INTRODUCTION
Essential oils (EOs) have long been recognized to have anti-inflammatory, immuno-modulatory, bronchodilatory, and antiviral activities, and they are now thought to have efficacy against the SARC-CoV-2 virus [1,2]. Eucalyptus is one of the sources of EOs used for many usages and remedies, including antiviral. Eucalyptus oil is an antibacterial agent and has long been used to treat colds, influenza, respiratory infections, rhinitis, and sinusitis. This reveals that 47 compounds were discovered in the EOs, with eucalyptol (1.8 cineol) being the major constituent [3]. Among many kinds of Eucalyptus oil, Eucalyptus citriodora oil proved the most efficacious antioxidant and antibacterial activity [4]. The major component of E. citriodora grown in Thailand leaf EO was citronellal (64.1%), followed by citronellol (10.06%); the content of 1.8 cineol or eucalyptol is only 1.64%. Meanwhile, other Eucalyptus, such as Eucalyptus urophyla and Eucalyptus deglupta, have a high eucalyptol content of 40.78% and 15.72%, respectively [5]. The yield of EO from E. citriodora leaves grown in Indonesia is higher than that of other Eucalyptus species, at 2.88%. Citronellal accounts for 68.35% of the EO, followed by isopulegol (10.14%) and citronellol (3.99%), with 1.8 cineol accounting for only 0.43% [6].
Eucalyptus is gaining popularity as one of the COVID-19 adjuvant therapeutics recently developed based on in vitro, and a clinical study showed effectiveness in inhibiting virus replication [7]. Other promising products to help with throat illness derived from Eucalyptus are lozenges candy [8], or mouthwash. Eucalyptus mouthwash is a good alternative for helping relieve throat illness or sanitizing the mouth and throat for its antimicrobial activity.
Eucalyptus and peppermint (Mentha piperita L) oil have been noted to prevent the growth of certain microorganisms. Eucalyptus oil inhibits the augmentation of Escherichia coli, Staphylococcus aureus, and Streptococcus mutans [9]. Meanwhile, peppermint oil inhibits the growth of the S. mutans bacterium [10]. Based on the antibacterial activity test, the clear zones produced in E. coli and S. aureus bacteria were 13.1 and 17.4 mm, and the inhibition was 25.92 ± 2.66 mm [11].
Eucalyptol was considered not to be an ocular corrosive or severe irritant. Eucalyptol is also renowned for its mucolytic and bronchodilatory effects. In a long-term oral trial with mice, eucalyptol was investigated as a toothpaste ingredient. Most studies using Eucalyptus oil in mouthwash formulas do not mention the Eucalyptus variety used. The most widely used eucalyptus oil on the market is the E. globulus. E. citriodora oil was used in this study since it has good antioxidant and antibacterial activity. Eucalyptus oil mouthwash could be an excellent and affordable oral hygiene product [12].
Many mouthwashes use alcohol as one of the ingredients. High alcohol concentration in mouthwash can induce mucosal ulceration, gingivitis, discomfort, and possibly an increased chance of developing oral cancer [13]; it reduces salivation and causes lousy breath problems [14]. Alcohol also causes the dissolution of polymers used for dental restorations [15]. An alcohol-free mouth rinse including EOs—listerine zero (LZ) and an alcohol-based EOs mouth rinse (EO+) demonstrated the same inhibitory activity on plaque regrowth [16].
The mouthwash formula was prepared in the form of nanoemulsion. Nanoemulsion preparation provides distinct advantages, such as improved oil mixture clarity, stability, and efficacy. Besides, nanoemulsions with small particles have more ability and are more constant than nonemulsions [16]. Mouthwash’s clearness and stability are likely more pleasant than turbid emulsion. Nanoemulsion may further enhance the antibacterial capabilities of these oils [17]. The nanoemulsion formula usually demands a surfactant and co-surfactant, such as Tween-80 and PEG 400. The ratio between these emulsifiers needs to be set to get proper properties. The ratio of surfactant and co-surfactant in preparation of mouthwash nanoemulsion derived from EO and heat shock treatment on the resulting product has not undergone in-depth research. The study aimed to determine the effect of emulsifier ratio and shock treatment effect in nanoemulsion formulation of mouthwashes based on eucalyptus and peppermint oil.
MATERIALS AND METHODS
The material used was eucalyptus oil of E. citriodora from the Laboratory of Indonesian Research Institute of Spice and Medicinal Crops (Balittro), Bogor. The supported chemicals were emulsifier Tween-80 and PEG-400, peppermint, glycerin, Na saccharin, Na benzoate, natural coloring, aquadest, and other chemicals for analysis.
Mouthwash preparation
Preparation of the mouthwash uses a formula according to Yosephine et al. [18] with minor modifications. The total volume base was 100 ml. The nano-emulsification process used low energy utilizing spontaneous diffusion and phase inversion. A nanoemulsion is formed when the dispersed phase (a mixture of oil and emulsifier) diffuses spontaneously into the dispersing phase (water). The volumes of eucalyptus and peppermint oil were equal, each 0.5 ml, so the total volume of EO was 1.0 ml. Variations were made on the volume of surfactant and cosurfactant, where the total volume of surfactant and cosurfactant was 5.0 ml. The ratio variations between surfactants (Tween 80) and cosurfactant (PEG 400) were 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5, respectively, as shown in Table 1.
Preparation of emulsion was carried out by mixing the oil phase (in this formulation, eucalyptus oil and peppermint oil) with surfactant (Tween 80) and cosurfactant (PEG 400). The oil phase was homogenized in a glass beaker using a continuous stirrer at 700 RPM for 5 minutes. The water phase, consisting of distilled water, Na-saccharine, and Na benzoate, was homogenized in a separate beaker glass using a continuous stirrer for 1 minute at 700 RPM. Then, add 94 ml water phase to the oil phase and agitate at 800 RPM simultaneously until all of the water phase volume is inserted into the oil phase. The mixed solution/emulsion was stirred at 800 RPM and added with green food coloring 0.02 ml while stirring.The mouthwash formulas were tested physically after production, which included clarity using a spectrophotometer, pH using a pH meter, color determination using a Chromameter (Minolta), determination of density using a pycnometer, particle size using distribution particle size analyzer (PSA). Moreover, the microbiological analysis was carried out by determining the inhibition activity using S. aureus. The stability test was done after the mouthwash products were stored for about one week at ambient temperature. The stability test used the thermic shock method. After that, the products were tested again for their particle size distribution characteristics, poly-dispersity index (PdI), specific gravity (SG), RI, and color.Organoleptic tests involving 30 respondents, including aroma, clarity, taste, relief feel, and overall liking were carried out on four product formulations that had high clarity compared to commercial mouthwash with herbal labels. The organoleptic tests used an assessment sheet with a score range of 1–5 (very dislike, dislike, fair, like, very like).
Emulsion transmittance
The clarity/transmittance of the mouthwash emulsion formed was observed using a spectrophotometer with the % transmittance (%T) parameter at a wavelength of 650 nm—the clarity measurement used distilled water as a comparison.
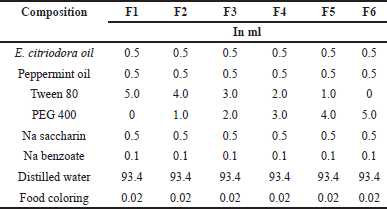 | Table 1. Formulation of 100 ml eucalyptus and peppermint oil-based mouthwash. [Click here to view] |
Stability test
A stability test using the thermic shock method was carried out by storing the mouthwash at a high temperature of 60°C and a low temperature of 4°C alternately, for one day each (for seven days), then allowed to stand at room temperature. After seven days, all samples were stored at ambient temperature until being analyzed [18].
Statistical analysis
The data were statistically analyzed using the software IBM SPSS version 23. Data measurements were subjected to analysis of variance (ANOVA) to assess the emulsifier’s varietal ratio during the stability test. The Duncan’s multiple range test was performed to differentiate means of data with significant differences (p < 0.05) were observed. Meanwhile, the organoleptic test data were evaluated using Kruskal Wallis, followed by the Mann-Whitney test at a 5% level to determine if there was a significant difference.
RESULTS AND DISCUSSION
Physical characteristics
The preparation of mouthwash based on using nano-emulsification is a low-energy process using spontaneous diffusion and phase inversion. In this process, a nanoemulsion was formed by diffusion of the dispersed phase (a mixture of oils and emulsifiers) into the dispersing phase (water), which occurred spontaneously. This diffusion process produced nano-scale oil droplets in the water phase (oil in water or o/w). In the inversion phase mechanism, the formation of nanoemulsions occurs in two stages: water formation in oil (w/o) emulsion, which then reverses the phase to o/w. The mouthwash in this study was green in color, homogeneous, generally transparent, had a pleasant flavor, smelled of a distinctive fresh aroma of lemon eucalyptus oil, had a pH of around 5.6–5.9, and was low in density, as shown in Figure 1. The appearance of the mouthwash showed clear solutions except F5 with the ratio of Tween and PEG 1.0:4.0. The clear solution indicates a suitable emulsion with a small droplet size. Nanoemulsions with a small size particle/droplet (nm) may produce clear or transparent preparations or are slightly cloudy. Their small-sized particle make them ideal for food and drug industries due to it has large surface area will help in the effective transportation of active substances through a semipermeable membrane [19].
Based on the research results, the best ratio that produces the highest clarity is the formula F3 percentage of oil: Tween and PEG = 1:3:2 (17.5%:52.5%:35%) (Table 2). The Tween and PEG 3:2 ratio gave the highest clarity value of 70.35%. The particle size of the mouthwash formula F3 was 13.45 nm with a shallow polydispersity index (PdI) of 0.08 (Table 3), indicating that the emulsion droplet size was uniform. The PdI is a significant factor because it is related to the size uniformity of the nanoemulsion; a small PdI value indicates better size uniformity. A PdI below 0.3 indicates good mono dispersity or size uniformity [20]. The particle size of mouthwash ranges from 13.3 to 108.71 nm. This range agrees with Serrano et al. [21] that the oil combination of nanoemulsion mouthwash formula should have a particle size of 20–200 nm. Table 3 shows that the higher the surfactant ratio of Tween 80, the smaller the particle size, and vice versa. Although surfactant T-80 is an excellent emulsifier for tiny emulsion droplets, a high concentration is required to form a stable emulsion. The addition of co-surfactants can lead to greater penetration of the oil phase in the hydrophobic region of the surfactant monomer and thus lower the interfacial voltage [22]. PEG-400, a co-surfactant, influences emulsification time more than droplet size reduction [23]. The co-surfactant will tuck and form a space between the surfactants, resulting in a more swollen shape with high fluidity that can create nanoemulsions faster. Research by Juniatik et al. [24] showed that the average particle size of the mouthwash formula using Tween and PEG 400 was 21.4 nm with a PdI of 0.324.
 | Figure 1. The appearance of mouthwash with different ratios of emulsifier (sequentially from left to right, i.e., F1 to F6). [Click here to view] |
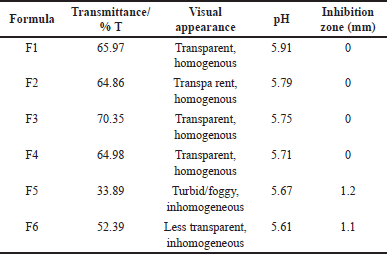 | Table 2. Physical and microbiological properties of the mouthwash. [Click here to view] |
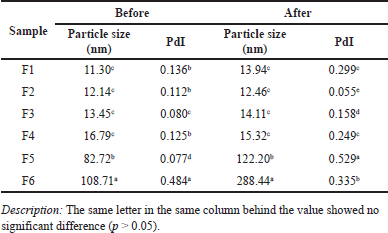 | Table 3. Particle size and Pdl of mouthwash formula before and after stability test. [Click here to view] |
The size of the dispersed phases considerably affected the emulsion’s appearance. When the light passes through the emulsion system with tiny droplet size, the light beam is forwarded so that the color of the solution appears clear, and the resulting transmittance increases [25]. Furthermore, a high transmittance rating reflects the clarity of a given mixture. The smaller a mixture’s particle size, the higher its transmittance value. Besides the droplet size, the concentration of EO also influences turbidity. The interaction of EO components and additives causes turbidity to increase with increasing concentration. The visual appearance of the mouthwash showed that almost all mouthwashs are clear except formula 5 with the ratio of Tween and PEG = 4:1. Figure 1 shows the appearance of all samples. The turbid solution is also shown from the lowest value of % transmittance.
pH
Differences in the ratio of surfactant and co-surfactant have no effect on the pH of the mouthwash produced. The pH value ranges from 5.61 to 5.91. PEG 400 has a pH of 4–7.5 [26]. Meanwhile, Tween 80 has a higher pH in the range of 6–8. Since the pH range of surfactant and co-surfactant is not so different, the pH of the resulting mouthwash is in the narrow range. Moreover, the pH of the mouthwash may be affected by many factors, not only the emulsifier’s pH but also the interaction of the materials used in the formula. This result is different from the research, which showed that the higher the concentration of PEG 400 in the kepayang oil nanoemulsion formula, the lower the pH of the preparation [27]. The pH of a solution impacts the type of bacteria that can thrive. The mouthwash’s pH generated is within the oral cavity’s pH range, ensuring that it does not irritate the oral mucosa when swallowed. As a result, the pH of a mouth rinse solution should be between 5.5 and 7.9, which corresponds to the pH of the oral cavity [28]. According to The Herbal Medicines Quality Standards, the pH of herbal mouth rinse shall range from 5 to 7 [29].
Antibacterial test
The result of the antibacterial inhibitory test against S. aureus showed that the mouthwash containing eucalyptus oil had no inhibition except for F5 and F6 treatments, with the inhibition zone being 1.2 and 1.1 mm (Fig. 2). This might happen because the inhibitory zone was lower than 1 mm. The concentration of both EOs was relatively low, only 0.5% each. Although eucalyptus and peppermint oil possess antimicrobial solid agents, the mouthwash formula’s inhibition activity becomes too small.
 | Figure 2. The inhibition zone of control, F5, F6, and F2, F3 treatment and pure eucalyptus oil. [Click here to view] |
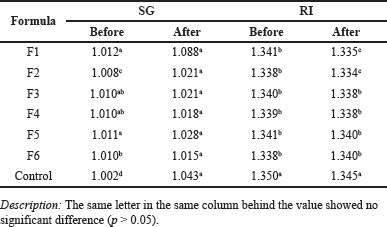 | Table 4. SG and RI before and after the stability test. [Click here to view] |
Characteristics after the stability test
The mouthwash formula produced was very stable, as evidenced by the characterization results before and after the stability test. It occurred for all parameters namely particle size, SG, and index of refraction (Tables 3 and 4).
Particle size
For particle size and PdI, there was a slight increase in particle size in almost all formulas. The rise in PdI was quite significant for F5, while for F1, F3, and F4, it nearly doubled but fell in F2 and F6. The increase in PdI was probably due to the agglomeration of emulsion droplets, but it still showed a reasonably small particle size below 20 nm for F1–F4. The results of this study were the droplet size of the mouthwash emulsion.
Tween-80 has the effect of lowering transmittance and increasing viscosity, while PEG has the opposite effect. Tween is a surfactant, and PEG is an anionic cosurfactant. Combining cosurfactants with surfactants will produce smaller and more stable particle sizes than single surfactants [30]. Moreover, using surfactants alone cannot reduce surface tension, so cosurfactants are added to increase the film’s flexibility [31].
Specific gravity
The research results’ SG is 1.008–1.012 before the stability test and 1.015–1.088 after experienced shock thermic treatment. The high and low exposure temperatures seem to affect the mass weight of the products. There was a significant difference according to the different emulsifier ratios on the SG of mouthwash. However, the SG was statistically similar after shock treatments (Table 4). Compared with the control, the commercial mouthwash labeled with herbal, the SG was the lowest at 1.002. The lower the Tween emulsifier and the higher PEG tend to give lower SG. The SG is similar to another research using lime peel oil-based mouthwash with a particular gravity value between 0.986 and 1.006 [31].
While the SG tends to increase after the stability test treatment, the refractive index (RI) tends not to change, as shown in Table 4. The RI of control (commercial herbal mouthwash) was higher than that of the mouthwash of the research. The RI value ranges from 1.338 to 1.341 before the stability test and becomes 1.335–1.340 after thermic treatment. The RI tends to increase by increasing PEG and decreasing Tween. Water content can raise the RI value of EOs. The higher the water content there is, the lower the RI. This is because of the nature of the water, which readily refracts the incidence light [32].
Color
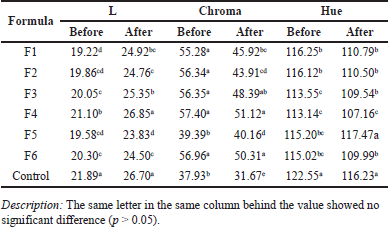
The color test using a chromameter showed that brightness (L) has a significantly different value between the samples and the control, where the clarity of the control, which was a commercial mouthwash, was higher than that of the samples. Meanwhile, after the stability test, the clarity values for all samples and controls tend to increase where the clarity of formula 4 is the highest and becomes similar to the control value. Thermic shock treatment by cooling and heating will likely evaporate some of the impurities so that the color of the solution becomes brighter. The L value was a bit different from the measurement of transmittance shown in Table 2, in which the highest clarity was F3, followed by F1 and F4. However, the L values of F3 and F4 are statistically different (25.353 and 26.853) (Table 5).
The Hue value has the same tendency as the L value, where the control has a higher value and is statistically different. The hue value is a value that indicates the dominant wavelength that determines the color of the material, namely red, blue, green, or yellow. Meanwhile, the Chroma value has a different pattern where the control value is the lowest, much lower than the sample. The value of C also tends to decrease after the stability test. The chroma value indicates the intensity of a color. The lowest C value suggests that the color of the low-intensity material is getting weaker or faded; on the contrary, the higher it is, the more striking red–yellow–green color is. The addition of green food coloring in mouthwash is 0.02 ml for 100 ml, which is the same volume for all formulas. The interaction between EOs and other additives and coloring agents only slightly affects the clarity but affects the chroma. The color intensity is related to the solubility and stability of the resulting material; the higher the intensity and brightness of the color, the better the solubility and stability of the material so that it does not quickly settle [33]. After the stability test, the L increases, but the a and b decrease. This phenomenon is the opposite with green tea ready-to-drink, in which the L and b values decrease during storage, but a value increases [34]total catechins (4-dimethylaminocinnamaldehyde.
Organoleptic test
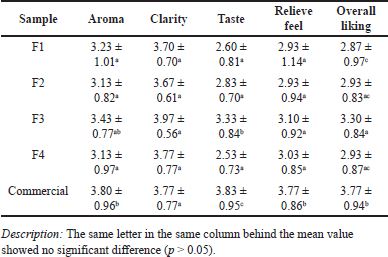
Organoleptic tests using 30 respondents were carried out on four formulation samples with high clarity, namely F1, F2, F3, and F4, in comparison with commercial mouthwash labeled “herbal.” From the results of the organoleptic test, it can be concluded that the level of preference is still below the commercial mouthwash, as shown in Table 6. The clarity attribute follows consumer acceptance, but for other attributes, it ranges from slight dislike or somewhat like. In the aroma attribute, the mouthwash formulations tested were not significantly different from one another, but on the contrary, when compared to commercial. The highest level of respondents’ preference for the aroma was on formulation F3, which is not significantly different from commercial mouthwash. EOs of Eucalyptus and peppermint oil have a robust aroma, even at a low percentage of 0.5% each. The use of emulsifiers also affects organoleptic acceptability. Tween-80 has a reasonably strong aroma and a bitter taste that causes a decrease in respondents’ acceptance of the taste. Taste is a biological perception, such as sensations produced by aroma receptors in the nose and taste receptors in the mouth [35]. The overall liking of the tested formulation offers a score range of 2.87–3.30 (dislike slightly–neither like nor dislike), with formulation F3 having the highest preference, although still below the commercial preference.
 | Table 5. L value, chroma, and hue of mouthwash before and after the stability test. [Click here to view] |
 | Table 6. Mean of organoleptic properties for mouthwash compared with commercial sample mouthwash. [Click here to view] |
CONCLUSION
The ratio 3:2 of Tween and PEG emulsifier gave a higher clarity value, with smaller particle size. After thermal shock treatment, the mouthwash nanoemulsion produced stable and only slight physical characteristics changes. The pH of the emulsion was 5.61–5.91, which is still the range of mucosa pH. The results of the inhibitory test against S. aureus showed that the eucalyptus oil treatment, in general, has not demonstrated inhibition except for F5 and F6 treatments. The best treatment based on physicochemical characteristics was the ratio Tween 80:PEG 400 = 3:2.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research is a part of the research and innovation consortium funding program to accelerate the handling of Corona Virus Disease 2019 (COVID-19) with the title: Production of Antiviral Natural Bioactive Compounds from Endophytic Microbes of Eucalyptus Origin. Funded by the National Priority Program of the Kemenristek – BRIN through the LPDP Project and the Ministry of Finance. Grant number: 24/FI/P-KCOVID-19.2B3/IX/2020
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The study protocol was approved by the Ethics Committee of the Research Organization for Agriculture and Food, National Research and Innovation Agency, Indonesia with [Approval No. 66/Kpts/OT.050/H.4.3/XI/2020].
DATA AVAILABILITY
All the data generated and analyzed are included in this report.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Mieres-Castro D, Ahmar S, Shabbir R, Mora-Poblete F. Antiviral activities of eucalyptus essential oils: their effectiveness as therapeutic targets against human viruses. Pharmaceuticals. 2021;14:1210. CrossRef
2. Asif M, Saleem M, Saadullah M, Yaseen HS, Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28:1153–61. CrossRef
3. Warnke PH, Sherry E, Russo PAJ, Açil Y, Wiltfang J, Sivananthan S, et al. Antibacterial essential oils in malodorous cancer patients: clinical observations in 30 patients. Phytomedicine. 2006;13:463–7. CrossRef
4. Chahomchuen T, Insuan O, Insuan W. Chemical profile of leaf essential oils from four Eucalyptus species from Thailand and their biological activities. Microchem J 2020;158:105248. CrossRef
5. Insuan W, Chahomchuen T. Chemical composition and antimicrobial activity of essential oil extracted from Eucalyptus citriodora leaf. Microbiol Biotechnol Lett 2020;48:148–57. CrossRef
6. Zulnely Z, Gusmailina G, Kusmiati E. Prospek Eucaliptus citriodora sebagai minyak atsiri potensial. Pros Semin Nas Masy Biodiversiti Indones. 2015;1:120–6. CrossRef
7. Chandorkar N, Tambe S, Amin P, Madankar C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phytomed Plus. 2021;1:100089. CrossRef
8. Winarti C, Utami R, Wahyuningsih K, Gusmaini KW, Agustaningrum CA. Formulasi dan karakterisasi hard candy pelega tenggorokan berbasis nanoenkapsulat minyak eukaliptus (Eucalyptus citriodora). J Littri 2021;27:58–68.
9. Astiani DP, Jayuska A, Arreneuz S. Uji aktivitas antibakteri minyak atsiri eucalyptus pellita terhadap bakteri Escherichia coli dan Staphylococcus aureus. J Kim Khatulistiwa 2014;3:49–53.
10. Pratiwi R. The difference of inhibition zones toward Streptococcus mutans among several herbal toothpaste. Dent J. (Majalah Kedokt Gigi) 2005;38:64. CrossRef
11. Widyastuti W, Fantari HR, Putri VR, Pertiwi I. Formulasi pasta gigi ekstrak kulit jeruk (Citrus sp.) dan daun mint (Mentha piperita L.) serta aktivitas terhadap bakteri Streptococcus mutans. J Pharmascience 2019;06:111–9.
12. Juergens U, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med 2003;97:250–6. CrossRef
13. Ragul P, Dhanraj M, Jain AR. Efficacy of eucalyptus oil over chlorhexidine mouthwash in dental practice. Drug Invent Today 2018;10:638–41.
14. McCullough MJ, Farah CS. The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashes. Aust Dent J 2008;53:302–5. CrossRef
15. Marchetti E, Tecco S, Caterini E, Casalena F, Quinzi V, Mattei A, et al. Alcohol-free essential oils containing mouthrinse efficacy on three-day supragingival plaque regrowth: a randomized crossover clinical trial. Trials 2017;18:154. CrossRef
16. Sharma N, Bansal M, Visht S, Sharma P, Kulkarni G. Nanoemulsion: a new concept of delivery system. Chronicles Young Sci. 2010;1:2–6.
17. Mason TG, JWilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter 2007;19:79001. CrossRef
18. Yosephine AD, Wulanjati MP, Saifullah TN, Astuti P. Mouthwash formulation of basil oil (Ocimum basilicum L.) and in vitro antibacterial and antibiofilm activities against Streptococcus mutans. Tradit Med J. 2013;18:2013.
19. McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 2011;7:2297–316. CrossRef
20. Badran M. Formulation and in vitro evaluation of flufenamic acid loaded deformable liposomes for improved skin delivery. Dig J Nanomater Biostruct. 2014;9:83–91.
21. Odriozola-Serrano I, Oms-Oliu G, Martín-Belloso O. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front Nutr. 2014;1:1–4. CrossRef
22. Villar AMS, Naveros BC, Campmany ACC, Trenchs MA, Rocabert CB, Bellowa LH. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int J Pharm. 2012;431:161–75. CrossRef
23. Wahyuningsih I, Putranti W. Optimaziton of tween 80 and polyethyleneglycol 400 for black cumin seed oil in self nanoemulsifying drug delivery system (SNEDDS) formula. Pharmacy 2015;12:223–41.
24. Juniatik M, Hidayati K, Priskaningtyas Wulandari F, Pangestuti N, Munawaroh I, Martien R, et al. Formulation of nanoemulsion mouthwash combination of lemongrass oil (Cymbopogon citratus) and Kaffir Lime Oil (Citrus hystrix) against Candida albicans ATCC 10231. Tradit Med J. 2017;22:7–15.
25. Lalwani JT, Thakkar VT, Patel HV. Enhancement of solubility and oral bioavailability of ezetimibe by a novel solid self nano emulsifying drug delivery system (SNEDDS). Int J Pharm Pharm Sci. 2013;5:513–22.
26. Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of pharmaceutical excipients. 6th ed. London, UK: Pharmaceutical Press and The American Pharmacist Association; 2009.
27. Rismarika R, Maharini I, Yusnelti Y. Pengaruh konsentrasi PEG 400 sebagai kosurfaktan pada formulasi nanoemulsi minyak kepayang. Chempublish J 2020;5:1–14. CrossRef
28. Barman I, Umesh CPG. Effects of habitual arecanut and tobacco chewing on resting salivary flow rate and pH. Int J Oral Heal Med Res 2015;2:13–8.
29. Hutadjulu TJ, Retnoningrum DS, Fudholi A, Sumirtapura YC, Ibrahim S. Suplemen I Farmakope Indonesia. VI ed. Jakarta, Indonesia: Kementerian Kesehatan, RI; 2021
30. Polychniatou V, Tzia C. Study of formulation and stability of co-surfactant free water-in-olive oil nano- and submicron emulsions with food grade non-ionic surfactants. JAOCS, J Am Oil Chem Soc 2014;91:79–88. CrossRef
31. Priya S, Koland M, Suchetha Kumari N. Nanoemulsion components screening of quetiapine fumarate: effect of surfactant and co surfactant. Asian J Pharm Clin Res 2015;8:136–40.
32. Mulyanti S, Laela DS, Julaeha E, Suwargiani AA, Aripin D. Formulation of mouth rinse from the essential oils of lime (Citrus aurantifolia) and its inhibitory efficacy on the growth of Streptococcus mutans—in vitro. Padjadjaran J Dent 2020;32:39. CrossRef
33. Christiana M, Radiati L, Purwadi P. Effect of gum arabic on organoleptic, color, pH, viscosity, and turbidity of apple concentrated honey drink. J Ilmu Dan Teknol Has Ternak 2015;10:46–53. CrossRef
34. Urías-Orona V, Niño-Medina G. Changes in phenolics and antioxidant capacity during short storage of ready-to-drink green tea (Camellia sinensis) beverage at commercial conditions. Bragantia 2019;78:141–5. CrossRef
35. Tarwendah IP. Comparative study of sensory attributes and brand awareness in food product?: a review. J Pangan Dan Agroindustri 2017;5:66–73.