graphostromabisabol A (57) and graphostromabisabol B (58) [13], and the third report, succeed isolated nine compounds from the Spiromastix sp. strain. MCCC 3A00308, which all were identified as new, namely spiromaterpene A-I (59–67) [12] (Fig. 2). In bioactivity assays, 25, 32–33, 39–40, 42, 46–47, 50–58, and 62–64 showed greater to moderate for anti-inflammatory (IC50: 8.6 to 50.0 µM) (Fig. 8). The remaining five compounds of sesquiterpenes were isolated from Penicillium commune MCCC 3A00940 (4 compounds) and Phomopsis lithocarpus FS508, namely conidiogenone J–K (68–69), conidiogenol B (70), cephalosporolide J (71) [14], and lithocarin A (72) [15], respectively (Fig. 2).
Diterpenes
Diterpenes are interesting compounds with structure diversity and significant bioactivities. This review reported, 81 new diterpenes isolated from two strains (Aspergillus wentii SD-310 and Botryotinia fuckeliana MCCC 3A00494) were described in 3 papers, which designate that the novelty of diterpenes from sea sediment-derived fungus is still very high. Respectively, aspewentin I-L (73–76) [16], wentinoid A-F (77–82) [17], and aphidicolin A1-A71 (83–153) [18] (Fig. 3). Bioactivity assays showed, 73–76 have antibacterial activity with Minimum Inhibitory Concentration (MIC) of 8–32.0 µg/ml) (Fig. 6), 77 showed antifungal activity against plant-pathogenic fungi and 90 showed significantly induced apoptosis on T24 and HL-60 (IC50: 2.5 and 6.1 μM, respectively) (Fig. 9).
Other terpenes
As many as 11 other new terpenes belong to steroids (6 compounds), monoterpenoids (4 compounds), and triterpene (2 compounds), they are 7b,8b-epoxy-(22E,24R)-24-methylcholesta-4,22-diene-3,6-dione (154) [19], penicisteroid D–H (155–159) [20], pestalotiolactone C and D (160 and 161) [9], aspermonoterpenoid A and B (162 and 163) [21], 1,4,23-trihydroxy-hopane-22,30-diol (164) [22], and lithocarin D (165) [23] (Fig. 3). They are generally isolated from Aspergillus and Penicillium, except 164 which is isolated from Phomopsis lithocarpus. Compounds 154, 160, 161, and 164 showed antibacterial activity (MIC: 16–32.0 µg/ml) (Fig. 6). 156, 161, and 163 inhibitory effects selectively against the A549 cancer cell line and 162–163 showed inhibitory Nitric Oxide (NO) production (Fig. 8). Moreover, 162 possessed a novel chained monoterpenoid skeleton.
 | Figure 2. Structures of the 1–72. [Click here to view] |
 | Figure 3. Structures of the 73–209. [Click here to view] |
Chromones
According to structure, 44 compounds can be roughly classified as chromone, including benzophenone (9 compounds), anthraquinone (7 compounds), tetramic acids (4 compounds), citrinin, phenylhydrazone, phthalide (each 3 compounds), and others. Namely, tenellone D–H (166–170) [15], tenellone J–M (171–174) [24], arthone A–C (175–177) [25], emerixanthone E (178) [26], phaseolorin I (179) [27], oxisterigmatocystin D (180) [28], 3,8-dihydroxy-2-methyl-9-oxoxanthene-4-carboxylic acid methyl ester (181) [29], cladosin H–K (182–185) [30], cladosporin A–D (186–188) [31], penoxahydrazone A–C (189–191) [32], farnesylemefuranone D–F (192–194) [7], engyodontiumin A (195) [33], sarcopodinol A–B (196–197) [34], arthone D and E (198 and 199) [25], coniochaetone J (200) [29], 5,5-dichloro-1-(3,5- dimethoxyphenyl)-1,4-dihydroxypentan-2-one (201), 2,3,4-trihydroxybutyl cinnamate (202) [35], diaporindene E–I (203–207) [24], 5-Hydroxy-dihydrodemethylsorbicillin (208) [36], and aladothalen (209) [37] (Fig. 3). The chromones were isolated from a diverse of marine sediments fungi inclusive to 10 genera as Arthrinium sp., Aspergillus sp., Cladosporium sp., Cystobasidium laryngis, Diaporthe phaseolorum, Emericella sp., Engyodontium album, Penicillium sp., Phomopsis lithocarpus, and Sarcopodium sp.
 | Figure 4. Structures of the 210–296. [Click here to view] |
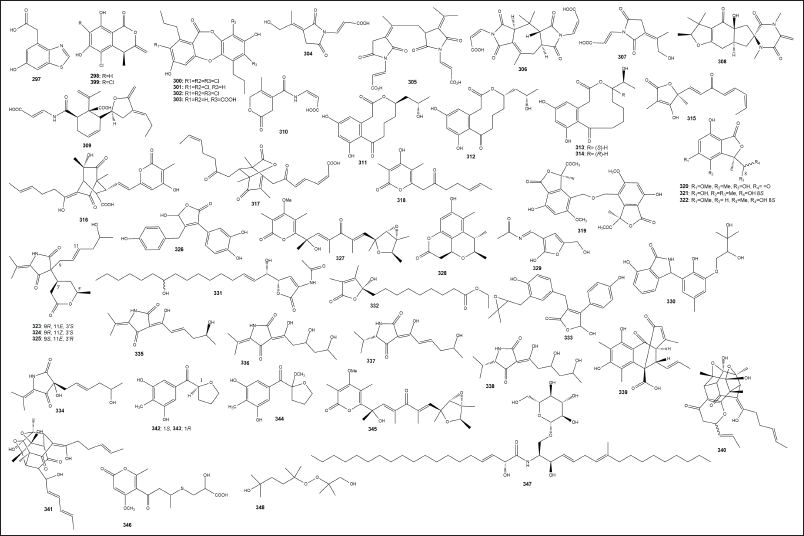 | Figure 5. Structures of the 297–348. [Click here to view] |
In the bioassays, more than 50% (29 compounds) of the chromones showed biological activities including cytotoxic (15 compounds), antimicrobial (9 compounds), anti-inflammatory (3 compounds), antioxidants (3 compounds), and anti-diabetes (1 compound). Compounds 188 and 208 showed to have 2 biological activities, respectively, cytotoxicity with antioxidant and antibacterial with anti-diabetes (Fig. 9). The 208 showed further strong anti-diabetes activity than control (acarbose) with IC50 value of 36.0 μM (Fig. 9). Based on the MIC value, compounds 189–194, 208, 209, and 178 have moderate to weak antibacterial activities with broad-spectrum (Fig. 6). Based on cytotoxic activities, 183–185 showed potential cytotoxicity (IC50: 2.8, 6.8, and 5.9 μM, respectively) and 170, 172–174, 186–188, 196, 197, 200, 201, and 206 have moderate to weak cytotoxicity (Fig. 7). Compounds 177 and 188 showed strong antioxidant effects on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and 180 has moderate antioxidant effect (Fig. 9).
Alkaloids
A total of 44 alkaloids were reported in this review, divided into derivatives thiodiketopiperazine (13 Compounds), diphenazine (6 compounds), azaphilone, tricyclic cyclopiazonic acid, phenazine, quinazoline (each, 3 compounds), diketopiperazine (2 compounds), roquefortine (1 compound), and other, were characterized from the fungus Aspergillus, Chaetomium, Cystobasidium, Epicoccum, Eutypella, and Penicillium. These compounds are, (±)-5-hydroxydiphenylalazine A (210), 5’-hydroxy-6’-ene-epicoccin G (211), 7’-demethoxyrostratin C (212) [38], 7-dehydroxyepicoccin H (213), 7-hydroxyeutypellazine F (214) [39], 7-methoxy-7’-hydroxyepicoccin G (215), 8’-acetoxyepicoccin D (216) [38], eutypellazine N–S (217–222) [40], phenazostatin E–J (223–228) [41], N-glutarylchaetoviridin A–C (229–231) [42], asperorydine N–P (232–234) [43], 6-[1-(2-aminobenzoyloxy)ethyl]-1-phenazinecarboxylic acid (235), saphenic amide (236), saphenol (237) [44], 29-hydroxyfumiquinazoline C (238) [22], penoxazolone A and B (239 and 240) [32], secofumitremorgin A and B (241 and 242) [22], roquefortine J (243) [45], 10R-15-Methylpseurotin A (244) [22], 5-Deoxypyroglutamyl-pyroglutamylleucinmethylester (245) [46], acremolin D (246) [47], aculeaquamide A (247) [48], adeninylpyrenocine (248) [35], aspergillusine A (249) [28], aurantiomoate C (250), methyl-2-hydroxy-3-methylbutanoyl-L-leucinate (251) [46], ozazino-cyclo-(2,3-dihydroxyl-trp-tyr) (252) [35], and penigrisamide (253) [46] (Fig. 4).
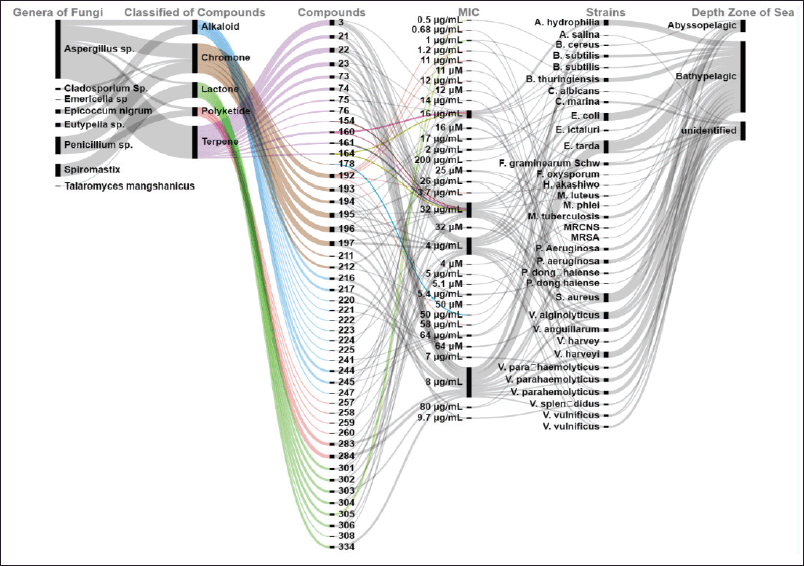 | Figure 6. Distributions of antibacterial activities of new compounds produced by marine sediments fungi. [Click here to view] |
Compounds 213, 214, 217–222, 238, 241–242, and 244 (12 compounds) have antibacterial activity (MIC in Fig. 9). Cytotoxic assay showed, 212, 228, 231, and 247 have potent activity with IC50 of 9.52, 1.0, 6.6, and 1.9 μM, respectively, and 243 and 246 moderate cytotoxicity (IC50 of 19.5 and 20 μM, respectively) (Fig. 7). Other, 235 and 237 have anti-inflammatory by NO production inhibition activity (Fig. 8), meanwhile 239 and 240 have strong anti-phytoplankton (MIC: 0.57 and 1.2 µg/ml, respectively) (Fig. 9).
Polyketides
There were 13 studies that reported the discovery of novel polyketides, comprising 43 compounds, namely fiscpropionate A–F (254–259) [49], aspertriol A and B (260 and 261) [50], aspercoumarine acid (262), asperphenylpyrone (263), graphostrin A–I (264–272) [51], hawatide A–G (273–279) [52], 1,2-didehydropeaurantiogriseol E (280), 9-dehydroxysargassopenilline A (281) [53], 6,7-Dihydroxy-3,7-dimethyloctanamide (282), 9-Hydroxy-3,7-epoxydecanoic acid (283), methyl-3,7,9-trihydroxydecanate (284) [43], 5-[(2R/S)-2-hydroxypropane-1-yl]-2,6-dimethlbenzene-1,3-diol (285), coniochaetone L (286) [54], 4,8-dimethoxy-1-naphthol (287), 1’-hydroxy-4’,8,8’-trimethoxy[2,2’]binaphthalenyl-1,4-dione (288), hypoxone A (289) [55], 12β-Chloro-3,9α,11β,13β,16-pentahydroxy-8,9,10,11,12,13-hexahydro-6(7H)-one (290), 3,11α,12β,13β,16-Pentahydroxy-11,12-dihydroperylen-6(13H)-one (291) [56], phaseolorin G and H (292 and 293) [27], 2’-hydroxy bisdechlorogeodin (294), globosuxanthone F (295) [57], and myrothin (296) [58] (Fig. 4). The compounds inclusive to 9 genera as Alternaria, Aspergillus, Diaporthe, Graphostroma, Hypoxylon, Myrothecium, Paraconiothyrium, Penicillium, and Pleosporales.
Liu et al. reported, 254–257 potent inhibitory against Mycobacterium tuberculosis (MIC = 5.1, 12, 4.0, and 11 μM, respectively) and 280 and 281 inhibited pathogenic bacteria (MIC in Fig. 6). The 276, 287–289, and 295 showed have cytotoxic activity of which 288 and 295 have potent activity (IC50: 1.9 and 0.45 μM, respectively) (Fig. 7). Other, 262, 263, and 291 showed moderate anti-inflammatory and 259 inhibitory activities against α-glucosidase (Fig. 8).
Lactones
Thirteen reports by Niu et al. [59], Zhang et al. [60], Wu et al. [61], Pang et al. [36], Luo et al. [62], Huang et al. [63], Xing et al. [46], Amin et al. [31], Ding et al. [56], Hu et al. [23], Yan et al. [22], Yang et al. [64], and Zeng et al. [65] were reported as 37 new lactones compounds, namely spiromastibenzothiazole A (297), spiromastimellein A and B (398 and 399), spiromastixone P–S (300–303), 10-hydroxy-8-demethyltalaromydine (304), ditalaromylectones A and B (305 and 306), 11-hydroxy-8-demethyltalaromydine (307), talaromanloid A (308), talaromydene (309), talaromylectone (310), sumalactone A–D (311–314), 5,6-Dihydrovertinolide (315), bisorbicillpyrone A (316), dihydrotrichodermolidic acid (317), sorbicillpyrone A (318), leptosphaerin J–M (319–322), cladosporiumin A–C (323–325), 8-Hydroxyhelvafuranone (326), verrucosidinol B (327), cladosporin C (328), 2-(N-Vinylacetamide)-4-hydroxymethyl-3-ene-butyrolactone (329), lithocarlactam A (330), sphingofungin I (331), sinulolide I (332), and (±)-asperteretal F (333) (Fig. 5). Twelve of them have biological activity, including antibacterial (compounds 298–303, 305, and 331) (Fig. 6), cytotoxicity (compounds 328 and 330) (Fig. 7), antidiabetes (compound 316) (Fig. 9), and antifungal (compound 332) (Fig. 9). The 302 potent activities with MIC of 0.5–1.0 mg/ ml (Fig. 6).
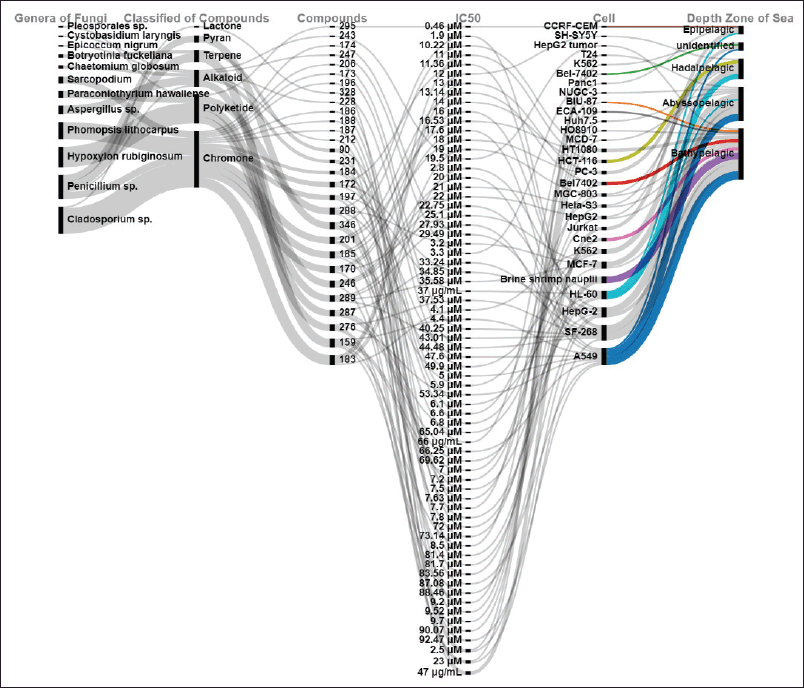 | Figure 7. Distributions of cytotoxicity activities of new compounds produced by marine sediments fungi. [Click here to view] |
Other compounds
Five tetramic acid isolated from Cladosporium sp. SCSIO z0025, namely cladosporiumin D–H (334–338) [63], without cytotoxic, antibacterial, and acetylcholinesterase (AChE) inhibitory activities. Moreover, three new bisorbicillinoids, 10-Methylsorbiterrin A (339), epitetrahydrotrichodimer ether (340), and demethyldihydrotrichodimerol (341) [36] (Fig. 5) produced of Penicillium sp. SCSIO06871., which 341 moderate inhibitory activity against α-glycosidase (Fig. 8). Three new furans were reported by Lu et al. [58] (−)-1S-myrothecol (342), (+)-1R-myrothecol (343), and methoxy-myrothecol (344) from fermented of Myrothecium sp. BZO-L062 (Fig. 5). The 342 and 343 exhibited anti-inflammatory and antioxidant activities (Fig. 8). Two new pyran, reported by Xing et al. [46] and Tang et al. [35] verrucosidinol A (345) and 2-hydroxyl-3- pyrenocine-thio propanoic acid (346) isolated from P. griseofulvum MCCC 3A00225 and P. citreonigrum XT20-134, deep-sea-derived fungus, respectively (Fig. 5), which the 346 potent cytotoxicity (IC50 = 7.63 and 10.22 µM) activity against tumors cell hepatoma Bel7402 and human fibrosarcoma HT1080, respectively (Fig. 7). In addition, Ding et al. [56] and Fengyi et al. [66] reported a compound cerebroside and acyclic peroxide, namely chrysogeside F (347), and asperoxide A (348), respectively (Fig. 5).
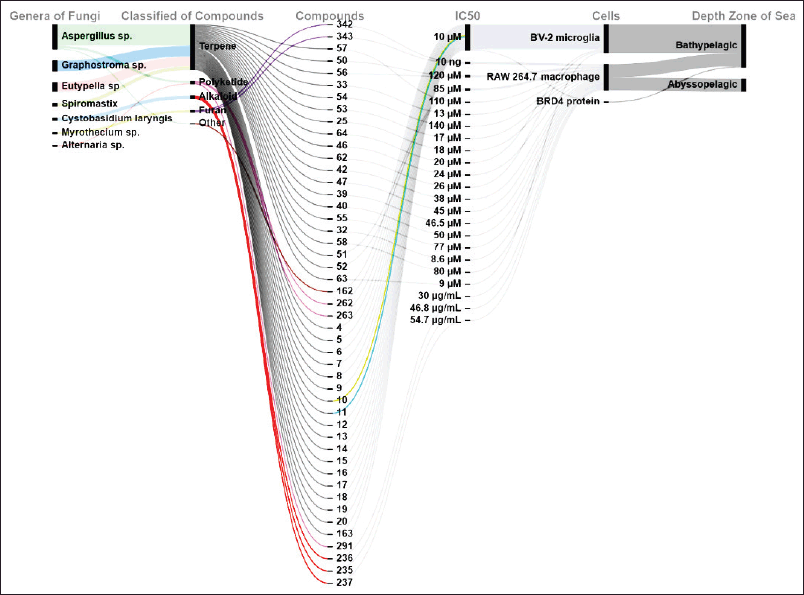 | Figure 8. Distributions of anti-inflammatory activities of new compounds produced by marine sediments fungi. [Click here to view] |
DIVERSITY OF ACTIVITIES
Antibacterial
Marine fungi represent a future source for the development of new antibiotics and investigation into sea-deep ecosystems is obligatory to meet the important demand for new powerful antibiotics [67]. There is a significantly higher possibility of discovering new antibacterial drug leads in sediment marine-derived fungi than in terrestrial environments [68]. According to this study, Aspergillus fungi produced a significant of antibacterial compounds, accounting for more than 50%. The new antibacterial was classified into terpenes, alkaloids, chromones, polyketides, alkaloids, and lactones. Among them, the fungi from the bathypelagic zone are the dominant producers of new compounds that have antibacterial activity, comprising more than 60% of total antibacterial compounds. Most of the compounds showed broad-spectrum antibacterial. Compounds 305 and 192 showed powerful antibacterial with (MIC shown in Fig. 6).
Cytotoxicity
Cytotoxicity is one of the biological activities approved by NPs produced by deep-sea-derived fungi [68]. Deep-sea-derived fungi are unusually adapted to hard environmental conditions, which empowers them to produce cytotoxic compounds [68]. In this report, the cytotoxic compounds were dominated by genera of Cladosporium, Penicillium, Hypoxylon, and Phomopsis. Most of the cytotoxic compounds (more than 50%) were classified as choromones, polyketides, and alkaloids. About 41% of them were isolated from fungi in the bathypelagic zone (Fig. 7).
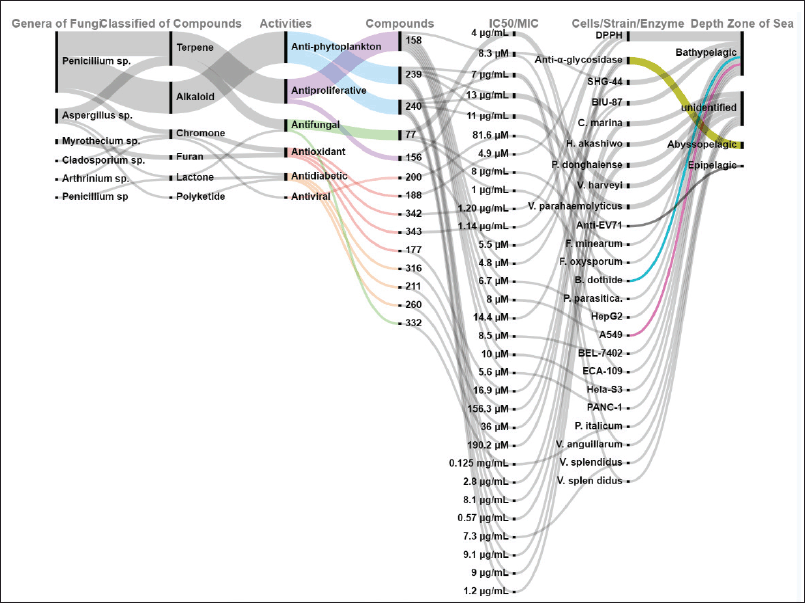 | Figure 9. Distributions of others activities of new compounds produced by marine sediments fungi. [Click here to view] |
Anti-inflammatory and other activities
Marine compounds obtained from deep-sea fungi are an important source of anti-inflammatory agents [69]. The compounds demonstrate inhibition of several inflammatory agents including enzymes [69]. According to this review, 47 compounds showed anti-inflammatory activity, with 38 of them classified as terpenes. Most of them showed production inhibition activity on BV-2 microglia and RAW 264.7 macrophage cells. All of the inflammatory compounds were isolated from fungi isolated in the abyssopelagic and bathypelagic zones. The IC50 of all compounds is shown in Figure 8. Other activities include antidiabetic, antifungal, antioxidant, anti-phytoplankton, antiproliferative, antiproliferative, and antiviral (Fig. 9).
CONCLUSION
The studies of marine NPs have highlighted that have unique structural scaffolds, including from marine-sediment fungi. These fungi have been found at depths zone with characteristics of strong environments that typical absence of light, low oxygen, and high pressure. To survive in at strong environment, these organisms have developed unique metabolic pathways and their NPs can have chemical and bioactivity diversity. In addition, the novelty of NPs from the marine-sediment fungi is still quite high.
This report provides an overview of the diversity of compounds isolated from the marine sediment fungi. At depths, the zone showed the characteristics and novelty of compounds that differ both in structure and activity. However, the activity of each of these compounds still needs further testing considering that the tests carried out are still at the early stages of proving activity, not yet on various activities. Further activity tests will show potential activity in compounds that do not yet have activity in this report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
Supported by grants from Ministry of Education, Culture, Research, and Technology of Indonesia (No.200/SPK/D.D4/PPK.01.APTV/VI/2023 and 3547/LL8/AL.04/2023).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hasan S, Ansari MI, Ahmad A, Mishra M. Major bioactive metabolites from marine fungi: a review. Bioinformation. 2015;11(4):176–81. CrossRef
2. Safwan S, Sucilawaty R, Wardani, A K. Diversity of source, chemistry, and bioactivities of secondary metabolites from algae-associated and sponge-associated fungi. 2023;10:45–58. CrossRef
3. Pham TT, Dinh KV, Nguyen VD. Biodiversity and enzyme activity of marine fungi with 28 new records from the tropical coastal ecosystems in Vietnam. Mycobiology. 2021;49(6):559–81. CrossRef
4. Saide A, Lauritano C, Ianora A. A treasure of bioactive compounds from the deep sea. Biomedicines. 2021;9(11):1556. CrossRef
5. Skropeta D, Wei L. Recent advances in deep-sea natural products. Nat Prod Rep. 2014;31(8):999–1025. CrossRef
6. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. CrossRef
7. Chi LP, Li XM, Wan YP, Li X, Wang BG. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J Nat Prod. 2020;83(12):3652–60. CrossRef
8. Niu S, Yang L, Zhang G, Chen T, Hong B, Pei S, et al. Phenolic bisabolane and cuparene sesquiterpenoids with anti-inflammatory activities from the deep-sea-derived Aspergillus sydowii MCCC 3A00324 fungus. Bioorg Chem. 2020;105:104420. CrossRef
9. Li XD, Li XM, Yin XL, Li X, Wang BG. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Marine Drugs. 2019;17(10):563. CrossRef
10. Li XD, Li X, Li XM, Yin XL, Wang BG. Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat Prod Res. 2021;35(22):4265–71. CrossRef
11. Niu S, Liu D, Shao Z, Proksch P, Lin W. Eremophilane-type sesquiterpenoids in a deep-sea fungus Eutypella sp. activated by chemical epigenetic manipulation. Tetrahedron. 2018;74(51):7310–25. CrossRef
12. Guo X, Meng Q, Niu S, Liu J, Guo X, Sun Z, et al. Epigenetic manipulation to trigger production of guaiane-type sesquiterpenes from a marine-derived Spiromastix sp. fungus with antineuroinflammatory effects. J Nat Prod. 2021;84(7):1993–2003. CrossRef
13. Niu S, Xie CL, Zhong T, Xu W, Luo ZH, Shao Z, et al. Sesquiterpenes from a deep-sea-derived fungus Graphostroma sp. MCCC 3A00421. Tetrahedron. 2017;73(52):7267–73. CrossRef
14. Niu S, Fan Z, Tang X, Liu Q, Shao Z, Liu G, et al. Cyclopiane-type diterpenes from the deep-sea-derived fungus Penicillium commune MCCC 3A00940. Tetrahedron Let–. 2018;59(4):375–8. CrossRef
15. Xu JL, Liu HX, Chen YC, Tan HB, Guo H, Xu LQ, et al. Highly substituted benzophenone aldehydes and eremophilane derivatives from the deep-sea derived fungus Phomopsis lithocarpus FS508. Marine Drugs. 2018;16(9):329. CrossRef
16. Li XD, Li X, Li XM, Xu GM, Liu Y, Wang BG. 20-nor-isopimarane epimers produced by Aspergillus wentii SD-310, a fungal strain obtained from deep sea sediment. Marine Drugs. 2018;16(11):440. CrossRef
17. Li X, Li XD, Li XM, Xu GM, Liu Y, Wang BG. Wentinoids A–F, six new isopimarane diterpenoids from Aspergillus wentii SD-310, a deep-sea sediment derived fungus. RSC Advances. 2017;7(8):4387–94. CrossRef
18. Niu S, Xia JM, Li Z, Yang LH, Yi ZW, Xie CL, et al. Aphidicolin chemistry of the deep-sea-derived fungus Botryotinia fuckeliana MCCC 3A00494. J Nat Prod. 2019;82(8):2307–31. CrossRef
19. Chi LP, Yang SQ, Li XM, Li XD, Wang BG, Li X. A new steroid with 7β,8β-epoxidation from the deep sea-derived fungus Aspergillus penicillioides SD-311. J Asia Nat Prod Res. 2021;23(9):884–91. CrossRef
20. Xie CL, Zhang D, Xia JM, Hu CC, Lin T, Lin YK, et al. Steroids from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475 induced apoptosis via retinoid X receptor (RXR)-α pathway. Marine Drugs. 2019;17(3):178. CrossRef
21. Niu S, Yang L, Chen T, Hong B, Pei S, Shao Z, et al. New monoterpenoids and polyketides from the deep-sea sediment-derived fungus Aspergillus sydowii MCCC 3A00324. Marine Drugs. 2020;18(11):561. CrossRef
22. Yan LH, Li XM, Chi LP, Li X, Wang BG. Six new antimicrobial metabolites from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Marine Drugs. 2022;20(1):4. CrossRef
23. Hu J, Wang N, Liu H, Li S, Liu Z, Zhang W, et al. Secondary metabolites from a deep-sea derived fungal strain of Phomopsis lithocarpus FS508. Nat Prod Res. 2023;37(14):2351–8. CrossRef
24. Liu HB, Liu ZM, Chen YC, Tan HB, Li SN, Li DL, et al. Cytotoxic diaporindene and tenellone derivatives from the fungus Phomopsis lithocarpus. Chin J Nat Med. 2021;19(11):874–80. CrossRef
25. Bao J, He F, Yu JH, Zhai H, Cheng ZQ, Jiang CS, et al. New chromones from a marine-derived fungus, Arthrinium sp., and their biological activity. Molecules. 2018;23(8):1982. CrossRef
26. Fredimoses M, Zhou X, Ai W, Tian X, Yang B, Lin X, et al. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp SCSIO 05240. Nat Prod Res. 2019;33(14):2088–94. CrossRef
27. Niu Z, Chen Y, Guo H, Li SN, Li HH, Liu HX, et al. Cytotoxic polyketides from a deep-sea sediment derived fungus Diaporthe phaseolorum FS431. Molecules. 2019;24(17):3062. CrossRef
28. Wu ZH, Liu D, Xu Y, Chen JL, Lin WH. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin J Nat Med. 2018;16(3):219–24. CrossRef
29. Liu Fa, Lin X, Zhou X, Chen M, Huang X, Yang B, et al. Xanthones and quinolones derivatives produced by the deep-sea-derived fungus Penicillium sp. SCSIO Ind16F01. Molecules. 2017;22(12):1999. CrossRef
30. Zhang Z, He X, Wu G, Liu C, Lu C, Gu Q, et al. Aniline-tetramic acids from the deep-sea-derived fungus Cladosporium sphaerospermum L3P3 cultured with the HDAC inhibitor SAHA. J Nat Prod. 2018;81(7):1651–7. CrossRef
31. Amin M, Zhang XY, Xu XY, Qi SH. New citrinin derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z015. Nat Prod Res. 2020;34(9):1219–26. CrossRef
32. Liu YP, Fang ST, Shi ZZ, Wang BG, Li XN, Ji NY. Phenylhydrazone and quinazoline derivatives from the cold-seep-derived fungus Penicillium oxalicum. Marine Drugs. 2021;19(1):9. CrossRef
33. Wang W, Li S, Chen Z, Li Z, Liao Y, Chen J. Secondary metabolites produced by the deep-sea-derived fungus Engyodontium album. Chem Nat Compd. 2017;53(2):224–6. CrossRef
34. Matsuo H, Nonaka K, Nagano Y, Yabuki A, Fujikura K, Takahashi Y, et al. New metabolites, sarcopodinols A and B, isolated from deep-sea derived fungal strain Sarcopodium sp. FKJ-0025. Biosci Biotechnol Biochem. 2018;82(8):1323–6. CrossRef
35. Tang XX, Liu SZ, Yan X, Tang BW, Fang MJ, Wang XM, et al. Two new cytotoxic compounds from a deep-sea Penicillum citreonigrum XT20-134. Marine Drugs. 2019;17(9):509. CrossRef
36. Pang X, Zhou X, Lin X, Yang B, Tian X, Wang J, et al. Structurally various sorbicillinoids from the deep-sea sediment derived fungus Penicillium sp. SCSIO06871. Bioorg Chem. 2021;107:104600. CrossRef
37. Fan C, Zhou G, Wang W, Zhang G, Zhu T, Che Q, et al. Tetralone derivatives from a deep-sea-derived fungus Cladosporium Sp. HDN17-58. Nat Prod Commun. 2021;16(4):1934578X211008322. CrossRef
38. Chi LP, Li XM, Li L, Li X, Wang BG. Cytotoxic Thiodiketopiperazine derivatives from the deep sea-derived fungus Epicoccum nigrum SD-388. Marine Drugs. 2020;18(3):160. CrossRef
39. Chi LP, Li XM, Li X, Wang BG. New antibacterial thiodiketopiperazines from the deep sea sediment-derived fungus Epicoccum nigrum SD-388. Chem Biodiversity. 2020;17(8):e2000320. CrossRef
40. Niu S, Liu D, Shao Z, Proksch P, Lin W. Eutypellazines N−S, new thiodiketopiperazines from a deep sea sediment derived fungus Eutypella sp. with anti-VRE activities. Tetrahedron Lett. 2017;58(38):3695–9. CrossRef
41. Lee HS, Kang JS, Cho DY, Choi DK, Shin HJ. Isolation, structure determination, and semisynthesis of diphenazine compounds from a deep-sea-derived strain of the fungus Cystobasidium laryngis and their biological activities. J Nat Prod. 2022;85(4):857–65. CrossRef
42. Sun C, Ge X, Mudassir S, Zhou L, Yu G, Che Q, et al. New glutamine-containing azaphilone alkaloids from deep-sea-derived fungus Chaetomium globosum HDN151398. Marine Drugs. 2019;17(5):253. CrossRef
43. Xiang Y, Zeng Q, Mai ZM, Chen YC, Shi XF, Chen XY, et al. Asperorydines N-P, three new cyclopiazonic acid alkaloids from the marine-derived fungus Aspergillus flavus SCSIO F025. Fitoterapia. 2021;150:104839. CrossRef
44. Lee HS, Kang JS, Choi BK, Lee HS, Lee YJ, Lee J, et al. Phenazine derivatives with anti-inflammatory activity from the deep-sea sediment-derived yeast-like fungus Cystobasidium laryngis IV17-028. Marine Drugs. 2019;17(8):482. CrossRef
45. Niu S, Wang N, Xie CL, Fan Z, Luo Z, Chen HF, et al. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J Antibiot. 2018;71(7):658–61. CrossRef
46. Xing CP, Chen D, Xie CL, Liu Q, Zhong TH, Shao Z, et al. Anti-food allergic compounds from Penicillium griseofulvum MCCC 3A00225, a deep-sea-derived fungus. Marine Drugs. 2021;19(4):224. CrossRef
47. Niu S, Chen Z, Pei S, Shao Z, Zhang G, Hong B. Acremolin D, a new acremolin alkaloid from the deep-sea sediment derived Aspergillus sydowii fungus. Nat Prod Res. 2022;36(19):4936–42. CrossRef
48. Wu J, Wang F, He LM, Zhou SY, Wang SB, Jia J, et al. Aculeaquamide A, cytotoxic paraherquamide from the marine fungus Aspergillus aculeatinus WHUF0198. Nat Prod Res. 2022;36(17):4382–7. CrossRef
49. Liu Z, Wang Q, Li S, Cui H, Sun Z, Chen D, et al. Polypropionate derivatives with mycobacterium tuberculosis protein tyrosine phosphatase B inhibitory activities from the deep-sea-derived fungus Aspergillus fischeri FS452. Jf Nat Prod. 2019;82(12):3440–9. CrossRef
50. Pan MH, Tian ZY, Hui Y, Xu W, Pan C, Cheng Z, et al. Two new compounds from the deep-sea-serived fungus Aspergillus sp. YPGA8. Rec Nat Prod. 2020;14:307–11. CrossRef
51. Niu S, Liu Q, Xia JM, Xie CL, Luo ZH, Shao Z, et al. Polyketides from the deep-sea-derived fungus Graphostroma sp. MCCC 3A00421 showed potent antifood allergic activities. J Agric Food Chem. 2018;66(6):1369–76. CrossRef
52. Chen S, Chen Y, Li S, Liu H, Li D, Liu Z, et al. Hawatides A–G, new polyketides from the deep-sea-derived fungus Paraconiothyrium hawaiiense FS482. Tetrahedron. 2021;93:132303. CrossRef
53. Li YH, Li XM, Li X, Yang SQ, Shi XS, Li HL, et al. Antibacterial alkaloids and polyketide derivatives from the deep sea-derived fungus Penicillium cyclopium SD-413. Marine Drugs. 2020;18(11):553. CrossRef
54. Guo C, Lin XP, Liao SR, Yang B, Zhou XF, Yang XW, et al. Two new aromatic polyketides from a deep-sea fungus Penicillium sp. SCSIO 06720. Nat Prod Res. 2020;34(9):1197–205. CrossRef
55. Zhang J, Chen Y, Liu Z, Bohong G, Gao X, Liu H, et al. Cytotoxic secondary metabolites from a sea-derived fungal strain of Hypoxylon rubiginosum FS521. Chin J Org Chem. 2020;40:1367. CrossRef
56. Ding H, Zhang D, Zhou B, Ma Z. Inhibitors of BRD4 protein from a marine-derived fungus Alternaria sp. NH-F6. Marine Drugs. 2017;15(3):76. CrossRef
57. Zhou J, Zhang H, Ye J, Wu X, Wang W, Lin H, et al. Cytotoxic polyketide metabolites from a marine mesophotic zone chalinidae sponge-associated fungus Pleosporales sp. NBUF144. Marine Drugs. 2021;19(4):186. CrossRef
58. Lu X, He J, Wu Y, Du N, Li X, Ju J, et al. Isolation and characterization of new anti-inflammatory and antioxidant components from deep marine-derived fungus Myrothecium sp. Bzo-l062. Marine Drugs. 2020;18(12):597. CrossRef
59. Niu S, Liu D, Shao Z, Huang J, Fan A, Lin W. Chlorinated metabolites with antibacterial activities from a deep-sea-derived Spiromastix fungus. RSC Advances. 2021;11(47):29661–7. CrossRef
60. Zhang K, Zhang X, Lin R, Yang H, Song F, Xu X, et al. New secondary metabolites from the marine-derived fungus Talaromyces mangshanicus BTBU20211089. Marine Drugs. 2022;20(2):79. CrossRef
61. Wu YH, Zhang ZH, Zhong Y, Huang JJ, Li XX, Jiang JY, et al. Sumalactones A–D, four new curvularin-type macrolides from a marine deep sea fungus Penicillium Sumatrense. RSC Advances. 2017;7(63):40015–9. CrossRef
62. Luo X, Lin X, Salendra L, Pang X, Dai Y, Yang B, et al. Isobenzofuranones andisochromenones from the deep-sea derived fungus Leptosphaeria sp. SCSIO 41005. Marine Drugs. 2017;15(7):204. CrossRef
63. Huang ZH, Nong XH, Liang X, Qi SH. New tetramic acid derivatives from the deep-sea-derived fungus Cladosporium sp. SCSIO z0025. Tetrahedron. 2018;74(21):2620–6. CrossRef
64. Yang Z, Kaliaperumal K, Zhang J, Liang Y, Guo C, Zhang J, et al. Antifungal fatty acid derivatives against Penicillium italicum from the deep-sea fungus Aspergillus terreus SCSIO 41202. Nat Prod Res. 2021;35(22):4394–401. CrossRef
65. Zeng Q, Zhong WM, Chen YC, Xiang Y, Chen XY, Tian XP, et al. A new butenolide derivative from the deep-sea fungus Aspergillus terreus SCSIO FZQ028. Nat Prod Res. 2020;34(14):1984–91. CrossRef
66. Lü F, Li X, Chi L, Meng L, Wang B. A new acyclic peroxide from Aspergillus nidulans SD-531, a fungus obtained from deep-sea sediment of cold spring in the South China Sea. J Oceanol Limnol. 2020;38(4):1225–32. CrossRef
67. Silber J, Kramer A, Labes A, Tasdemir D. From discovery to production: biotechnology of marine fungi for the production of new antibiotics. Marine Drugs. 2016;14(7):137. CrossRef
68. Zhao G, Tang W, Zhang J, Shi P, Li Y, Wang J, et al. Deep-sea-derived fungi as valuable producers of cytotoxic secondary metabolites and their leads potential. Front Mar Sci. 2022;9:929561. CrossRef
69. Xu J, Yi M, Ding L, He S. A Review of anti-inflammatory compounds from marine fungi, 2000–2018. Marine Drugs. 2019;17(11):636. CrossRef